Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
A Case Report of Zosteriform Cutaneous Metastases from Breast Carcinoma
Authors Xu L, Wang Y, Mu Y, Huang Q, Shuai W , Yang H
Received 10 October 2023
Accepted for publication 16 January 2024
Published 24 January 2024 Volume 2024:17 Pages 205—209
DOI https://doi.org/10.2147/CCID.S444101
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Anne-Claire Fougerousse
Liuli Xu,* Yujuan Wang,* Yunzhu Mu, Qing Huang, Wenlong Shuai, Hao Yang
Department of Dermatology, Affiliated Hospital of North Sichuan Medical College, Nanchong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hao Yang, Department of Dermatology, Affiliated Hospital of North Sichuan Medical College, No. 1 Maoyuan South Road, Shunqing District, Nanchong, Sichuan Province, 63700, People’s Republic of China, Tel +8615882626833, Email [email protected]
Abstract: We described a 58-year-old female diagnosed with zosteriform cutaneous metastases from breast carcinoma. She was initially diagnosed with herpes zoster. Correct diagnosis was obtained after pathological biopsy. Various forms of cutaneous metastases have various forms, which require careful discrimination by dermatologists to reduce the rate of misdiagnosis.
Keywords: breast carcinoma, zosteriform, cutaneous metastasis
Introduction
Skin cancers are mostly primary ones including basal cell carcinoma (BCC), squamous cell carcinoma (SCC) and melanoma. But cutaneous metastases from internal malignancies continue to be an underappreciated but important factor, especially as the overall incidence of cancer rises and more cancer survivors need to be monitored for recurrence and metastasis.1 In addition, incidence rate of cutaneous metastases is also gradually increasing.2 Among female patients, approximately 70% of cutaneous metastases related to the breast carcinoma. The metastatic location occurred more near to the primary tumor, either as a result of lymphatic dissemination or direct extension. The majority of lesions of cutaneous metastases from breast carcinoma are painless nodules, single or multiple.3 However, a zosteriform pattern with erythema and nodules is rare. The authors here report a woman with weakened immune system who had lesions along her dermatome and was initially misdiagnosed as having herpes zoster.
Case Report
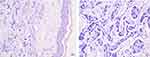
A 58-year-old female was admitted with one-month-old painful and pruritic erythema and nodules on the right thoracic wall, armpit and back. Three and a half years before admission, the patient underwent left breast cancer radical mastectomy and regional lymph node dissection in the local hospital due to the discovery of left breast mass. Postoperative pathological diagnosis was left breast invasive ductal carcinoma, and cancer cells were also found in axillary lymph nodes. Immunohistochemistry showed ER (+), PR (+), HER-2 (-), P120 (+), E-ca (+), CK5/6 (-), Ki-67 (+), CD34 (vascular endothelial cells +). She had received treatment with multiple courses of chemotherapy after the operation. Half a year before admission, the patient was found to have small masses on both sides of the neck and gradually increased. Histopathological examination revealed metastasis to the right breast and lymph nodes. The patient had an appellate rash before admission, accompanied by pain, and was misdiagnosed as herpes zoster in the local hospital. After antiviral treatment, the erythema and nodules did not disappear significantly, and finally, she was admitted to our hospital for treatment. At the current visit, we performed a physical examination of the patient. The right chest wall, axilla, and back were localized in a zosteriform pattern with erythema and scattered or dense red-hard nodules. Some of the nodules are fused with each other (Figure 1). The laboratory testing showed tumor markers (carcinoembryonic antigen and CA-125) obviously increased. A pathological biopsy of the right breast skin revealed that nested arrangement of cancer cells was seen in the dermis, which was compatible with the origin of breast carcinoma (Figure 2). The final diagnosis was zosteriform cutaneous metastases from breast carcinoma. Currently, the female is undergoing endocrine therapy combined with local skin radiotherapy and close follow-up.
 |
Figure 1 Figure 1a–1c: Scattered or clustered erythema and nodules almost on the right thoracic wall, armpit and back, distribution like girdle-shaped. |
Discussion
The term “cutaneous metastases” refers to lesions made up of cancerous cells that have spread from a primary tumor elsewhere in the body.4 About 5% to 10% of solid tumors develop cutaneous metastases as a first sign of malignancy or in the advanced stages of the disease, including lung cancer, melanoma, breast carcinoma, and cancers of the upper respiratory tract (oral cavity, nasal sinuses, and larynx), lung cancer, colon and rectal cancers.5,6 Metastases frequently develop close to the site of the primary malignancy. Breast and lung cancers usually metastasize to the chest wall, while bowel, ovarian and bladder cancers most often metastasize to the abdominal skin. However, distant metastases are not rare.7
Cutaneous metastases usually present as firm, painless, erythematous nodules or masses, while zosteriform cutaneous metastases are less common. A meta-analysis developed by Savoia P et al5 showed a total of 56 cases with zosteriform cutaneous metastases of primary malignant tumors, including lymphoma, breast cancer, squamous cell carcinoma, digestive tumors, respiratory tumors, and urinary tract tumors. In women, the majority of causes of metastatic skin disease are related to breast cancer.3,8 The most typical symptom of cutaneous metastases from breast cancer are firm, asymptomatic nodules. Telangiectatic carcinoma, carcinoma en cuirasse or scirrhous carcinoma, and inflammatory metastatic carcinoma (carcinoma erysipeloid or erysipelatoides) are other clinical manifestations that can look like illnesses like mastitis and cellulitis.3,9 A few cases of zosteriform cutaneous metastases from breast carcinoma have been tabulated in Table 1.
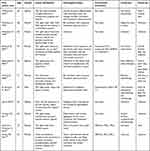 |
Table 1 Case Reports of Zosteriform Cutaneous Metastases from Breast Carcinoma |
Our patient was presented with painful and pruritic erythema and nodules with zosteriform distribution along the T2-T6 dermatome on the thoracic wall, armpit and back of the right side, which was initially misdiagnosed as herpes zoster. First of all, an impairment of the immunological function of tumor patients can easily activate the latent varicella-zoster virus (VZV) in the body. In addition, the rash showed a unilateral zosteriform distribution, and nodules were densely distributed on the basis of erythema with obvious pain, which closely resembled the clinical manifestations of herpes zoster.21 It is still unclear how cancer cells propagate in zosteriform skin metastases. Malignant cells spread through lymphatic vessels, which are consistent with the nerve distribution. Thus, the rash has a zosteriform distribution. In addition, cutaneous metastases sometimes occur at the site of previous herpes zoster, that is Koebner-like reaction. The skin barrier is damaged, and the tumor cells are more likely to infiltrate the nerves invaded by the varicella-zoster virus. Thirdly, tumor cells infiltrate the nerve roots and spread from the dorsal root ganglia to the cutaneous nerves’ perineural lymphatics, so patients are more likely to be painful. Surgical implantation of tumor cells is the significant cause, though our patient’s rash occurs at the opposite site.5,14,22
Typically, cutaneous metastases from internal cancers indicate a bad prognosis. Thus, the presence of widespread cutaneous metastases requires systemic therapy. Tan AR9 thought that the therapy of metastatic cutaneous lesions takes into account the tumor’s hormone receptor (HR), including estrogen receptor (ER) and progesterone receptor (PR) and human epidermal growth factor (HER-2) status, similarly to how visceral metastases are handled. Endocrine medications can be used to treat a tumor that is HR-positive. Chemotherapy is used for cutaneous metastases that are HR-negative and/or growing quickly. Depending on the severity of the disease, a HER-2-positive tumor should either get chemotherapy or HER-2-directed therapy. External beam radiation therapy is a palliative alternative for the local management of skin metastases. Combined with the immunohistochemical analysis of our patient, endocrine therapy and local skin radiotherapy were finally selected.
Conclusion
In short, the clinical manifestations of cutaneous metastases are not specific. If patients with malignant tumors have obvious skin lesions, histopathological examination should be performed to confirm the diagnosis. For patients without a history of cancer, we should also be vigilant and prompt a biopsy to exclude internal malignancies and avoid misdiagnosis.
Ethics Statement
The patient in this manuscript provided written informed consent to the publication of the case details and images. Publication of the case details did not require institutional consent.
Acknowledgments
We appreciate the patient and physicians for participating in our study.
Funding
The work was supported by the Research and Development Project of Affiliated Hospital of North Sichuan Medical College (2020JC010).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Choate E-A, Nobori A, Worswick S. Cutaneous metastasis of internal tumors. Dermatol Clin. 2019;37(4):545–554. doi:10.1016/j.det.2019.05.012
2. Gan E-Y, Chio M-T, Tan W-P. A retrospective review of cutaneous metastases at the national skin centre Singapore. Australas J Dermatol. 2015;56(1):1–6. doi:10.1111/ajd.12194
3. Gonzalez-Martinez S, Pizarro D, Perez-Mies B, et al. Clinical, pathological, and molecular features of breast carcinoma cutaneous metastasis. Cancers. 2021;13(21):5416. doi:10.3390/cancers13215416
4. Jaros J, Hunt S, Mose E, et al. Cutaneous metastases: a great imitator. Clin Dermatol. 2020;38(2):216–222. doi:10.1016/j.clindermatol.2019.10.004
5. Savoia PAOLA, Paolo FAVA, Tommaso DEBOLI, et al. Zosteriform cutaneous metastases: a literature meta‐analysis and a clinical report of three melanoma cases. Dermatol Surg. 2009;35(9):1355–1363. doi:10.1111/j.1524-4725.2009.01241.x
6. Lookingbill D-P, Spangler N, Helm K-F. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2 Pt 1):228–236. doi:10.1016/0190-9622(93)70173-Q
7. Habermehl G, Ko J. Cutaneous metastases: a review and diagnostic approach to tumors of unknown origin. Arch Pathol Lab Med. 2019;143(8):943–957. doi:10.5858/arpa.2018-0051-RA
8. Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34(4):347–393. doi:10.1097/DAD.0b013e31823069cf
9. Tan A-R. Cutaneous manifestations of breast cancer. Semin Oncol. 2016;43(3):331–334. doi:10.1053/j.seminoncol.2016.02.030
10. Williams L-R, Laurie-J L, Young-C K. Cutaneous malignancies mimicking herpes zoster. Int J Dermatol. 1991;30(6):432–434. doi:10.1111/j.1365-4362.1991.tb03900.x
11. Manteaux A, Cohen P-R, Rapini R-P. Zosteriform and epidermotropic metastasis: report of two cases. J dermatol surg oncol. 1992;18(2):97–100. doi:10.1111/j.1524-4725.1992.tb02440.x
12. Heckmann M, Volkenandt M, Lengyel E-R, et al. Cytological diagnosis of zosteriform skin metastases in undiagnosed breast carcinoma. Br J Dermatol. 1996;135(3):502–503. doi:10.1111/j.1365-2133.1996.tb01538.x
13. Brasanac D, Boricic I, Todorovic V. Epidermotropic metastases from breast carcinoma showing different clinical and histopathological features on the trunk and on the scalp in a single patient. J Cutan Pathol. 2003;30(10):641–646. doi:10.1034/j.1600-0560.2003.00130.x
14. Bassioukas K, Nakuci M, Dimou S, et al. Zosteriform cutaneous metastases from breast adenocarcinoma. J Eur Acad Dermatol Venereol. 2005;19(5):593–596. doi:10.1111/j.1468-3083.2005.01205.x
15. Torchia D, Giovanni-M P, Margherita T, et al. Ulcerative carcinoma of the breast with zosteriform skin metastases. Breast J. 2006;12(4):385. doi:10.1111/j.1075-122X.2006.00285.x
16. Al Zouman A, Harthi Fahad A. Male breast carcinoma with zosteriform metastasis. Breast J. 2010;16(1):88–89. doi:10.1111/j.1524-4741.2009.00826.x
17. Rao R, Balachandran C, Rao L. Zosteriform cutaneous metastases: a case report and brief review of literature. Indian J Dermatol Venereol Leprol. 2010;76(4):447. doi:10.4103/0378-6323.66605
18. Virmani N-C, Sharma Y-K, Panicker N-K, et al. Zosteriform skin metastases: clue to an undiagnosed breast cancer. Indian J Dermatol. 2011;56(6):726–727. doi:10.4103/0019-5154.91838
19. Del P-T-C, Munoz-Leiva D, Jaque-Silva A, et al. Breast cancer metastasis misdiagnosed as an angiokeratomatous eruption. an infrequent presentation. case report. Am J Dermatopathol. 2016;38(4):302–304. doi:10.1097/DAD.0000000000000487
20. Cohen P-R. Pleomorphic appearance of breast cancer cutaneous metastases. Cureus. 2021;13(12):e20301. doi:10.7759/cureus.20301
21. Chiang A, Salomon N, Gaikwad R, et al. A case of cutaneous metastasis mimicking herpes zoster rash. IDCases. 2018; 20: 12167–12168.
22. Apalla Z, Chassioti V, Ioannides D, et al. Zosteriform cutaneous metastasis of breast carcinoma in a male patient. Int J Dermatol. 2014;53(7):e358–e359. doi:10.1111/ijd.12327
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.