Back to Journals » International Medical Case Reports Journal » Volume 16
A Case of Tumor-Induced Osteomalacia Detected by Venous Sampling
Authors Horinouchi Y, Shiota S , Kaimori R, Yoshimura K, Utsunomiya-Nishimizu R, Yamamoto K , Miyazaki E
Received 12 June 2023
Accepted for publication 21 September 2023
Published 10 October 2023 Volume 2023:16 Pages 659—665
DOI https://doi.org/10.2147/IMCRJ.S425599
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Yasuo Horinouchi, Seiji Shiota, Ryo Kaimori, Katsuhiko Yoshimura, Rie Utsunomiya-Nishimizu, Kyoko Yamamoto, Eishi Miyazaki
Department of General Medicine, Oita University Faculty of Medicine, Yufu, Oita, 879-5593, Japan
Correspondence: Seiji Shiota, Department of General Medicine, Oita University Faculty of Medicine, 1-1 Idaigaoka, Hasama-machi, Yufu, Oita, 879-5593, Japan, Tel +81-97-586-5106, Fax +81-97-586-5573, Email [email protected]
Abstract: Tumor-induced osteomalacia (TIO) can cause osteomalacia due to excessive production of fibroblast growth factor 23 (FGF23) by the tumor. Since TIO is a very rare disease, it is often misdiagnosed as intervertebral disc herniation, spondyloarthritis, or osteoporosis. We report a 65-year-old man who developed generalized arthralgia and difficulty walking two years ago and was diagnosed with multiple fractures throughout his body. He was initially diagnosed with osteoporosis and was treated with calcitriol. However, he was referred to our hospital since his symptoms did not improve. We diagnosed tumor-induced osteomalacia based on low serum phosphorus, high bone-type alkaline phosphatase, high FGF23 levels, and the presence of two tumors. The responsible tumor was identified using FGF23 levels in venous sampling. As the location of the tumor made surgical resection difficult, we selected treatment with burosumab, a human monoclonal antibody against FGF23, leading to improvement in the hypophosphatemia and pain, such that he was able to walk with a cane. In cases of osteoporosis with hypophosphatemia, general physicians should keep TIO in mind, and attempt to identify the responsible tumor lesion.
Keywords: hypophosphatemia, TIO, FGF23, burosumab
Introduction
Rickets/osteomalacia causes bone fractures and bone pain due to impaired calcification of bone and cartilage. Tumor-induced osteomalacia (TIO) results from excessive production of fibroblast growth factor 23 (FGF23) by a tumor, leading to hypophosphatemia. FGF23 is a hormone produced in bone that suppresses the expression of type 2a and 2c sodium–phosphate cotransporters in renal tubules and inhibits proximal tubular phosphate reabsorption.1,2 In addition, FGF23 reduces serum 1.25-dihydroxyvitamin D [1,25 (OH)2 vitamin D] levels by modifying the expression of vitamin D-metabolizing enzymes.1 Since 1.25 (OH)2 vitamin D increases intestinal phosphate absorption, increased FGF23 reduces serum phosphate levels by inhibiting both renal tubular phosphate reabsorption and intestinal phosphate absorption.1 TIO is a rare paraneoplastic disease, in which most of the primary tumors are classified as benign phosphaturic mesenchymal tumors.3 TIO is often misdiagnosed as intervertebral disc herniation, spondyloarthritis, or osteoporosis, because of its rarity and its under-recognition by clinicians.4 Therefore, delayed diagnosis of TIO is common in clinical practice.
Here, we report a case of TIO that was initially diagnosed as osteoporosis. Although the tumor responsible for TIO was identified by venous sampling, the patient was treated using burosumab, a human monoclonal antibody that blocks the action of FGF23, because surgical resection was not possible. In cases of hypophosphatemia in patients with osteoporosis, general physicians should keep TIO in mind and attempt to identify the responsible tumor.
Case Report
A 65-year-old man with independence in activities of daily living (ADL) and no remarkable medical history presented with generalized joint pain. Approximately two years prior to visiting our department, he began experiencing right ankle joint pain and right hip-thigh pain when walking uphill. As the pain intensified, walking became progressively more difficult, and he experienced several falls. About 8 months before his visit to our hospital, he visited his previous physician because the pain had spread to almost all of his joints. Examination revealed multiple fractures of the sacrum, vertebrae, and ribs, low vitamin D levels, and low bone mineral density (Dual energy X-ray T scores of −4.3 SD) in the femoral bone. He was diagnosed with osteoporosis, and calcitriol treatment was started. However, he was referred to our hospital because his symptoms did not improve despite rehabilitation and administration of calcitriol and analgesics. At that time, he complained of pain in his hip joints, knees, ankles, ribs, and other parts of his body during exertion, such as standing up and walking. Neurological examination did not reveal any abnormal findings. Manual muscle testing (MMT) of his upper and lower limbs indicated a muscle strength of 5/5. His gait was somewhat steady with a walker, but he was not able to walk long distances even with a cane.
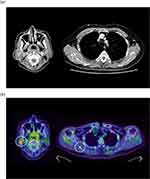
We suspected osteomalacia because blood tests showed low phosphorus (1.58 mg/dL, reference range: 2.7–4.6 mg/dL), high alkaline phosphatase (ALP) (724 U/L, reference range: 38–113 U/L), high bone-type ALP (70.6%, reference range: 25–59%), low 1.25 (OH)2 vitamin D (21 pg/mL, reference range: 20–60 pg/mL) and low 25 (OH) vitamin D (9.7 ng/mL, reference range: >20 ng/mL) levels (Table 1). Urinary phosphorus reabsorption was decreased (ratio of maximal tubular reabsorption of phosphate to glomerular filtration rate, TmP/GFR 1.6 mg/dL, reference range: 2.3–4.3 mg/dL). PTH-intact levels and renal function were within the reference range, ruling out primary hyperparathyroidism and renal failure. As his serum FGF23 level was high (108.0 pg/mL; reference range: <30 pg/mL), a diagnosis of FGF23-related hypophosphatemic osteomalacia was made. Based on his medical history, we determined that his condition was likely acquired rather than congenital, and therefore attempted to identify the responsible tumor. Upper and lower gastrointestinal endoscopy revealed no specific findings. Contrast-enhanced computed tomography (CT) revealed a 6 × 4 mm tumor on the anterior surface of the right scapula, and a 13 mm tumor in the right parotid gland (Figure 1a). 18FDG PET/CT showed FDG accumulation at the same site as the tumors on the anterior surface of the right scapula (SUVmax 3.5) and in the right parotid gland (SUVmax 6.3) (Figure 1b), although qualitative evaluation and tumor differentiation were difficult with 18FDG PET/CT. Somatostatin receptor scintigraphy was also performed, although no abnormal accumulations were observed. The otorhinolaryngology and orthopedics departments were consulted regarding performing tumor biopsy; however, needle biopsy of the parotid gland provided insufficient sample volume to make a diagnosis. Although total tumor excision is necessary for pathological diagnosis, excision of the tumor in the right parotid gland was deemed to carry a high risk of facial nerve palsy, and the approach to the tumor in the right anterior scapula for excision would have been difficult. Therefore, it was necessary to identify the responsible tumor before surgical intervention.
 |
Table 1 Baseline Laboratory Findings |
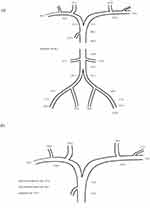
To identify the responsible tumor, venous sampling of FGF23 levels was performed at 26 locations throughout the body, which revealed significantly high FGF23 levels in the right subclavian vein (Figure 2a). However, since the external jugular vein feeds parotid tumors and external jugular venous sampling was not performed, it was not possible to identify the responsible tumor. Therefore, venous sampling was performed again in the upper body, including the external jugular vein (Figure 2b). Since the FGF23 level was significantly higher in the subclavian vein than the right external jugular vein, the responsible tumor was identified as the tumor on the anterior surface of the scapula.
Three months later, follow-up contrast-enhanced CT showed that the right parotid tumor had shrunk slightly, but the tumor on the anterior surface of the right scapula had increased to 11 × 7 mm. Although the orthopedics department was consulted regarding surgical treatment, it was concluded that surgery should not be performed because of the high risk of nerve damage and postoperative pain, so treatment with burosumab was selected.
Since it was difficult for the patient to live alone at home, he was transferred to another hospital for rehabilitation. After the first dose of burosumab, his phosphorus level improved to 2.5 mg/dL, and the knee joint and lumbar pain also tended to improve. Figure 3 shows the subsequent changes in phosphorus levels, which gradually increased with each dose, remaining above normal limits from the second dose onwards. Serum calcium levels also remained normal. With progressive improvement of the pain, the patient was able to walk with a cane. After the fourth dose of burosumab, he was well enough to be discharged to home. On the follow-up contrast-enhanced CT 6 months later, the tumor on the anterior surface of the right scapula had slightly enlarged to 11 × 10 mm. At this time, based on shared decision-making, our patient selected monitoring of tumor size by imaging rather than surgery for its treatment. We plan to continue treatment with burosumab, with follow-up contrast-enhanced CT to monitor for tumor progression. Written informed consent was obtained from the patient for publication of this case report.
Discussion
We experienced a case of TIO that was initially diagnosed as osteoporosis. Subsequently, low serum phosphorus, high bone-type ALP, and high FGF23 levels led to the suspicion of FGF23-related hypophosphatemic osteomalacia. Two tumors were identified on imaging, and the tumor responsible for TIO was subsequently determined by venous sampling. TIO should be suspected in patients with musculoskeletal symptoms, difficulty in walking, and low serum phosphorus levels.4
TIO is a very rare condition that typically presents with non-specific symptoms, such as fatigue, bone pain, and musculoskeletal weakness. Therefore, most cases of TIO are initially misdiagnosed as intervertebral disc herniation, spondyloarthritis, or osteoporosis.4 In addition, the diagnosis of TIO is often missed despite hypophosphatemia, as seen in our case. In fact, a previous study showed that 43.1% of cases of hypophosphatemia observed on laboratory charts were neglected and the diagnosis was missed.4 Furthermore, only 11.8% of TIO patients were tested for serum phosphorus levels at the initial visit.4 Hypophosphatemia and elevated alkaline phosphatase can be important diagnostic clues for TIO.
Detection of the responsible tumor in TIO is sometimes difficult because the tumor might be small and can appear anywhere in the body, mainly in bone and soft tissue. In a report of 895 cases of neoplastic osteomalacia, the median diameter of the tumor was 27 mm, the smallest diameter was 5 mm, and the limbs were the most common tumor site (46.4%), followed by the head and neck (25.7%).5 Tumors in the scapular region, as in our case, have also been reported.6,7 CT and magnetic resonance imaging (MRI) failed to detect the culprit tumors in several studies, although these imaging modalities are generally recommended when planning for surgery.8 In addition to anatomical imaging, such as CT and MRI, functional imaging, such as 18FDG-PET/CT and SSTR imaging (octreotide scan and 68Ga-DOTATOC, -DOTANOC, DOTATATE PET/CT), are useful for localization of the responsible tumor in TIO.3,5 Recently, PET/CT using 68Ga was considered to have the best diagnostic performance,3,5,8,9 with one report showing that 68Ga-DOTATATE PET/CT has a sensitivity of 100% and specificity of 90.9%.10 However, most facilities, including our hospital, lack the equipment for performing this imaging.
To determine whether the tumors identified by these imaging modalities actually produce FGF23, venous sampling to examine the FGF23 levels is useful.3 In particular, when multiple tumors are identified on imaging, as in our case, it is useful for identifying the tumor responsible for the TIO.3,11 Although it was not possible to identify the responsible tumor from imaging data alone, we suspected the tumor in the right parotid gland as being the responsible tumor, because it was larger in diameter and demonstrated higher FDG accumulation. However, venous sampling revealed that the responsible tumor was actually the tumor on the anterior surface of the right scapula rather than the tumor in the parotid gland.
The first choice of treatment for TIO is surgical removal of the tumor. When surgery is difficult due to inability to localize the tumor or for other reasons, treatment with phosphorus and vitamin D supplementation is the second choice, although it is associated with the risk of nephrocalcinosis, nephrolithiasis, hypoadrenocorticism, and hypercalciuria.3,12 Since 2018, burosumab has become clinically available in Europe and other countries for the treatment of X-linked hypophosphatemia in children.13 Furthermore, burosumab resulted in increased serum phosphorus levels and improved fracture healing, bone pain, functional mobility, and health-related quality of life in patients with TIO in Phase 2 studies.14,15 In Japan, burosumab became clinically available for the treatment of FGF23-related hypophosphatemic osteomalacia in February 2019.1,16 The efficacy of burosumab as an alternative to conventional therapy for non-remission TIO in Japan was first reported by Miyaoka et al.17 However, there are only a few previous reports from Japan on the efficacy of burosumab. In previous reports, burosumab treatment led to improvement of serum phosphorus levels,18–20 improvement of pain and physical performance,18,20 partial healing of pseudofractures, and extension of the distance covered in the 6-min walk test.19 In our case, improvement in pain allowed the patient to walk with a cane, which facilitated his discharge to home. Although there are reports on the safety of long-term administration of burosumab,18 it is still considered a novel drug and confirmation of its long-term therapeutic effects is needed. Additionally, since treatment with burosumab does not eradicate the tumor, careful follow-up is necessary to monitor for tumor progression.
In conclusion, we experienced a case of TIO that caused whole-body joint pain. We suspected TIO in our patient based on low serum phosphorus, high bone-type ALP, and high FGF23 levels. Two tumors were observed on imaging, with the larger one suspected as being the tumor responsible for TIO. However, the actual tumor responsible for TIO was identified by venous sampling of FGF23 levels. Venous sampling is considered especially useful when multiple tumors are present. Although surgical resection was not possible in this case, the patient’s symptoms and ADL improved with burosumab treatment. In cases of osteoporosis with hypophosphatemia and elevated alkaline phosphatase levels, TIO should be considered in the differential diagnosis, and attempts should be made to find the culprit tumor using several tools.
Data Sharing Statement
The datasets used during the current study are available from the corresponding author on reasonable request.
Ethics Statement
Based on the regulations of the Oita University Faculty of Medicine, institutional review board approval is not required for case reports.
Consent for Publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Acknowledgments
We would like to express our sincere gratitude to Dr. Nobuaki Ito of the Department of Nephrology and Endocrinology, Faculty of Medicine, The University of Tokyo, for his cooperation in performing the venous sampling.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors have no competing interests to declare.
References
1. Fukumoto S. FGF23-related hypophosphatemic rickets/osteomalacia: diagnosis and new treatment. J Mol Endocrinol. 2021;66(2):R57–R65. doi:10.1530/JME-20-0089
2. Cianferotti L. Osteomalacia is not a single disease. Int J Mol Sci. 2022;23:23.
3. Jan de Beur SM, Minisola S, Xia WB, et al. Global guidance for the recognition, diagnosis, and management of tumor-induced osteomalacia. J Intern Med. 2023;293(3):309–328. doi:10.1111/joim.13593
4. Feng J, Jiang Y, Wang O, et al. The diagnostic dilemma of tumor induced osteomalacia: a retrospective analysis of 144 cases. Endocr J. 2017;64(7):675–683. doi:10.1507/endocrj.EJ16-0587
5. Bosman A, Palermo A, Vanderhulst J, et al. Tumor-induced osteomalacia: a systematic clinical review of 895 cases. Calcif Tissue Int. 2022;111(4):367–379. doi:10.1007/s00223-022-01005-8
6. Nawrot-Wawrzyniak K, Varga F, Nader A, et al. Effects of tumor-induced osteomalacia on the bone mineralization process. Calcif Tissue Int. 2009;84(4):313–323. doi:10.1007/s00223-009-9216-z
7. Kawai S, Ariyasu H, Furukawa Y, et al. Effective localization in tumor-induced osteomalacia using (68)Ga-DOTATOC-PET/CT, venous sampling and 3T-MRI. Endocrinol Diabetes Metab Case Rep. 2017;2017. doi:10.1530/EDM-17-0005
8. Meyer M, Nicod Lalonde M, Testart N, et al. Detection rate of culprit tumors causing osteomalacia using somatostatin receptor PET/CT: systematic review and meta-analysis. Diagnostics. 2019;10:1.
9. Agrawal K, Padhy BM, Meher BR, Mohanty RR. Diagnostic utility of Ga-68 DOTA-SSTR and F-18 FDG PET/CT in the detection of culprit tumours causing osteomalacia: a systematic review and meta-analysis. Nucl Med Commun. 2021;42(6):646–655. doi:10.1097/MNM.0000000000001379
10. Zhang J, Zhu Z, Zhong D, et al. 68Ga DOTATATE PET/CT is an accurate imaging modality in the detection of culprit tumors causing osteomalacia. Clin Nucl Med. 2015;40(8):642–646. doi:10.1097/RLU.0000000000000854
11. Andreopoulou P, Dumitrescu CE, Kelly MH, et al. Selective venous catheterization for the localization of phosphaturic mesenchymal tumors. J Bone Miner Res. 2011;26(6):1295–1302. doi:10.1002/jbmr.316
12. Marques JVO, Moreira CA, Borba VZC. New treatments for rare bone diseases: hypophosphatemic rickets/osteomalacia. Arch Endocrinol Metab. 2022;66(5):658–665. doi:10.20945/2359-3997000000555
13. Carpenter TO, Whyte MP, Imel EA, et al. Burosumab therapy in children with X-linked hypophosphatemia. N Engl J Med. 2018;378(21):1987–1998. doi:10.1056/NEJMoa1714641
14. Imanishi Y, Ito N, Rhee Y, et al. Interim analysis of a phase 2 open-label trial assessing burosumab efficacy and safety in patients with tumor-induced osteomalacia. J Bone Miner Res. 2021;36(2):262–270. doi:10.1002/jbmr.4184
15. de Beur SM J, Miller PD, Weber TJ, et al. Burosumab for the treatment of tumor-induced osteomalacia. J Bone Miner Res. 2021;36(4):627–635. doi:10.1002/jbmr.4233
16. Minisola S, Peacock M, Fukumoto S, et al. Tumour-induced osteomalacia. Nat Rev Dis Primers. 2017;3:17044. doi:10.1038/nrdp.2017.44
17. Miyaoka D, Imanishi Y, Yano M, et al. Effects of burosumab on osteocalcin and bone mineral density in patient with 15-year history of nonremission tumor-induced osteomalacia initially treated with conventional therapy: case report. Bone Rep. 2020;13:100736. doi:10.1016/j.bonr.2020.100736
18. Crotti C, Zucchi F, Alfieri C, Caporali R, Varenna M. Long-term use of burosumab for the treatment of tumor-induced osteomalacia. Osteoporos Int. 2023;34(1):201–206. doi:10.1007/s00198-022-06516-6
19. Oe Y, Kameda H, Nomoto H, et al. Favorable effects of burosumab on tumor-induced osteomalacia caused by an undetectable tumor: a case report. Medicine. 2021;100(46):e27895. doi:10.1097/MD.0000000000027895
20. Day AL, Gutierrez OM, Guthrie BL, Saag KG. Burosumab in tumor-induced osteomalacia: a case report. Joint Bone Spine. 2020;87(1):81–83. doi:10.1016/j.jbspin.2019.07.012
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.