Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
A Case of Multiple Myeloma-Associated Systemic Amyloidosis with Multiple Skin Manifestations as the First Symptom
Authors Yao S , Wang S, Yi R, Ran L , Zhang C
Received 18 January 2023
Accepted for publication 30 March 2023
Published 9 April 2023 Volume 2023:16 Pages 987—993
DOI https://doi.org/10.2147/CCID.S405330
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Shunyu Yao,1,2 Suxia Wang,3,* Runxi Yi,1,4,* Liwei Ran,5 Cang Zhang2
1Chinese Traditional Medicine, Beijing University of Chinese Medicine, Beijing, People’s Republic of China; 2Department of Dermatology, Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing, People’s Republic of China; 3Laboratory of Electron Microscopy, Pathological Center, Peking University First Hospital, Beijing, People’s Republic of China; 4Department of Oncology, Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing, People’s Republic of China; 5Department of Dermatology, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Liwei Ran, Department of Dermatology, Beijing Chao-Yang Hospital, Capital Medical University, 8 Gongren Tiyuchang Nanlu, Chaoyang District, Beijing, 100020, People’s Republic of China, Tel +86 010 85231374, Email [email protected] Cang Zhang, Department of Dermatology, Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, 23 Back Street of Art Gallery, Dongcheng District, Beijing, 100010, People’s Republic of China, Tel +86 13693219292, Email [email protected]
Abstract: An 81-year-old woman presented with purpura, petechiae, ecchymoses, flesh or brown-colored waxy, smooth, papules, warty plaque, nail dystrophy and palmodigital erythematous swelling for more than 6 years. She was diagnosed as multiple myeloma-associated systemic amyloidosis after skin subcutaneous histopathological examinations and relevant examinations such as blood and bone marrow. Systemic amyloidosis is closely related with multiple myeloma (MM). Multiple and pleomorphic skin lesions are not usual among patients with multiple myeloma or systemic amyloidosis.
Keywords: multiple myeloma, primary systemic amyloidosis, monoclonal immunoglobulin
Case Report
An 81-year-old woman initially presented to a dermatology clinic with recurrent petechiae, ecchymoses, sometimes hemorrhagic blisters which were not easily ruptured, all over the body for 6 years, without pain or itching. Almost at the same time, the patient found nail abnormality, erythematous, swollen palms, buttocks papules, and progressively relaxation of the skin. She had no recent significant weight change.
Physical examination showed multiple petechia, ecchymosis with negative Nissl sign all over the body, abnormal skin wrinkling and laxity, symmetrical periorbital edema accompanied with purpura, and multiple flesh or brown-colored waxy, smooth, papules on the face and the anterior cervical region (Figures 1–4), hard warty plaque and papules on the left buttock (Figure 5), multiple fingernails dystrophy, palmodigital erythematous swelling.
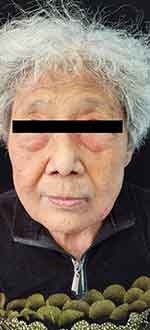 |
Figure 1 Symmetrical periorbital edema accompanied with purpura, and multiple flesh or brown-colored waxy, smooth, papules were on the patient’s face. |
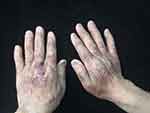 |
Figure 2 Purpura, petechiae and ecchymoses of the skin and fingernails dystrophy. |
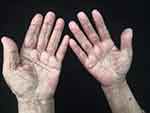 |
Figure 3 Palmodigital erythematous swelling. |
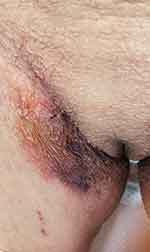 |
Figure 4 The patient’s right groin showed ecchymosis without obvious cause. |
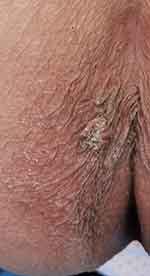 |
Figure 5 There was a hard warty plaque on the left buttock at 3 o’clock. Bleeding could occur after crushing. |
Blood tests showed serum IgG 3.73g/L(8.60–17.40g/L), IgA 34.40g/L(1.00–4.20g/L), IgM 0.08g/L(0.50–2.80g/L), kappa light chain 3.45g/L(6.29–13.50g/L), lambda light chain 22.90 g/L(3.13–7.23g/L), κ/λ 0.15(1.53–3.29), coagulation factor X 48.70%(77–131%).
Protein electrophoresis and immunofixation electrophoresis showed albumin 48.2%(54.0–65.7%), β2-globulin 32.3%(8.2–13.8%), γ-globulin 1.9%(10.6–23.5%), monoclonal IgA-α and monoclonal λ light chain. M protein was positive which was IgA-λ type. The serum free light chains showed κ 6.2mg/L(3.30–19.40 mg/L), λ 307.5mg/L(5.71–26.3mg/L), Fκ/Fλ 0.020(0.26–1.65), and dFLC 301.3mg/L/L. Urine examination showed that protein quantitation 0.32g/L, albumin 98.3%, positive monoclonal IgA-α, monoclonal λ, λ type Bence–Jones protein, λ light chain 7.44mg/dl(0.00–5.00mg/dl) and 24h urine total λ light chains quantification 130mg.
X-ray examination showed no abnormality in bilateral femur and humerus. CT scanning showed no obvious puncture-like bone destruction in the skull, no obvious bone destruction in the whole spine, ribs, sternum and pelvic bones, but cervical and lumbar degeneration.
Bone marrow biopsy showed hyper-cellularity with plasma-cell hyperplasia, which was consistent with multiple myeloma.
The histopathological features of abdominal skin showed that the eosinophilic, morphous, fissured material was present in the dermis which was positive for Congo red stain (Figures 6 and 7). Electron microscopy showed that sheets of fine fibers 10nm in diameter were disorderly distributed in the dermis (Figure 8). The features were consistent with amyloidosis.
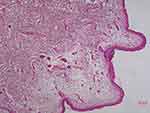 |
Figure 6 Histological features. |
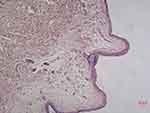 |
Figure 7 Congo red staining (+). |
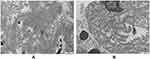 |
Figure 8 (A and B) Electron microscopy showed that sheets of fine fibers with a diameter of about 10 nm were disorderly distributed in the dermis, which is in accord with amyloidosis. |
The patient was diagnosed as multiple myeloma and multiple myeloma-associated systemic amyloidosis. After treatment with BD regimen (a regimen of bortezomib in combination with dexamethasone), no significant improvement of skin lesions was observed. The patient refused further treatment due to financial reasons.
Discussion
Immunoglobulin light chain (AL) amyloidosis (known as primary amyloidosis in history) is an uncommon disease, and the exact incidence is still unknown. In the US, the incidence seems to be stable at about 9 to 14 cases per million person-years.1–3 Olders are more susceptible to AL amyloidosis. As with other plasma cell dyscrasias, the age-specific incidence rates increase in each decade of life after age 40 years.1 AL amyloidosis in which the fibrils are composed of fragments of monoclonal light chains. Affected patients may suffer from amyloidosis alone, or may be accompanied by other plasma cell dyscrasias (multiple myeloma, Waldenström macroglobulinemia). This portion of AL amyloidosis is related to multiple myeloma, in which the deposited light chains come from plasma cells in the bone marrow, leading to extensive organ deposition and dysfunction.4–6 There exist complex interactions between immunoglobulin-derived proteins, including light and heavy chains, and elastic tissue components, leading to different types of impairment of the latter.7 Therefore, AL amyloidosis could have various clinical manifestations of dermatology, including purpura, waxy thickening, ecchymosis and cutis laxa.7,8
Amyloid purpura is suspected to be associated with reduction of coagulation factor X, but this connection is not made. It has been observed that in 36 patients, low levels of factor X activity were observed in about 1 out of 4 samples.9 Because AL amyloidosis is a clonal plasma cell disorder, it is treated with chemotherapy to eradicate the underlying clone. AL amyloidosis must be differentiated from other forms of amyloidosis (eg, AA amyloidosis, ATTRmt amyloidosis, and ATTRwt amyloidosis) since the latter are non-neoplastic and will not benefit from chemotherapy. Compared with normal conditions, MM cells can express lower or higher levels of microRNAs (miRs), which are used as tumor suppressors or oncogenes. Since the expression of tumor suppressor miRs is low in cancer, it may provide therapeutic benefits to restore their normal levels through miRs replacement strategy.10
Conclusion
Our patient presented with purpura, flesh or brown-colored waxy, smooth, papules, warty plaque, hemorrhagic blisters, and nail dystrophy in at least five different forms of skin lesions at the same time. We confirmed the presence of amyloid fibrils using Congo red staining for identity. We also observed under the electron microscope, and found sheets of fine fibers with a diameter of about 10nm distributed disorderly in the dermis, consistent with amyloid fibrils. Multiple myeloma was diagnosed through bone marrow biopsy, according to the updated criteria for the diagnosis of multiple myeloma by the International Myeloma Working Group, so the final diagnosis of this patient was multiple myeloma-associated systemic amyloidosis.
We report a case of multiple myeloma-associated systemic amyloidosis, in which the simultaneous presentation of multiple skin lesions at different sites in one patient is clinically rare. It is difficult to identify the same skin disease due to the diverse morphology of the skin lesions. Regarding the skin manifestations, since the morphology of the rash depends on the site of amyloid deposition, if it is deposited in small vessels of the skin, the skin lesions present as petechiae, ecchymosis and purpura; if it is deposited in the superficial layer of dermis, it will present with waxy papules; and if it is deposited in the dermal elastic tissue, it will present with cutis laxa.Initial skin manifestations included multiple skin manifestations in addition to the typical signs of amyloidosis. Petechiae and ecchymoses were the first symptoms in our patient.
Ethics Statements
Written informed consent for publication of their clinical details and clinical images was obtained from the patient. No institutional approval was required. This case report was prepared following the CARE Guidelines.11
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kyle RA, Linos A, Beard CM, et al. Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. Blood. 1992;79(7):1817. doi:10.1182/blood.V79.7.1817.1817
2. Quock TP, Yan T, Chang E, et al. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv. 2018;2(10):1046. doi:10.1182/bloodadvances.2018016402
3. Kyle RA, Larson DR, Kurtin PJ, et al. Incidence of AL Amyloidosis in Olmsted County, Minnesota, 1990 through 2015. Mayo Clin Proc. 2019;94(3):465–471. doi:10.1016/j.mayocp.2018.08.041
4. Hazenberg BP. Amyloidosis: a clinical overview. Rheum Dis Clin North Am. 2013;39(2):323–345. doi:10.1016/j.rdc.2013.02.012
5. Rosenzweig M, Landau H. Light chain (AL) amyloidosis: update on diagnosis and management. J Hematol Oncol. 2011;18(4):47. doi:10.1186/1756-8722-4-47
6. Bahlis NJ, Lazarus HM. Multiple myeloma-associated AL amyloidosis: is a distinctive therapeutic approach warranted? Bone Marrow Transplant. 2006;38(1):7–15. doi:10.1038/sj.bmt.1705395
7. Ruszczak Z, Wozniak L. Localized nodular amyloidosis cutis with anetoderma. Hautarzt. 1961;12:254–259.
8. Eder L, Bitterman H. Image in clinical medicine. Amyloid purpura. N Engl J Med. 2007;356(23):2406. doi:10.1056/NEJMicm061510
9. Gamba G, Montani N, Anesi E, et al. Clotting alterations in primary systemic amyloidosis. Haematologica. 2000;85(3):289–292.
10. Desantis V, Saltarella I, Lamanuzzi A, et al. MicroRNAs-based nano-strategies as new therapeutic approach in multiple myeloma to overcome disease progression and drug resistance. Int J Mol Sci. 2020;21(9):3084. doi:10.3390/ijms21093084
11. Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. JclinEpi. 2017;89:218–235.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
