Back to Journals » International Medical Case Reports Journal » Volume 12
A case of Legionnaire’s endophthalmitis
Authors Yu JH, Avaylon J , Kil H , Kim JK, Gallemore RP
Received 15 August 2018
Accepted for publication 15 March 2019
Published 14 June 2019 Volume 2019:12 Pages 173—177
DOI https://doi.org/10.2147/IMCRJ.S184046
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Jea H Yu,1 Jaycob Avaylon,1 Hyein Kil,1 Jisoo K Kim,1 Ron P Gallemore1,2
1Department of Clinical Research, Retina Macula Institute, Torrance, CA, USA; 2Department of Ophthalmology, University of California, Los Angeles, CA, USA
Purpose: To report a case of endophthalmitis associated with Legionella Pneumophila.
Case Report: A 46-year-old, highly myopic male with a complex history of recurrent retinal detachments, macular hole, cataract surgery and an infected scleral buckle in the left eye, presented with pain, redness, hypopyon and vision loss in the left eye, 14 days following blunt head trauma. Empirical treatment for endophthalmitis with intravitreal injections of Vancomycin and Ceftazidime afforded minimal improvement. He developed recurrent hypopyon and underwent vitrectomy surgery with intravitreal antibiotic injections at the time of surgery and had improvement. Intraoperative culture was positive for Legionella Pneumophila. He had continued episodes of recurrent inflammation which were quelled by intravitreal moxifloxacin injections performed every 3–10 days. He developed a recurrent RD with proliferative vitreoretinopathy (PVR) and underwent vitrectomy with silicone oil. The retina was reattached but had no light perception vision in the affected eye.
Conclusion: When endophthalmitis is contracted in a work-place setting, a culture for L. pneumophila should be considered. A combination of intravitreal moxifloxacin and oral azithromycin may be effective.
Keywords: endophthalmitis, Legionella Pneumophila, Legionnaire’s disease
Introduction
L. pneumophila is a gram negative bacillus associated with several pathological conditions such as Pontiac Fever and most famously, Legionnaires’ disease.1 It is most commonly found in outdoor water sources such as lakes and rivers but may also be found in hot tubs and air conditioning/evaporative condensing units. Generally, older patients, smokers and immunocompromised patients are most susceptible to a Legionella infection. Aspiration is the most common route of entry for the bacteria and hence, smokers (see above) are particularly susceptible to the disease. For our patient, it is possible that an infected scleral buckle was a possible source/entry point for the bacteria. It is difficult to identify Legionella as a causative agent, if and when it does cause disease, as it has very specific growth requirements and it needs to be cultured on a cysteine infused medium such as a buffered charcoal yeast extract, as opposed to more common media such as blood agar.2 Here we report the first case of L. pneumophila endophthalmitis.
Methods
Retrospective chart review of a patient being treated for a case of endophthalmitis associated with L. pneumophila following a history of a recurrent retinal detachment, macular hole and cataract surgery.
Case report
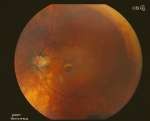
A 46 year old highly myopic Hispanic male presented for a follow-up five months after pneumatic retinopexy and cryotherapy for a macula-off retinal detachment (RD) in his right eye (OD) with no complaints and a normal post-operative course. Visual acuity (VA) was 20/60 OD 20/400 OS and intraocular pressure (IOP) was 17 mmHg OD 15 mmHg OS. Three months prior to the visit, the patient had undergone cataract surgery in his left eye which made visualizing the fundus possible for the first time since his initial presentation. Upon exam of his left eye, there was a full thickness macular hole (Figure 1), cystoid macular edema (CME), cells in the anterior chamber (AC) and vitreous with evidence of prior retinal tears and a scleral buckle. Following a discussion of the options, the patient elected to proceed with vitrectomy with C3F8 Gas OS for the macular hole. The post-operative course was generally unremarkable with VA stabilized at 20/100 and the retina flat and attached (Figure 2). Post-operative inflammation was managed with a tapering course of topical loteprednol etabonate ophthalmic gel 0.5% (Lotemax, BAUSCH + LOMB, Tampa, FL) and IOP was managed with topical brimonidine tartrate/timolol maleate ophthalmic solution 0.2%/0.5% (Combigan, Allergan, Irvine, CA).
 | Figure 1 Fundus photo showing pre-operative full thickness macular hole. |
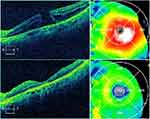 | Figure 2 OCT images of the macula OS showing a full thickness macular hole (above) and subsequent closure of the macular hole with decreased edema 3 months post-operatively (below). |
The patient was regularly seen for follow-up and his inflammation slowly resolved on the drop regimen. The patient presented 5 months after with recurrent inflammation with 2+cells in the AC and vitreous, cystoid macular edema (CME), an IOP of 23 mmHg, a decrease in VA from 20/100 to 20/200 and 6/10 pain in the left eye. His IOP was elevated at 23 mmHg, despite his use of topical brimonidine tartrate/timolol maleate ophthalmic solution 0.2%/0.5% (Combigan, Allergan, Irvine, CA) and topical Dorzolamide HCL ophthalmic solution 2% (Hi-Tech Pharmaceutical, Amityville, NY). Oral acetazolamide 500 mg was added for IOP control. A month later, his VA had improved to 20/60 but his IOP remained elevated at 22 mmHg. Given the persistent CME noted on fluorescein angiogram, a posterior sub-tenon kenalog (Kenalog, Bristol-Myers Squibb, NY) was administered resulting in a reduction of the CME. However, his VA dropped to 20/80 OS and the IOP increased to 26 mmHg. Topical Pilocarpine Hydrochloride Ophthalmic Solution 4% (Akorn, NY) was added for IOP control.
Two months following the kenalog injection, the patient presented with sudden onset of blurry vision and irritation OS. On exam there was an infected scleral buckle OS and the patient underwent surgery two days later. The scleral buckle was removed and subconjunctival vancomycin and dexamethasone injection was given at the time of surgery. The infected buckle was sent for culture, under the hood, at the time of surgery but the results were not obtained due to a processing error. The patient’s VA improved over the next month to 20/60 OS with no reported pain or distortion while taking topical besifloxacin ophthalmic suspension 0.6% (Besivance, BAUSCH + LOMB, Tampa, Florida), topical brimonidine tartrate/timolol maleate ophthalmic solution 0.2%/0.5% (Combigan, Allergan, Irvine, CA), topical Dorzolamide HCL ophthalmic solution 2% (Hi-Tech Pharmaceutical, Amityville, NY), topical loteprednol etabonate ophthalmic gel 0.5% (Lotemax, BAUSCH + LOMB, Tampa, FL), topical pilocarpine Hydrochloride Ophthalmic Solution 4% (Akorn, NY), and oral acetazolamide 500 mg. There was persistent but improved CME with active leakage on fluorescein angiogram (FA) studies and persistence of 1+cells in the AC and all drops were continued.
Four weeks later, days after being hit two times on the left side of the head at work with a deep frying basket, the patient presented urgently with throbbing pain and his VA decreased to hand motion OS. On exam, his IOP was 11 mmHg OS and there was diffuse hyphema, vitreous hemorrhage, and 4+red blood cells in the AC and vitreous. A B-scan was scheduled after completion of a one week course of methylprednisolone which was started for the hyphema. The topical loteprednol etabonate ophthalmic gel 0.5% (Lotemax, BAUSCH + LOMB, Tampa, FL) was increased and the patient continued the topical brimonidine tartrate/timolol maleate ophthalmic solution 0.2%/0.5% (Combigan, Allergan, Irvine, CA), topical Dorzolamide HCL ophthalmic solution 2% (Hi-Tech Pharmaceutical, Amityville, NY), topical pilocarpine Hydrochloride Ophthalmic Solution 4% (Akorn, NY) and oral acetazolamide 500 mg. The B-scan showed diffuse vitreous hemorrhage and the topical pilocarpine Hydrochloride Ophthalmic Solution 4% (Akorn, NY) was discontinued while the topical difluprednate ophthalmic solution 0.05% (Durezol, Alcon, Irvine, CA) was added to the regimen to alternate with the topical loteprednol etabonate ophthalmic gel 0.5% (Lotemax, BAUSCH + LOMB, Tampa, FL). Over the course of the next week, the patient developed pain, hypopyon with fibrin deposits OS and his VA was reduced to light perception and his IOP was 20 mmHg. The diagnosis of endophthalmitis was established and empirically treated with intravitreal (IV) injection of vancomycin and ceftazidime, Tissue plasminogen activator (TPA), along with intravitreal bevacizumab. Oral prednisone (40 mg) was added to the medication regimen and the prognosis was guarded, at the time.
Daily follow-up for endophthalmitis, post-injection, showed resolution of the hypopyon and fibrin deposits on exam but persistent debris on subsequent B-scans with no improvement of VA beyond light perception OS. Two weeks following the initial treatment for endophthalmitis the patient returned urgently once again due to a recurrent hypopyon with fibrin deposits, lid edema and increasing pain OS. On B-scan, there was a new retinal elevation in the macula which indicated a possible abscess or early detachment. The patient underwent vitrectomy with a second intravitreal injection of Vancomycin and Ceftazidime, TPA, along with another intravitreal bevacizumab. The retinal elevation associated with an abscess and vitreous fluid was sent for culture. The patient was managed post-operatively with oral moxifloxacin hydrochloride 400 mg (Avelox, Bayer, Sacramento, CA) oral doxycycline hyclate 100 mg, topical Atropine sulfate ophthalmic solution 1% (Akorn, NY), topical Brimonidine Tartrate ophthalmic solution 0.15% (Sandoz, Princeton, New Jersey), and topical Neomycin and Polymyxin B sulfates and Dexamethosone Ophthalmic Suspension (BAUSCH + LOMB, Tampa, FL) to treat the persistent infection.
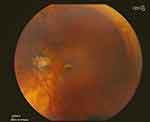
5 days post-operatively, a new rhegmatogenous RD was identified with an associated scleral defect and opacified AC, prompting the need for another vitrectomy with a scleral patch graft with IV injection of Vancomycin/Ceftazidime. A week after surgery, the retina appeared attached on exam but 3+necrotic debris, 2+fibrin deposits and 1+cell in the AC led to the patient’s fourth IV injection of vancomycin/ceftazidime. The inflammation persisted over the course of the next month with the patient developing proliferative vitreoretinopathy (PVR) with membranes over the retina causing contraction and re-detachment. The intraoperative culture report was finalized after 16 days after submission and was positive for L.pneumophila. The patient was re-started on oral moxifloxacin hydrochloride 400 mg (Avelox, Bayer, Sacramento, CA) as suggested by the health department and scheduled for vitrectomy surgery with silicone oil for the recurrent detachment along with IV injection of moxifloxacin for the Legionella. Despite another successful retinal reattachment, there were increasing cells, fibrin in the AC and retinitis on exam, so oral azithromycin 500 mg was added, given culture sensitivities, despite the reportedly poor ocular penetration shown in bactericidal cases of Legionnaire’s disease. The inflammation and hypopyon persisted and 0.05 cc of IV moxifloxacin was injected every 3–10 days for a total of six injections over the course of the month. The patient stabilized following the injections and showed resolution of the hypopyon. The retina remained attached but VA was no light perception OS (Figure 3).
 | Figure 3 High resolution fundus photo after resolution of Legionella endophthalmitis. |
Discussion
L.pneumophila endophthalmitis has not been reported in the literature and ocular complications of systemic Legionnaire’s disease have been rarely described.3 The patient’s complex history of multiple intraocular procedures and persistent inflammation make the origin of infection difficult to ascertain. The intraocular infection was clear from the start but the infective source and causative agent was difficult to identify. However, direct inoculation seems likely given the absence of any other systemic manifestations of Legionnaire’s disease, the longstanding post-surgical inflammation and the fact that extrapulmonary symptoms are extremely rare in immunocompetent individuals.4 Our theory is that, while at work, in the back of a fast-food restaurant, there was an introduction of the bacteria through a defect in the conjunctiva associated with the scleral buckle erosion. The subsequent trauma to the head may have to lead to occult scleral rupture and introduction of L. pneumophila into the eye.
The most common method of transmission of L. pneumophila involves a contaminated aerosol; however, there are indeed reports of Legionnaire’s disease outbreaks linked to restaurants without aerosol sources.5 Various methods that are used in the identification of L. pneumophila include serology assays, urinary antigen test, PCR, as well as culture and isolation. Our intravitreal aqueous humor sample was prepared for culture-based identification using a buffered charcoal-yeast extract medium containing α-ketoglutarate, activated charcoal to sequester peroxides, and ±L-cysteine to select for cysteine autotrophy of the Legionella genus.6
Effective treatment of Legionella depends on early recognition of the organism via culture and selection of appropriate antibiotics.7 In this unique case, the intracellular nature of the bacteria means choosing antibiotic therapy with effective intracellular penetration and also appropriate intravitreal penetration. Typically, the macrolides (azithromycin, erythromycin, roxithromycin) and quinolones (ciprofloxacin, levofloxacin, moxifloxacin, gemifloxacin, trovofloxacin) have been shown to be effective for Legionella infection, so a combination of oral azithromycin and intravitreal moxifloxacin was employed as soon as the organism was identified.7 There have been reports of using intravitreal moxifloxacin injections in Ochrobactrum intermedium endophthalmitis as well as toxicity studies on human RPE cells.8 Repeat intravitreal moxifloxacin injections were needed as the half-life of moxifloxacin in vitreous is about 1.72 hrs in rabbit vitreous.8 The empiric treatment for endophthalmitis was not optimal in this case because L. pneumophila does not respond well to vancomycin (glycopeptide antibiotic) or ceftazidime (cephalosporin antibiotic) which led to ineffective clearance of the organism from the vitreous.9 The delay in initiating appropriate antibiotics due to the difficulty in identifying the organism, contributed to the poorer than expected outcome, the persistence of the infection and the poor outcome with eventual loss of light perception in the affected eye. In pulmonary Legionnaire’s disease, the course of infection varies case by case, even under treatment, and the ocular structures may well be destroyed before the infection can be brought under control. When endophthalmitis is contracted in the work-setting, cultures for Legionella and other microbes should be considered.
Patient Consent
We confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies. Written informed consent has been obtained from the patient to have case details and any accompanying images published.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1.
2. Winn WC
3. Friedland L
4. Yu V
5. O’Loughlin RE, Kightlinger L, Werpy MC, et al. Restaurant outbreak of Legionnaires’ disease associated with a decorative fountain: an environmental and case-control study. BMC Infect Dis. 2007;7:93. Accessed August 9, 2007. doi:10.1186/1471-2334-7-93
6. Mercante JW, Winchell JM. Current and emerging Legionella diagnostics for laboratory and outbreak investigations. Clin Microbiol Rev. 2015;28(1):95–133. doi:10.1128/CMR.00029-14
7.
8. Jacobs DJ, Grube TJ, Flynn HW, et al. Intravitreal moxifloxacin in the management of Ochrobactrum intermedium endophthalmitis due to metallic intraocular foreign body. Clin Ophthalmol. 2013;7:1727–1730. doi:10.2147/OPTH.S44212
9. Graham R
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
