Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 12
A Challenge in Diagnosis of Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome (TB-IRIS)
Authors Suryana K
Received 30 March 2020
Accepted for publication 27 June 2020
Published 21 July 2020 Volume 2020:12 Pages 263—269
DOI https://doi.org/10.2147/HIV.S254105
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bassel Sawaya
Ketut Suryana
Department of Internal Medicine, Merpati Clinic, HIV and Allergy - Clinical Immunology Services Unit, Wangaya Hospital, Denpasar, Bali, Indonesia
Correspondence: Ketut Suryana
Department of Internal Medicine; Merpati Clinic; HIV and Allergy - Clinical Immunology Services Unit, Wangaya Hospital, Akasia Street, VIII No. 22, Denpasar, Bali 80235, Indonesia
Tel +628 5953783944
Email [email protected]
Abstract: Tuberculosis immune reconstitution inflammatory syndrome (TB-IRIS) in HIV-infected patients is the sign and symptom of exacerbation, or radiological manifestation of Mycobacterium tuberculosis (Mtb) infection, can describe the improvement of the immune system after initiating highly active antiretroviral therapy (HAART). No approved or explicit symptomatic tests for TB-IRIS exist, the diagnosis depends on the clinical manifestations. Here we report a TB-IRIS case with diagnostic challenges.
Keywords: HIV infection, tuberculosis, HAART, TB-IRIS, diagnostic challenge
Introduction
Immune reconstitution inflammatory syndrome (IRIS) is an excessive immune response to a variety of pathogens in response to highly active antiretroviral therapy (HAART) mediated recovery of the immune system in HIV-infected patients.1,2 TB-IRIS is a worsening, or progress, of new tuberculosis disease in a patient who had TB treatment previously, after initiating HAART (paradoxical TB-IRIS). Another type, an unmasking TB-IRIS is a new confirm of TB with especially acute inflammatory manifestation after initiating HAART.3–7 Paradoxical reactions to TB involve enlargement of lymph nodes (large adenopathies), abscesses, and worsening radiological features of TB.2,5
Studies in the US and Europe found that the rate of TB-IRIS was from 11% to 45%.8
No specific test can rule out the diagnosis of TB-IRIS. Therefore, it may become a major challenge and it will have the potential to be misdiagnosed as a HAART or opportunistic infection (OI) treatment toxicity, OI drug resistance, other new OI and poor adherence to treatment. The paradoxical TB-IRIS diagnostic clue is the existence of symptoms and signs related to previous OI while the treatment is ongoing, to be worse, after an earlier response to the treatment prior to HAART.9,10
The International Network for the Study of HIV-associated IRIS (INSHI) in 2008 reported the criterion for TB-IRIS diagnosis which was aimed for usage with limitedlaboratory infrastructure.8 The diagnosis of TB-IRIS based on strict criteria should be applied. Delays in diagnosis and treatment can cause exaggerated inflammatory response and are associated with high mortality.5,9 Here, we report a case of tuberculosis immune Reconstitution inflammatory syndrome (TB-IRIS).
The patient who participated in this study specifically provided written informed consent for the publication of the case and any accompanying images. The study obtained ethical approval from the Ethical Committee of Wangaya Hospital in Denpasar, Bali, Indonesia with register number: 15/RSUDW/Litbang/2020 and has approved publication of the case details. The study was conducted in accordance with the Declaration of Helsinki.
Case Presentation
A 33 year-old male patient living with HIV/AIDS, visits Merpati Clinic at Wangaya Hospital in Denpasar, Bali, Indonesia, every month routinely. On May 6, 2019, he presented with prolonged fever, chronic cough, night sweats, decrease of appetite, decrease of body weight and no enlargement of lymph nodes. The past medical history was confirmed with HIV infection. There were other family members with the same complaint. Vital signs of the patients were: body temperature 37.8°C, arterial blood pressure: 120/70, and heart rate: 96/min regularly. On physical examination we found rales in chest auscultation at both sides, no lymph node enlargement. On the chest X-ray there were infiltrates in both of the lungs (Figure 1).
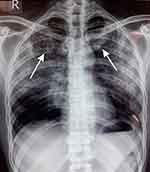 |
Figure 1 Chest X-ray shows bilateral infiltrates and no cardiomegaly. Left and right arrow showed the lesion (infiltrate). |
The laboratory results found no increase of white blood cells, no thrombocyte abnormality and no anemia. The peripheral smear showed no abnormality, without atypical cells. No hepatic function, no electrolyte and no renal function abnormalities were reported. Erythrocyte sedimentation level was 48 (normal range: 0–20 mm/h). With CD4 T cell depletion, the number of CD4 was 150 cell/µL and Xpert HIV-1 viral load of 5.3 log10 copies/mL.
The working diagnosis was HIV-infected patient (WHO stage IV) and confirmed with active pulmonary TB and he was treated with antituberculosis drugs (ATDs).
On July 3, 2019, at the end of the intensive phase (eight weeks after taking ATD treatment), the patient's condition had stabilized or improved; clinically he felt better (no cough, no fever, no night sweats, no decrease in appetite, no enlargement of lymph nodes). The number of CD4 was decreased: 150 to 53 cells/µL. Xpert HIV-1 viral load was increased to 5.8 log10 copies/mL. Chest X-ray showed no abnormality (Figure 2).
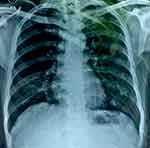 |
Figure 2 Chest X-ray was showed no abnormality. |
The conclusion was TB improvement (had stabilized) and he had been started on HAART with fixed dose combination/FDC (tenofovir, lamivudine and efavirenz).
On October 1, 2019; 12 weeks after taking HAART, he complained of an enlarged lymph node in the left anterior of his neck. On physical examination we found abscesses (cold abscesses), also experienced with weight loss: 53 kg to 50 kg (5.7%) in the last month, fever (37.6°C body temperature), night sweats and cough. Increased blood CD4 T cell count after taking HAART (53 cell/μL to 650 cell/μL). Viral load: HIV-1 not detected (Figure 3).
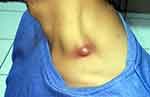 |
Figure 3 Enlarged lymph nodes in the left anterior of neck, the supraclavicular region, abscesses (cold abscesses). |
Worsening the radiologic feature of TB (chest X-ray showed a minimal infiltrate subclavicular in the right lung) (Figure 4). In this case, the Mantoux test was positive with induration about 25 mm (Figure 5)
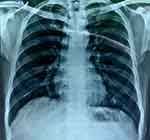 |
Figure 4 Chest X-ray showed a minimal infiltrate sub-clavicular in the right lung. |
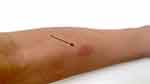 |
Figure 5 Mantoux test result (induration about 25 mm). The arrow shows mantoux test result. |
In this case, there were no signs and symptoms associated with other OIs, some conditions associated with drug toxicity to ATDs.
The patient has good adherence to both ATDs and antiretroviral (ARV). He always takes the drugs (ATD, ARV) every day routinely.
This case was an HIV-infected patient (WHO stage IV) and confirmed with active pulmonary TB with CD4 count 150 cell/µL and Xpert HIV-1 viral load of 5.3 log10 copies/mL. He was treated with ATD and after eight weeks of taking ATD treatment the patient was clinically stabilized or improved, but CD4 level was decreased: 150 to 53 cells/µL, Xpert HIV-1 viral load was increased to 5.8 log10 copies/mL. Twelve weeks after initiating HAART, CD4 cell level was increased rapidly (53 to 650 cells/µL) and rapid suppression of HIV viral load (from viral load of 5.8 log10 copies/mL to not detected) (Figure 6).
 |
Figure 6 The Viral load (HIV RNA copies/mL) and CD4 cell count over time in paradoxical TB-IRIS patient. |
Viral load (HIV RNA copies/mL) and the CD4 cell count over time in paradoxical TB-IRIS patient is shown in Figure 6.
However, the patient complained of an enlarged lymph node in the left anterior of his neck. On physical examination e abscesses were found (cold abscesses), weight loss: 53 kg to 50 kg (5.7%), fever (the patient was: 37.6°C body temperature), night sweats and cough and chest X-ray showed a minimal infiltrate.
The final conclusion of this case is paradoxical TB-IRIS in an HIV-infected patient (WHO Stage IV).
There is no consensus yet on the TB-IRIS standard treatment. This case was treated by continuing the ATD, HAART, and other symptomatic and supportive treatment. Corticosteroids at a starting dosage of 1 mg/kg body weight/day. The patient’s condition gradually improves day by day. The schematic of case progress note is shown in Figure 7.
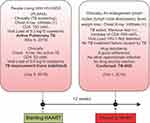 |
Figure 7 The schematic of case progress note. |
Discussion
Paradoxical TB-IRIS is a worsening or progress of new tuberculosis disease in patients taking ATD after initiating HAART.11–14 Paradoxical reactions mostly occur within 4–12 weeks of treatment initiation.8 This case occured 12 weeks after taking HAART. The paradoxical TB-IRIS incidence is estimated at 18% (95%CI 16–21%) or over 50% in high risk groups.3
The risk factors for the progress of IRIS mostly were reported by the case studies in a low CD4 count, a high viral load and an early initiation HAART to be close to the opportunistic infection treatment.8,15,16
In this case, the host-related risk factors were a low CD4 count at initiation of HAART (CD4 count was 53 cell/μL) and tuberculosis prior to HAART initiation. High pre-HAART HIV viral load (5.8 log10 copies/mL) is the risk factor of TB-IRIS. The shorter duration of OI treatment prior to starting HAART, in this case is eight weeks. Rapid HIV viral load rapid suppression (from a viral load of 5.8 log10 copies/mL to not detected) is another risk factor that we found in this case which was treatment related.
The most common clinical manifestations include systemic, pulmonary, and lymph node.3,17–19 In this case there is a systemic manifestation such as fever (the body temperature: 37.6°C), night sweats, cough and the patient also experienced weight loss 53 kg to 50 kg (5.7%), worsening of the radiologic feature of TB (chest X-ray showed a minimal infiltrate subclavicular in the right lung) and the enlargement of lymph node. In this case there is an enlarged lymph node in the left anterior of neck, the supraclavicular region.
There is no specific or validated diagnostic test for TB-IRIS and this clinical diagnosis therefore remains a major challenge, particularly in low-resource settings.3 The paradoxical reaction phenomenon during TB treatment in which existing disease worsens has been recognized prior to the association with HIV coinfection. Previous study reported a severity of paradoxical TB-IRIS and increasing frequency, both in patients taking HAART and those not on HAART. The “paradoxical IRIS” diagnosis requires a diagnosis of TB and an underlying reaction to ATD, along with at least one of four major or two of three minor clinical criteria and exclusion of alternative explanations (International Network for the Study of HIV-associated IRIS/INSHI).8,14,20–22
Definition case of paradoxical TB-IRIS for use in resource settings:8
- Antecedent requirements (both are must)
- TB diagnosis (to be in line with WHO criteria and made before starting of HAART)
- Initial response of TB treatment (before HAART initiation, patient conditions is stabilized or better or appropriate TB treatment)
- Clinical criteria such as:
- TB IRIS manifestations start should be within 12 weeks of HAART initiation, reinitiation or regimen change caused by treatment failure
- Needed minimum two minor clinical criteria or one major criterion
- Major criteria (initial or worsening radiological features of TB; initial or enlarging lymph nodes, other tissue involvement or cold, abscesses; initial or worsening serositis; initial or worsening central nervous system/CNS TB)
- Minor criteria (initial or worsening constitutional symptoms such as night sweats, fever, or weight loss; initial or worsening abdominal pain accompanied by hepatomegaly, splenomegaly, peritonitis, or abdominal adenopathy; initial or worsening respiratory symptoms such as cough, dyspnea or stridor)
- Excluded the alternative cause of clinical deterioration
- Drug toxicity or reaction
- TB treatment failure caused by TB drug resistance
- Another opportunistic infection or neoplasm
- Poor adherence to TB treatment
In this case the diagnosis of TB (fulfill WHO criteria) is made before starting HAART. He is treated by giving ATD (450 mg rifampicin; 400 mg isoniazid; 1000 mg pyrazinamide; 750 mg ethambutol; 25 mg pyridoxin); all of the regimens are given once a day orally. The TB treatment initial response: before HAART initiation, patients condition should be stabilized or improved on appropriate TB treatment. Based on the clinical criteria: the onset of TB associated IRIS manifestation is within one to three months of HAART initiation. In this case TB-IRIS is confirmed after three months (12 weeks) of taking HAART. The major criteria is new or enlarging lymph nodes with cold abscesses, new or worsening radiological features of TB (chest X-ray showed a minimal infiltrate subclavicular in the right lung) and with minor criteria is weight loss: 53 kg to 50 kg (5.7%) in the last month.
The other excluded causes of clinical deterioration are no TB treatment failure caused by resistance of TB drug, the patient with a good TB treatment adherence, no other OI or neoplasm and no reaction or toxicity of drug: ATD, ARV.23–25 In this case, clinically we did not find the clinical condition which shows side effects/toxicity caused by ATD or ARV.
Mantoux test (tuberculin test), although it is not used to diagnose latent TB infection or TB routinely, the conversion of a negative result of tuberculin test to be positive initially when IRIS occurs, has been recommended as a marker for IRIS.26
Mantoux test result: the reaction size of the Mantoux test, is associated with the future risk of developing TB disease. The result of the Mantoux test: 5 mm or more is positive in: recent contacts of active tuberculosis cases, HIV-positive patients, other immunosuppressed patients, organ transplant recipients and, patients on long-term systemic corticosteroid therapy, end stage renal disease.26 In this case, the HIV-positive patient the size of induration was 25 mm.
It is now considered that tuberculosis is as an indication for HAART irrespective of CD4 count, also HAART initiation does not mean without adverse effects especially in the first six months.9 CD4; the most common risk factor for the IRIS development, an early initiation HAART close to the opportunistic infection treatment when there is a low CD4 cell count. A high viral load and a low CD4 cell count are the most common risk factors for the IRIS development.8,9
Other data such as chest X-ray, showed a minimal infiltrate subclavicular in the right lung supports active TB (TB-IRIS). Clinically, this patient with the TB improvement, but with the high viral load (5.3 log10 copies/mL) and a low CD4 cell count (53 cell/µL) when the HAART was started. Excluded other causes of clinical deterioration: a good adherence of TB treatment, no TB drug resistance, no drug toxicity or reaction or no nother OI or neoplasm. Therefore, based on the clinical and laboratory findings, this case was confirmed as paradoxical TB-IRIS.
Management of paradoxical TB-IRIS involves the continuation of anti-TB therapy and HAART.27 Clarification to exclude certain conditions such as sepsis, supportive therapy, symptomatic therapy, surgical/percutaneous interventions (drainage of abscess) and inhibition of excessive immune responses by giving corticosteroids.3,9,28 After three months of treatment, the patient had good clinical condition.
Conclusions
The heterogeneity of TB-IRIS clinical manifestations and lack of a specific diagnostic test, may lead to TB-IRIS potentially being misdiagnosed and it remains a major challenge, especially in low-resource settings. The potential risk factors of TB-IRIS include low CD4 count, high HIV viral load at HAART initiation and shorter duration of OI treatment prior to starting HAART (paradoxical IRIS). A TB-IRIS diagnosis requires a TB diagnosis and an initial response to anti-TB therapy together with at least two of three minor clinical criteria and exclusion of alternative explanations or at least one of four major criteria. In consequence, the following strict criteria have to be applied to diagnose TB-IRIS. There is no standard treatment of TB-IRIS, corticosteroids significantly reduces days of hospitalization, more rapidly improving clinical manifestations, quality of life score and chest X-ray.
Acknowledgment
The author appreciates all of the doctors who are in duty at Emergency Unit, the Intensive Care Unit Crew and other colleagues; Wahyu Semara Pulmonologist, Novitasari Pathologist, Noviana Joenputri General Practitioner at Wangaya hospital in Denpasar, Bali, Indonesia.
Disclosure
The author declares that there is no conflict of interest.
References
1. Gopal R, Rapaka RR, Kolls JK. Immune reconstitution inflammatory syndrome associated with pulmonary pathogens. Eur Respir. 2017;26:160042. doi:10.1183/16000617.0042-2016
2. Okechukwu AA, Mamuna CC. Immune reconstitution inflammatory syndrome: case series from Abuja. J Dent Med Med Sci. 2011;1(1):001–004.
3. Walker NF, Stek C, Wasserman S, Wilkinson RJ, Meintjes G. The tuberculosis-associated immune reconstitution inflammatory syndrome: recent advances in clinical and pathogenesis research. Curr Opin HIV AIDS. 2018;13:512–521. doi:10.1097/COH.0000000000000502
4. Ali K, Klotz SA. The immune reconstitution inflammatory syndrome with tuberculosis: a common problem in Ethiopian HIV-infected patients beginning antiretroviral therapy. J Int Assoc Provid AIDS Care. 2012;11(3):198–202.
5. Lapadula G, Soria A, Bandera A, et al. Unmasking tuberculosis in the era of antiretroviral treatment. Eur Respir J. 2012;39:1064–1075. doi:10.1183/09031936.00116611
6. Rapose A, Karande S. Tuberculosis human immunodeficiency virus and the immune reconstitution inflammatory syndrome. J Postgrad Med. 2017;63(4):207–209. doi:10.4103/jpgm.JPGM_365_17
7. Spengler U, Lichterfeld M, Rockstroh JK. Antiretroviral drug toxicity – a challenge for the hepatologists? J Hepatol. 2002;36:283–294. doi:10.1016/S0168-8278(01)00311-7
8. Viskovic K, Begovac J. Tuberculosis-associated immune reconstruction inflammatory syndrome (TB-IRIS) in HIV-infected patients: report of two cases and the literature overview. Case Rep Infect Dis. 2013;2013:1–7. doi:10.1155/2013/323208
9. Walker NF, Scriven J, Meintjes G, Wilkinson RJ. Immune reconstitution inflammatory syndrome in HIV infected patients. HIV/AIDS-Res Palliative Care. 2015;7:49–64.
10. Meintjes GM, Stek C, Thienemann BF, et al. Prednison for the prevention of paradoxical tuberculosis-associated IRIS. N Engl J Med. 2018;379:1915–1925. doi:10.1056/NEJMoa1800762
11. Lanzafame M, Vento S. Tuberculosis-immune reconstitution inflammatory syndrome. J Clin Tuberc Other Mycobact Dis. 2016;3:6–9. doi:10.1016/j.jctube.2016.03.002
12. Namale PE, Abdullah LH, Fine S, Kamkuemah M, Wilkinson RJ, Meintjes G. Paradoxical TB-IRIS in HIV-infected adults: a systematic review and meta-analysis. Future Microbiol. 2015;10(6):1077–1099. doi:10.2217/fmb.15.9
13. Bell LCK, Breen R, Miller RF, Noursadeghi M, Lipman M. Paradoxical reaction and immune reconstitution inflammatory syndrome in tuberculosis. Int J Infect Dis. 2015;32:39–45. doi:10.1016/j.ijid.2014.12.030
14. Sarkar S, Ganguly A, Sunwoo HH. Current overview of anti tuberculosis drugs: metabolism and toxicities. Mycobact Dis. 2016;6:1–6. doi:10.4172/2161-1068.1000209
15. Padua CM, Braga LP, Mendicino CCP. Adverse reactions to antiretroviral therapy: a prevalent concern. Rev Panam Salud Publica. 2017;41:e84.
16. Bana TM, Lesosky M, Pepper DJ, et al. Prolonged tuberculosis-associated immune reconstitution inflammatory syndrome: characteristic and risk factors. BMC Infect Dis. 2016;16:518. doi:10.1186/s12879-016-1850-2
17. Narendran G, Andrade BB, Porter BO, et al. Paradoxical tuberculosis immune reconstitution inflammatory syndrome (TB-IRIS) in HIV patients with culture confirmed pulmonary tuberculosis in India and the potential role of IL-6 in prediction. PLoS One. 2013;8:e63541. doi:10.1371/journal.pone.0063541
18. De D, Sarkar RN, Phaujdar S, Bhattacharyya K, Pal HK. Incidence and risk factors of immune reconstitution inflammatory syndrome in HIV-TB coinfected patients. Braz J Infect Dis. 2011;15(6):553–559. doi:10.1016/S1413-8670(11)70250-1
19. Gopalan N, Andrade BB, Swaminathan S. Tuberculosis-immune reconstitution inflammatory syndrome in HIV: from pathogenesis to prediction. Expert Rev Clin Immunol. 2014;10:631–645.
20. Beishuizen SJE, Geerlings SE. Immune reconstitution inflammatory syndrome: immunopathogenesis, risk factors, diagnosis, treatment and prevention. Neth J Med. 2009;67:327–331.
21. Wilson IE, Havers F, Nachega JB, et al. Evaluation of paradoxical TB-associated IRIS with the use of standardized case definitions for resource-limited settings. J Int Assoc Physicians AIDS Care. 2010;9(2):104–108. doi:10.1177/1545109710361537
22. Karanja JK, Kiboi NG, Nebere SN, Achieng HO. Highly active antiretroviral therapy and anti-tuberculosis drug interactions with associated clinical implications: a review. J Drug Metab Toxicol. 2016;7:20.
23. Sharma R, Sharma VL. Review: treatment of toxicity caused by anti-tubercular drugs by use of different herbs. Int J Pharma Sci Res. 2015;6:1288–1294.
24. Tweed CD, Crooko AM, Amkoye EI, et al. Toxicity associated with tuberculosis chemotherapy in the REMoxTB study. BMC Infect Dis. 2018;18:317. doi:10.1186/s12879-018-3230-6
25. Meintjes G, Lawn SD, Scano F, et al. Tuberculosis associated immune reconstitution inflammatory syndrome: case definition for use in resource-limited settings. Lancet Infect Dis. 2008;8(8):516–523. doi:10.1016/S1473-3099(08)70184-1
26. Swaminathan S, Narendran G. HIV and tuberculosis in India. J Biosci. 2008;33(4):527–537. doi:10.1007/s12038-008-0071-2
27. Lai RPJ, Meintjes G, Wilkinson RJ. HIV-1 tuberculosis-associated reconstitution inflammatory syndrome. Semin Immunopathol. 2015. doi:10.1007/s00281-015-0532-2.
28. Narendran G, Souza DOD, Vinhaes CL, et al. Multifocal tuberculosis-associated immune reconstitution inflammatory syndrome – a case report of a complicated scenario. BMC Infect Dis. 2019;19:529. doi:10.1186/s12879-019-4182-1
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
