Back to Journals » OncoTargets and Therapy » Volume 15
A Case of Pneumonia Masking Pleural Malignancy
Authors Xu Q , Tian J, Huang L , Zhong Q, Xu Y , Liu L , Shi J
Received 14 March 2022
Accepted for publication 30 June 2022
Published 2 July 2022 Volume 2022:15 Pages 741—746
DOI https://doi.org/10.2147/OTT.S366221
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Arseniy Yuzhalin
Qian Xu,1,2,* Juanjuan Tian,3,* Lin Huang,3 Qilin Zhong,3 Yulin Xu,3 Linlin Liu,3 Jian Shi4
1Mianyang Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Mianyang, People’s Republic of China; 2Department of Clinical Medicine, Southwest Medical University, Luzhou, People’s Republic of China; 3Department of Internal Medicine, Beichuan Qiang Autonomous County People’s Hospital, Mianyang, People’s Republic of China; 4Department of Psychosomatic Medicine, Mianyang Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Mianyang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jian Shi, Department of Psychosomatic Medicine, Mianyang Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Mianyang, People’s Republic of China, Tel +86-15883756120, Email [email protected]
Abstract: The invasion of the pleural membrane by a malignant pleural tumor can lead to the production of malignant pleural effusion (MPE), resulting in the symptoms of dyspnea, and some patients have cough, sputum and other symptoms, which are easily confused with pneumonia. In this case, the initial diagnosis of the patient is pneumonia, and the final diagnosis is pneumonia combined with pleural malignancy. Therefore, if the patient has unexplained symptoms of bloody pleural effusion, it is necessary to examine for malignant tumors and should actively perform thoracentesis and drainage, look for malignant cells in the pleural effusion cell precipitation, evaluate the nature of pleural effusion, conduct pleural biopsy tissue examination, and determine the type and source of lung malignancy by the combined application of cell block technology and immunohistochemistry. Take the cytological examination results in pleural effusion seriously, and finally, surgical or immunotherapy can be performed.
Keywords: pleural effusion, pleural malignancy, tumor, bloody pleural effusion, encapsulating pleural effusion
Introduction
Pleural malignancy includes primary pleural tumors and tumors metastasized to the pleura at other sites; and primary pleural malignancy is represented by malignant pleural mesothelioma (MPM), and the average survival time for MPM patients who cannot operate is only 6 to 9 months.1 However, secondary pleural malignancy comes from pleural metastasis of various tumors, including breast cancer and lung cancer. The invasion of the pleural membrane by a malignant pleural tumor can lead to the production of malignant pleural effusion (MPE), decreased chest wall compliance, impaired ipsilateral diaphragm activity, mediastinal displacement and reduced lung volume, leading to symptoms such as dyspnea, cough, airtightness, etc. The symptoms are very similar to those of pneumonia, so we want to improve the differential diagnosis of pleural malignancy, then making doctors be wary of confusion between pneumonia and symptoms of pleural malignancy during clinical visits. Symptoms of chest pain are uncommon, and whether chest pain occurs is usually associated with malignant tumors involving the mural pleural membrane, ribs, and other intercostal tissues. In addition to respiratory symptoms, often accompanied by weight loss, fatigue, loss of appetite, and other systemic symptoms, late can appear cachexia. The emergence of MPE means that the patient enters the advanced tumor, has a poor survival rate, low quality of life, poor clinical prognosis, and a mean survival time of only 3 to 12 months. The estimated annual incidence of MPE is around 40,000 in the UK.2
Until now, little is known about the mechanism of MPE, and it is currently only established that pathological pleural effusion forms when fluid production in the pleural membrane exceeds absorption, and the absorption of pleural effusion depends mainly on the lymphatic canal system of the pleural and lungs. The absorption of pleural effusion was decreased differently when the tumor invaded the draining lymphatic vessels anywhere from the mural pleural membrane to the lung gate and mediastinal lymph nodes. On the other hand, when operating thoracoscopy, a large amount of fluid can be observed in rapid extravasation; that is, the amount of MPE is not necessarily associated with the degree of tumor invasion. Autopsies reveal that MPE3–7 was present only in 55%-66% of patients with pleural metastases. Blocking the absorption of the pleural effusion alone is not sufficient to explain the full mechanism of MPE formation.8 Thus, the penetration of massive plasma from the pleural vascular network with increased permeability into the pleural lumen becomes an important mechanism for MPE.
Herein, we report a patient who was initially diagnosed with pneumonia with a large effusion in the right thoracic cavity but was a patient with a pleural malignancy (metastatic low-differentiated pleural carcinoma) where no primary lesion was found.
Case Presentation
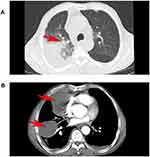
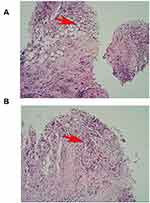
A 70-year-old male farmer, at 11:41 on January 20,2022, was admitted to the hospital with “repeated coughing and coughing sputum for 1 + years, aggravated with heart tiredness and tightness 1 week”, after the outpatient chest x-ray was perfected, he was admitted with “pneumonia” to the Respiratory and Critical Care Medicine Department of Beichuan Qiang Autonomous County People’s Hospital, asked about the medical history: plain and healthy, no history of hypertension, diabetes, kidney disease, heart disease, smoking and drinking for 40 + years. Physical examination: left lung smells of crackles and stridor, C-reactive protein quantitative 64.4mg/ L, blood analysis: leukocytes 11.5 * 109/ L, neutrophil percentage 85.9%, bound to chest CT: slight exudation, interstitial changes, and atelectasis in the upper lobe of the right lung, a medial segment of the medial lobe of the right lung, and lower lobe of the right lung. The two lungs were scattered in a few chronic inflammatory lesions. Bilateral local pleural thickening (Figure 1A). The initial diagnosis was pneumonia for elderly males, chronic course, acute onset, with repeated cough and left lung was filled with rales and wheezing. The patient had a long disease course, repeated coughing and sputum, considered the lower respiratory tract infections, and was mainly infected with Gram-negative bacteria. Therefore, cefazoxime sodium 2.0 iygtt q12h*7 days anti-infection therapy, while Lung contrast-enhanced CT also suggests a large encapsulated pleural effusion on the right side (Figure 1B), thoracentesis of the patient, can induce hemorrhagic turbid liquid, combined with carcinoembryonic antigen at 9.04ng/mL; CA125>1000.00U/ mL; CA15-367.2U/ mL; CA19-9>2000.00U/mL. Malignant cells were searched for in the pleural effusion precipitation, to further judge the nature of the pleural effusion, a pleural biopsy was checked in the direction of 2, 5, 7 and 9 o’clock, and submit it for pathological examination. The patient had a cough, airtightness, and other symptoms improved through the active anti-infection, release of pleural effusion and symptomatic cough treatment. Pathological biopsy: (right pleural) small tissue, hyperplastic cell nests (Figure 2A) and specific hyperplastic cells (Figure 2B) were found in fibrotic adipose tissue, prone to tumor involvement, immunohistochemistry is recommended. Pathological diagnosis: (right chest) liquid-based cell production, detection of specific hyperplasia cells, it is recommended to be cell blocks. Therefore, we diagnosed the malignant pleural effusion on the right side, submitted for immunohistochemistry, and actively sought out primary tumor lesions. On January 28, 2022, 50mL of bloody pleural effusion was drained from the pleural indwelling catheter, with 0.9% sodium chloride injection 500mL + cisplatin 60mg qw chest perfusion therapy, in order to prevent adverse reactions of chemotherapeutic drugs, famotidine gastric protection, granisetron and metoclopramide antiemetic are temporarily given. Immunohistochemistry review: wax block slices sent for examination, combined with HE and immunohistochemical results diagnosis is: biopsy specimens, low-differentiated carcinoma in the pleural tissue, mostly secondary. Lung adenocarcinoma, squamous cell carcinoma, small cell carcinoma, and mesothelial tumors are not currently supported. Immunohistochemical results: tumor cells CK (+), WT-1 (-), TTF-1 (-), NapsinA (-), P40 (-), CK5/6 (-), Syn (-) Ki67 about 80% (+). Therefore, the revised diagnosis was pneumonia combined with pleural malignancy. We perfected the full abdominal enhancement CT to observe the primary tumor lesions, but no primary lesions were ultimately identified. After a follow-up of one month, the patient still did not find the primary tumor lesion. At present, the patient’s quality of life is still acceptable, chemotherapy is continued, and we conducted the follow-up.
Discussion
It is generally believed that bloody pleural effusion is mostly malignant tumors, tuberculosis, etc. Therefore, when patients develop pleural effusion, performing thoracentesis and drainage with bloody pleural effusion, it is necessary to be vigilant against tumor induction. The formation of encapsulating pleural effusion is due to the pleura under inflammatory stimulation, and a large amount of fibrin is constantly exuding and settling, forming fibrous induration, and gradually thickening and adhesion of the pleura; or producing a sterile pleural effusion, protein substances and cell debris accumulate in the thoracic cavity and adhere to the surface of the pleura, appearing to separate and wrap. Henke, C. A. et al9 believe that inflammation causes aseptic pleural fluid protein substances and cell debris to accumulate in the thoracic cavity, and fibrin adheres to the involved pleural surface, which can quickly separate and wrap, resulting in poor drainage. The formation of the package is also related to the long course of the disease and repeated puncture and extraction. The examination of clinically enveloped effusion mainly distinguishes tuberculous and malignant tumor effusion. Further tests are required when patients develop encapsulating pleural effusion and are alert to malignancy.
The “gold standard” for diagnosing MPE is still finding malignant cells in pleural effusion cell precipitation, or the pathological changes of malignant tumors observed in pleural biopsy tissue. In addition, cancer ratio has a high diagnostic accuracy for predicting MPE, especially when used in combination with pleural carcinoembryonic antigen.10 To date, no reliable tumor marker has been found for the differential diagnosis of MPE. At present, the commonly used tumor markers such as Cancer embryo Antigen, cytokeratin fragment 21–1, carbohydrate antigen CA125, CA15-3, and CA19-9 do not have high sensitivity, and their specificity is not high enough to meet the clinical needs.11,12 Therefore, it is still necessary to develop more reliable indicators through immunology, molecular biology technique, and proteomics technique to improve the diagnostic rate of MPE. In this case, laboratory auxiliary examination: C-reactive protein quantification 64.4mg/ L; 9.04ng/mL; CA125>1000.00U/ mL; CA15-367.2U/ mL; CA19-9>2000.00U/mL; perform thoracentesis and drainage, pleural biopsy tissue examination, and the combination of cell block technology and immunohistochemistry to determine the type and source of malignancy. Pathological diagnosis: (right pleural cavity) liquid-based cell production, detection of specific hyperplasia cells, and immunohistochemical results show that low-differentiated carcinoma is found in the pleural tissue; most of them were secondary (Figure 2). To seek the primary tumor lesions for the next treatment, abdominal enhanced CT is examined, and the primary lesions have not been found yet.
At present, immunotherapy for tumors has matured, and the idea of immunotherapy for solid tumors can be referred to in the treatment of pleural malignancy. The pleural cavity is an extended space of the tumor microenvironment. The pleural cavity/pleural effusion contains macrophages, regulatory cells, regulatory T cells, CD4 + T cells and CD8 + T cells, etc., sharing a complex immunosuppressive factor network and promoting the formation of a tumor tolerance environment.13 Using tumor-associated macrophages (TAMS) as an example, it has been found that TAMS isolated from the pleural effusion of MPM patients tends to differentiate into a tumor-promoting M2 phenotype, and when co-cultured with activated CD4 + and CD8T cells, by reducing and inhibiting both proliferation from promoting tumor progression to worsen.14 Another study found that, in MPE, the number of regulatory T cells increases, and its enhanced immunosuppressive function may be related to the induction and maintenance of the tumor microenvironment of MPE.15 The above studies demonstrate that immunomodulation is closely related to MPM, and that immunotherapy can potentially change the immune composition of pleural effusion in patients with pleural malignancy and stimulate a specific immune response, exerting an anti-tumor effect and allowing patients to gain potential clinical benefit. The clinical advantages of thoracic immunotherapy include relatively low concentration and total dose of therapeutic drugs, relatively safe treatment; effective local treatment with the closure of the thoracic cavity; direct contact between the drug and tumor cells, directly acting on the tumor microenvironment, and give full play to the efficacy. Intrathoracic immunotherapy mainly includes direct cytokine-mediated immunotherapy in the chest cavity, congenital immunotherapy, cellular immunotherapy, viral vector-mediated immune stimulation, oncolytic virus gene delivery therapy, etc. Currently, palliative care is mainly used for pleural malignancy, aiming to improve patient quality of life, relieve dyspnea and/or chest pain, and prolong patient survival as long as possible.16
It is particularly emphasized that not the pleural effusion present in all patients with malignant tumors is malignant, which may be the peritumoral pleural effusion. The mechanism of the pleural effusion in these patients is different from MPE, there are no malignant cells in the pleural effusion, and there is no metastasis in the pleura. An earlier study showed that 5% of lung cancer patients presenting with pleural effusion still had surgical opportunities.17 Therefore, in the diagnosis and differential diagnosis of malignant tumors complicated with pleural effusion, we should be particularly careful to treat the patients with negative cytology examination results in the pleural effusion, as long as there is no evidence of pleural metastasis and other site metastasis, combined with other conditions, surgical conditions should be performed when there are surgical conditions. Patient immunohistochemistry in this case: wax block sections, combined with HE and immunohistochemistry results are diagnosed as biopsy specimens and poorly differentiated carcinoma in pleural tissue, most of which are secondary. Therefore, the operation cannot be performed.
Clinical physicians should deepen their cognition and understanding of MPE mechanism, and in the case of unexplained bloody pleural effusion diagnosis, can use internal medicine thoracoscopy or single-trocar technical,18,19 only for local anesthesia or sedation, manage biopsy of lesions of the chest wall, diaphragm, mediastinum, pericardium, and lungs, which is more conducive to pleural malignancy diagnosis, tissue classification and clinical stage than closed pleural biopsy. In this case, the patient’s chest CT showed multilocular encapsulating pleural effusion, which could inject fibrinolysis, reduce the viscosity of pleural effusion, remove pleural adhesion and separation, avoid or reduce multilocular encapsulating pleural effusion, and rarely such complications as immune-mediated adverse reactions or bleeding tendency.20
Conclusion
When patients appear encapsulating pleural effusion, and cannot be explained by other reasons, need to consider malignant tumor induction. If the patient meets the thoracic puncture drainage condition, give positive pleural effusion puncture and look for malignant cells in pleural effusion cell precipitation, determine pleural effusion properties, pleural biopsy tissue examination simultaneously, use cell block technique and immunohistochemistry to determine the type and source of lung malignancy, which is beneficial to improve the prognosis of patients. Thus, our aim is to suggest that patients with poorly treated pleural effusions should be diagnosed by pathology as soon as possible.
Abbreviations
MPE, malignant pleural effusion; MPM, Malignant pleural mesothelioma; CT, Computed Tomography; CA, carbohydrate antigen.
Ethics and Consent Statement
Written informed consent has been provided by the patient to have the case details and any accompanied images published. An institutional approval was not required for a case report.
Acknowledgments
We thank AJESCI (www.aje-cn.com) for editing the English text of a draft of this manuscript.
Funding
This work was supported by Sichuan Provincial Health and Family Planning Commission [grant number 17PJ088].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bibby AC, Maskell NA. Current treatments and trials in malignant pleural mesothelioma. Clin Respir J. 2018;12(7):2161–2169. doi:10.1111/crj.12938
2. Egan AM, McPhillips D, Sarkar S, Breen DP. Malignant pleural effusion. QJM. 2014;107(3):179–184. doi:10.1093/qjmed/hct245
3. Burrows CM, Mathews WC, Colt HG. Predicting survival in patients with recurrent symptomatic malignant pleural effusions: an assessment of the prognostic values of physiologic, morphologic, and quality of life measures of extent of disease. Chest. 2000;117(1):73–78. doi:10.1378/chest.117.1.73
4. Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ; Group BTSPDG. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(Suppl 2):ii32–40. doi:10.1136/thx.2010.136994
5. Hsu C. Cytologic detection of malignancy in pleural effusion: a review of 5255 samples from 3811 patients. Diagn Cytopathol. 1987;3(1):8–12. doi:10.1002/dc.2840030103
6. Salyer WR, Eggleston JC, Erozan YS. Efficacy of pleural needle biopsy and pleural fluid cytopathology in the diagnosis of malignant neoplasm involving the pleura. Chest. 1975;67(5):536–539. doi:10.1378/chest.67.5.536
7. Harris RJ, Kavuru MS, Rice TW, Kirby TJ. The diagnostic and therapeutic utility of thoracoscopy. Chest. 1995;108(3):828–841. doi:10.1378/chest.108.3.828
8. Stathopoulos GT, Kalomenidis I. Malignant pleural effusion: tumor-host interactions unleashed. Am J Respir Crit Care Med. 2012;186(6):487–492. doi:10.1164/rccm.201203-0465PP
9. Henke CA, Leatherman JW. Intrapleurally administered streptokinase in the treatment of acute loculated nonpurulent parapneumonic effusions. Am Rev Respir Dis. 1992;145(3):680–684. doi:10.1164/ajrccm/145.3.680
10. Zhang Y, Li X, Liu J, et al. Diagnostic accuracy of the cancer ratio for the prediction of malignant pleural effusion: evidence from a validation study and meta-analysis. Ann Med. 2021;53(1):558–566. doi:10.1080/07853890.2021.1906943
11. Shi HZ, Liang QL, Jiang J, Qin XJ, Yang HB. Diagnostic value of carcinoembryonic antigen in malignant pleural effusion: a meta-analysis. Respirology. 2008;13(4):518–527. doi:10.1111/j.1440-1843.2008.01291.x
12. Liang QL, Shi HZ, Qin XJ, Liang XD, Jiang J, Yang HB. Diagnostic accuracy of tumour markers for malignant pleural effusion: a meta-analysis. Thorax. 2008;63(1):35–41. doi:10.1136/thx.2007.077958
13. Salaroglio IC, Kopecka J, Napoli F, et al. Potential Diagnostic and Prognostic Role of Microenvironment in Malignant Pleural Mesothelioma. J Thorac Oncol. 2019;14(8):1458–1471. doi:10.1016/j.jtho.2019.03.029
14. Lievense LA, Cornelissen R, Bezemer K, Kaijen-Lambers ME, Hegmans JP, Aerts JG. Pleural Effusion of Patients with Malignant Mesothelioma Induces Macrophage-Mediated T Cell Suppression. J Thorac Oncol. 2016;11(10):1755–1764. doi:10.1016/j.jtho.2016.06.021
15. Budna J, Kaczmarek M, Kolecka-Bednarczyk A, et al. Enhanced Suppressive Activity of Regulatory T Cells in the Microenvironment of Malignant Pleural Effusions. J Immunol Res. 2018;2018:9876014. doi:10.1155/2018/9876014
16. Wahla AS, Uzbeck M, El Sameed YA, Zoumot Z. Managing malignant pleural effusion. Cleve Clin J Med. 2019;86(2):95–99. doi:10.3949/ccjm.86a.17095
17. Decker DA, Dines DE, Payne WS, Bernatz PE, Pairolero PC. The significance of a cytologically negative pleural effusion in bronchogenic carcinoma. Chest. 1978;74(6):640–642. doi:10.1378/chest.74.6.640
18. Migliore M. Efficacy and safety of single-trocar technique for minimally invasive surgery of the chest in the treatment of noncomplex pleural disease. J Thorac Cardiovasc Surg. 2003;126(5):1618–1623. doi:10.1016/s0022-5223(03
19. Özkaya M. Malign plevral efüzyonlarda tek port torakoskopik talk plöredez. Turkish J Clin Lab. 2019. doi:10.18663/tjcl.413937
20. Kim BS, Kim IJ, Kim SJ, Pak K, Kim K. Predictive value of F-18 FDG PET/CT for malignant pleural effusion in non-small cell lung cancer patients. Onkologie. 2011;34(6):298–303. doi:10.1159/000328793
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.