Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
A Case of Pustular Pyoderma Gangrenosum Misdiagnosed as Acute Febrile Neutrophilic Dermatosis in a Pediatric Patient
Authors Yang X, Wu Y, Jiang F, Deng D
Received 11 November 2023
Accepted for publication 18 February 2024
Published 28 February 2024 Volume 2024:17 Pages 493—498
DOI https://doi.org/10.2147/CCID.S449404
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Xiaoli Yang, Yongzhuo Wu, Fuqiong Jiang, Danqi Deng
Department of Dermatology, The Second Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, 650101, People’s Republic of China
Correspondence: Danqi Deng, Department of Dermatology, The Second Affiliated Hospital of Kunming Medical University, No. 374, Dianmian Avenue, Wuhua District, Kunming, Yunnan, Tel +8613888139863, Fax +86-871-65351214, Email [email protected]
Background: Pyoderma gangrenosum (PG) is a rare cause of skin ulcers in children, posing challenges in diagnosis and treatment. As the disease is often associated with conditions such as inflammatory bowel disease (IBD), rheumatoid arthritis, haematological disorders and other diseases, diagnosis and treatment often require cooperation with other medical departments. Accordingly, dissemination of information about the disease to doctors in departments other than dermatologists, especially paediatricians, can help in its early detection.
Case Presentation: The 11-year-old pediatric patient in the case initially diagnosed with acute febrile neutrophilic dermatosis was eventually confirmed as pustular PG through histopathological examinations of skin and other relevant examinations. The medical condition is lessened after treatment with a combination of glucocorticoids and adalimumab.
Conclusion: PG is relatively rare in clinical settings, particularly among pediatric patients exhibiting persistent high fever and signs of pustular pyoderma gangrenosum. This case underscores the importance of considering the potential diagnosis of pediatric pustular PG when confronted with a child presenting persistent high fever and pustules after trauma. Additionally, the proactive initiation of adalimumab emerges as a promising treatment option for pediatric IBD -associated pustular PG.
Keywords: pustular pyoderma gangrenosum, acute febrile neutrophilic dermatosis, misdiagnosis, adalimumab
Introduction
Pustular PG represents a rare etiology of skin ulcers in pediatric population, accounting for approximately 4% of global cases.1 The ulcerative (classic) subtypepredominates, typically addressed as the first-line treatment with systemic corticosteroids. However, the occurrence of the blistering variant of PG, especially in children, is relatively uncommon. The absence of well-defined diagnostic criteria, encompassing specific clinical, histopathological, and laboratory findings, contributes to the potential for misdiagnosis.2 The most common underlying etiologies in pediatric PG are inflammatory bowel diseases, hematologic malignancies, immune deficiencies and vasculitis. Thorough clinical examination is necessary to clarify the diagnosis and select appropriate treatment methods.3 Here, we present the case of an 11-year-old pediatric patient who was initially misdiagnosed with acute febrile neutrophilic dermatosis. Subsequent skin histopathology and relevant examinations confirmed the diagnosis of pustular PG. The timely initiation of a treatment combining glucocorticoids and Tumour necrosis factor antagonists (adalimumab) yielded notable improvement in systemic symptoms.
Case Presentation
An 11-year-old patient suffered a knee injury on May 6, 2023, followed by the onset of fever on May 9. Subsequently, on May 10, the patient developed pustules on the upper lip and sought medical attention at an external institution. The initial diagnosis was “bullous impetigo and ulcerative stomatitis”, and the patient received treatment involving intravenous drip administration of metronidazole and levofloxacin, along with the use of chlorhexidine mouthwash. However, the condition progressively deteriorated, leading to ulceration of the oral mucosa and difficulties in eating. On May 13, erythematous patches appeared on the patient’s back, accompanied by the development of pustules. The rash extended progressively to involve the face, limbs, anterior chest and buttocks. Persistent fever persisted throughout this period, reaching a maximum temperature of 39.8°C. Empirical treatment attempts with azithromycin, penicillin, and linezolid did not result in any improvement and the condition continued to worsen. On May 21, the patient was admitted to our department with the provisional diagnoses of “unexplained fever” and “acute febrile neutrophilic dermatosis”. The patient also presented with ongoing diarrhea and had recently undergone an 8kg weight loss. There was no significant medical history, allergies, or pertinent family medical history in the patient’s background.
Physical examination (Figure 1): Erythema, erosion, and crust formation are evident on the upper lip. The surface of the tongue exhibits shallow, map-like ulcers covered by purulent coating. Scattered edematous erythematous patches can be observed on the trunk, limbs, head and face. Within these erythematous patches, ring-shaped vesicles and pustules are present, accompanied by minimal crusting.
 |
Figure 1 Clinical photograph of the patient - Initial photo. |
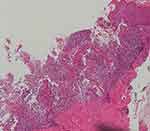
Histopathology of the skin lesions (Figures 2–4): Hyperkeratosis accompanied by hypertrophy of the spinous layer is observed. In the shallow to mid-dermal layers, there is a prominent infiltration of dense neutrophils in a band-like pattern. Around the blood vessels and adnexal structures in the dermal layer, a mild infiltration of lymphocytes and neutrophils is noted. Additionally, a dense proliferation of collagen fibers is observed in the dermal layer.
 |
Figure 4 Histopathology of the skin lesion (HEx400). |
Laboratory examination: Colonoscopy showed chronic non-atrophic gastritis (Helicobacter pylori-negative), multiple ulcers in the colon of a nature to be investigated, and pathological assessment showed chronic active inflammation of the mucosa in the ascending colon, mucosal ulceration, and chronic inflammation in the transverse colon with focal lymphoid follicle formation. Elevated levels were noted in leukocytes, neutrophil count percentage, neutrophils, erythrocyte sedimentation rate, and C-reactive protein. Additionally, the occult blood test yielded positive results, while pus culture, fungal culture, and respiratory syncytial virus infection were negative. The pharyngeal swab culture suggested Morganella morganii infection, and immunofluorescence results were negative.
Diagnosis: Pustular pyoderma gangrenosum and ulcerative colitis.
Treatment: The patient was admitted and initially prescribed prednisolone at a daily dosage of 40mg for anti-inflammatory purposes. Subsequently, there was a notable improvement in the skin lesions and a restoration of normal body temperature. However, upon reducing the steroid dosage to 30mg, the patient experienced a recurrence of low-grade fever. During this period, there was no noticeable improvement in the symptoms of cough and diarrhea. On June 3rd, the patient received treatment with adalimumab, and after one month, the symptoms improved significantly. The patient is currently being followed up (Figure 5).
 |
Figure 5 Clinical photograph of the patient at the 1-month follow-up visit. |
Discussion
PG is a rare neutrophilic dermatosis with skin inflammation and ulceration, comprising ulcerative, maculopapular, pustular and value-added types, which vary in clinical presentation, site of onset and associated diseases, with the ulcerative type being the most common clinically,4 affecting approximately 3–10 individuals per million population annually, with only 4% occurring in the pediatric population. The rarity and ambiguous diagnostic criteria often contribute to the misdiagnosis. The incidence of PG is higher in women. The incidence rate of PG in men and women is 1:3, and the average age of onset is 51 years old.5 About 50% of PG is accompanied by systemic diseases, of which IBD is the most common. Associated diseases in paediatrics include IBD, hematological malignancies, IgA monoclonal immunoglobulin disease, rheumatoid arthritis, and various immune deficiencies. The lower limbs and trunk are most commonly affected, while the head and neck are rarely affected, accounting for only 8% of cases.6 Pediatric PG can often be overlooked or misdiagnosed due to its low incidence and atypical involvement of areas such as the head and face. Timely diagnosis and treatment are crucial to avoid unsightly scarring and potential psychological distress.7
Notably, standardized diagnostic criteria for PG are currently lacking. PG is an exclusive diagnosis and should be differentiated from ischaemic ulcers, infections, vascular disease, other neutrophilic skin diseases and malignancies. Histologically, PG lesions are characterized by dense neutrophilic infiltration in the dermis, accompanied by evident tissue necrosis at the ulcer base.8 The child in this case underwent a thorough examination to rule out ulcers and any other associated diseases. Although the patient persistently have a fever, the use of multiple antibiotics is ineffective and the bacterial culture is negative, so infection is ruled out; vasculitis was excluded by negative ANCA and pathological findings. Combined with the child’s favourable response to glucocorticoids, this case fulfils the two primary and three secondary diagnoses of Su et al9 and the primary and four secondary criteria of the Delphi consensus of international experts.10
In our department, we initially considered an initial diagnosis of acute febrile neutrophilic dermatosis based on the patient’s elevated body temperature, increased leukocyte count, neutrophil percentage, neutrophil count, elevated erythrocyte sedimentation rate, elevated C-reactive protein, and positive response to glucocorticoids. However, after the patient’s steroid dose was reduced to 30mg/day, fever recurred, and the gastrointestinal symptoms remained unresolved. Eventually, through histopathological examination and gastroscopy, the patient was conclusively diagnosed with PG of the pustular type. This case highlights the importance of histopathological examination in cases with uncertain diagnoses, given the nonspecific histological features of PG.
It is noteworthy that up to 50% of pediatric PG cases are associated with IBD, making it indispensable to perform gastrointestinal endoscopy in these patients. In this case, the combined IBD was identified by timely gastroscopy, enhancing the recovery of the child. Due to the lack of well-controlled studies, especially in pediatric populations, there are no established treatment guidelines for PG. However, several cases of pediatric PG associated with IBD have shown complete clinical response with the use of Tumour necrosis factor antagonists, predominantly infliximab.8 In this case, the patient initially exhibited substantial improvement in systemic skin lesions following glucocorticoid treatment. However, persistent fever and unresolved diarrhea prompted a shift to adalimumab therapy, leading to a positive response. This outcome confirms the effectiveness of Tumour necrosis factor antagonists in patients with IBD-associated PG. It is essential to highlight that such experiences are notably limited in pediatric patients with pustular PG. Our case represents one of the most effective trials of biologic therapy for pediatric IBD-associated pustular PG to date, and the results indicate that adalimumab is a promising treatment option for this condition.
Conclusion
PG is relatively rare in clinical practice, especially among pediatric patients exhibiting persistent high fever and signs pustular pyoderma gangrenosum. Due to the lack of specificity in clinical, histopathological, and laboratory examinations, misdiagnosis is common. This case serves as a poignant reminder to consider the post pediatric pustular PG when a child with persistent high fever and pustules after trauma. It also underscores the necessity to contemplate the potential presence of IBD when confronted with persistent diarrhea. Timely skin biopsy and gastrointestinal endoscopy emerge as crucial diagnostic tools in such cases. Additionally, early use of adalimumab is a promising treatment option for pediatric IBD-associated pustular PG.
Abbreviations
PG, Pyoderma gangrenosum; IBD, inflammatory bowel disease; IgA, Immunoglobulin A; ANCA, anti-neutrophil cytoplasmic antibodies.
Ethics Approval and Consent to Participate
This case report was approved by the Ethics Committee.
Consent for Publication
Written informed consent was obtained from the patient and the patient’s parents to publish the case details and associated images.
Acknowledgments
The authors are grateful to the patient.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This article has no funding support.
Disclosure
The authors declare no competing interest in this work.
References
1. Chateau A, Makhubele J, Dlova N. Corticosteroid wraps as monotherapy in a child with extensive idiopathic pyoderma gangrenosum. Pediatr Dermatol. 2021;38(1):184–186. doi:10.1111/pde.14348
2. Chen B, Li W, Qu B. Practical aspects of the diagnosis and management of pyoderma gangrenosum. Front Med. 2023;10:1134939. doi:10.3389/fmed.2023.1134939
3. Kechichian E, Haber R, Mourad N, El Khoury R, Jabbour S, Tomb R. Pediatric pyoderma gangrenosum: a systematic review and update. Int J Dermatol. 2017;56(5):486–495. doi:10.1111/ijd.13584
4. Schøsler L, Fogh K, Bech R. Pyoderma gangrenosum: a retrospective study of clinical charac-teristics, comorbidities, response to treatment and mortality related to prednisone dose. Acta Derm Venereol. 2021;101(4):adv00431. doi:10.2340/00015555-3776
5. Ashchyan HJ, Butler DC, Nelson CA, et al. The association of age with clinical presentation and comorbidities of pyoderma gangrenosum. JAMA Dermatol. 2018;154(4):409–413. doi:10.1001/jamadermatol.2017.5978
6. Haimes H, Corey K, Mostaghimi A, Schmidt B, Gellis SE, Liang MG. Pediatric facial pyoderma gangrenosum preceding the diagnosis of inflammatory bowel disease. Pediatr Dermatol. 2020;37(4):764–766. doi:10.1111/pde.14186
7. Chen J, Wang H, Ren F. Dramatic case of paediatric pyoderma gangrenosum. Arch Dis Child. 2021;106(10):1001. doi:10.1136/archdischild-2020-320598
8. Vaidy K, Winderman R, Rabinowitz SS, Schwarz SM. Treatment of pyoderma gangrenosum in pediatric inflammatory bowel disease. JPGN Rep. 2020;1(2):e008. doi:10.1097/PG9.0000000000000008
9. Su WP, Davis MD, Weenig RH, Powell FC, Perry HO. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43(11):790–800. doi:10.1111/j.1365-4632.2004.02128.x
10. Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a delphi consensus of international experts. JAMA Dermatol. 2018;154(4):461–466. doi:10.1001/jamadermatol.2017.5980
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.