Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
A Case of Isotopic Response Presented with Eosinophilic Pustular Folliculitis
Authors Xv L, Wang B , Zhu Q , Zhang G
Received 20 April 2023
Accepted for publication 21 June 2023
Published 7 July 2023 Volume 2023:16 Pages 1749—1752
DOI https://doi.org/10.2147/CCID.S415322
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Anne-Claire Fougerousse
Lulu Xv,* Bin Wang,* Qing Zhu, Guoqiang Zhang*
The First Hospital of Hebei Medical University, Shijiazhuang, China
*These authors contributed equally to this work
Correspondence: Guoqiang Zhang, No. 89, DongGang Road, Shijiazhuang, Hebei Province, People’s Republic of China, Tel +8618633888122, Email [email protected]
Abstract: This is a 58-year-old woman who has had itching on her right back for 10 days; a month ago she developed blisters on her right back with no apparent cause and pain, and was diagnosed with “herpes zoster”. Erythema and blisters with itching reappeared 10 days ago on the right side of the back, where the original blisters had subsided. The histopathological manifestations of the lesions were: mild hyperkeratosis of the epidermis, pustules within the stratum corneum, irregular proliferation of the spinous layer, spongiosis, local destruction of the basal layer, infiltration of eosinophils, neutrophils, histiocytes and scattered plasma cells in the dermis and subcutis. According to the medical history, clinical manifestations and histopathology of the lesions, the diagnosis was Wolf’s isotopic response after herpes zoster.
Keywords: Wolf’s isotopic response, herpes zoster, eosinophilic pustular folliculitis
Clinical Information
The patient developed blisters with pain on the right side of the back without obvious precipitating factor 1 month ago. The patient went to the local hospital and was diagnosed as “herpes zoster”. After half a month of antiviral treatment, the pain was relieved and the blisters subsided. Ten days ago, erythema and blisters with itching appeared again at the site where the original blisters had subsided. She had a past medical history of hypertension for 10 years and she denied history of food or drug allergy. Dermatologic examination revealed scattered erythema with clusters of corn-sized papules, and pustules on the right side of the back, with no obvious ulceration, and the lesions distributed unilaterally (Figure 1a).
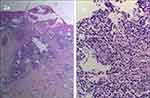 |
Figure 2 (a) HE staining*40; (b) HE staining*100, eosinophil and neutrophil infiltration be seen in the dermis and subcutis. |
Examination and laboratory tests: Results of routine laboratory tests were all within normal limits, no bacteria growth on bacterial culture and HIV test was negative.
Histopathological manifestations: mild hyperkeratosis of the epidermis, pustules within the stratum corneum, irregular proliferation of the spinous layer, spongiosis, local destruction of the basal layer, infiltration of eosinophils, neutrophils, histiocytes and scattered plasma cells in the dermis and subcutis (Figure 2).
Diagnosis: Eosinophilic pustular folliculitis secondary to healed herpes zoster (Wolf’s isotopic response).
Treatment: Compound glycyrrhizin injection (40mg once/day) was given intravenously, compound polymyxin B ointment (2 times/day) was treated externally, and the affected area was irradiated with UVB light therapy (2 times/week). After 7 days of treatment, the lesions improved and the patient was discharged. After 15 days of follow-up, the rash had basically subsided (Figure 1b). The follow up at 5 months showed no recurrence. We have followed up to find out that this woman did not have post herpetic neuralgia (PHN).
Discussion
In 1955, Wyburn Mason described for the first time that a new type of skin disease occurred at the site of a cured skin disease.1 It was not until 1995 that Wolf et al proposed the “Wolf’s Isotopic Response”.2 The pathogenesis of the disease is still unclear. At present, studies believe that the pathogenesis of isotopic response has four hypotheses: viral, immunogenic, vascular and neurogenic. The most widely accepted of these is the neurogenic hypothesis that the Valicella-Zoster virus (VZV) causes damage to nerve fibers (myelinated group Aδ and unmyelinated group C) in the lower and middle dermis, causing neuropeptide release and nerve signal delivery through direct and/or indirect effects, which may be responsible for triggering Wolf’s isotopic response. These neuropeptides include substance P, bradykinin, 5-hydroxytryptamine, vasoactive intestinal peptide (VIP), calcitonin gene-related peptide (CGRP), and alpha-melanocyte-stimulating hormone (MSH). However, the occurrence of new skin diseases still depends on the balance between the pro-inflammatory and anti-inflammatory effects of the neuropeptides themselves. Neuropeptides may produce symptoms such as itching and pain on their own, but their main effect is the abnormal activation of the immune system. This causes dysregulation of the immune system and the formation of isotopic responses. Common diseases currently found in Wolf’s homoeostasis are lichen planus, dermatitis herpetiformis, papular dermatitis, SJS, chronic graft-versus-host disease (GVHD), and acne.3
Wolf’s isotopic response is most commonly seen following the primary onset of herpes zoster, but no Wolf’s isotopic response of eosinophilic pustular folliculitis (EPF) following herpes zoster has been reported. Herpes zoster accompanied with eosinophilic pustular folliculitis has been reported,4 the difference in this case is the occurrence of eosinophilic pustular folliculitis was at the site of the previous and already healed herpes zoster, which is a Wolf’s isotope reaction.
EPF is a rare skin disease with pruritic follicular papules and pustules as the main manifestation, first reported by Ofu-ji et al, and its etiology is unknown. The pathogenesis of EPF may be the result of a multifactorial immune response pattern to antigenic stimuli of various origins, which leads to altered immune homeostasis in the sebaceous microenvironment, resulting in the production of prostaglandin D2 (PGD2),5 the latter and its metabolites 15-deoxy-deta12, 14-prostaglandin12 induce up regulation of eotaxin-3 (a chemoattractant of eosinophils) in sebum and lead to eosinophil infiltration around the sebaceous glandular unit of the hair follicle.6,7 The typical EPF rash is characterized by an annular plaque over which follicular papules and aseptic pustules are seen, fading centrally and expanding peripherally. Histopathologically, an inflammatory cell infiltrate dominated by eosinophils was seen in the superficial dermis and around the sebaceous glandular units of the hair follicles. For the treatment of EPF, non steroidal anti-inflammatory drugs such as indomethacin (oral and topical) are preferred, but other methods such as glucocorticoids, antibiotics, dapsone, colchicine, etc. can also be tried. There are also some refractory cases where the use of phototherapy (UVA, UVB) and other methods are effective.8 It has also been reported that cyclooxygenase (COX) inhibitors, which are involved in prostaglandin synthesis, have been shown to be effective in the treatment of eosinophilic pustular folliculitis.9 We have learned that compounds isolated from licorice exert their anti-inflammatory activity by inhibiting COX, cytokines and their receptors and nuclear transcription factors, as well as by removing oxygen-free radicals, and in this case treatment with compound glycyrrhizin was used to treat eosinophilic pustular folliculitis.10
Conclusion
This disease should be differentiated from acne, impetigo herpetiformis, and dermatitis herpetiformis, etc. Therefore, relevant tests should be completed as early as possible to clarify the diagnosis and help shorten the treatment time.
Abbreviations
PHN, post herpetic neuralgia; VZV, Varicella-zoster virus; VIP, vasoactive intestinal peptide; CGRP, calcitonin gene-related peptide; MSH, alpha-melanocyte-stimulating hormone; GVHD, graft-versus-host disease; EPF, eosinophilic pustular folliculitis; PGD2, prostaglandin D2; COX, cyclooxygenase.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wyburn-Mason R. Malignant change arising in tissues affected by herpes. Br Med J. 1955;2(4948):1106–1109. doi:10.1136/bmj.2.4948.1106
2. Wolf R, Brenner S, Ruocco V, Filioli FG. Isotopic response. Int J Dermatol. 1995;34(5):341–348. doi:10.1111/j.1365-4362.1995.tb03616.x
3. Mahajan R, Saikia U, Mahajan R. Wolf’s isotopic response: report of a case and review of literature. Indian J Dermatol. 2014;59(3):275–282. doi:10.4103/0019-5154.131401
4. Lee J-H, Lee S-K, Kim J-H, Kim H-Y, Myoung-Shin K, Lee U-H. A case of eosinophilic pustular folliculitis associated with herpes zoster. Am J Dermatopathol. 2021;43(4):298–299. doi:10.1097/DAD.0000000000001818
5. Nomura T, Katoh M, Yamamoto Y, Miyachi Y, Kabashima K. Eosinophilic pustular folliculitis: a proposal of diagnostic and therapeutic algorithms. J Dermatol. 2016;43(11):1301–1306. doi:10.1111/1346-8138.13359
6. Amerio P, Frezzolini A, Feliciani C, et al. Eotaxins and CCR3 receptor in inflammatory and allergic skin diseases: therapeutical implications. Curr Drug Targets Inflamm Allergy. 2003;2(1):81–94. doi:10.2174/1568010033344480
7. Nakahigashi K, Doi H, Otsuka A, et al. PGD2 induces eotaxin-3 via PPARγ from sebocytes: a possible pathogenesis of eosinophilic pustular folliculitis. J Allergy Clin Immunol. 2012;29(2):536–543. doi:10.1016/j.jaci.2011.11.034
8. Monastirli A, Antoniades G, Kapranos N, Pasmatzi E, Badavanis G, Tsambaos D. Classic form of eosinophilic pustular folliculitis in an immunocompetent girl: rapid and complete resolution after low-dose oral indomethacin treatment. Dermatol Online J. 2017;23:11. PMID: 29447640. doi:10.5070/D32311037269
9. Simon D, Simon H-U. Therapeutic strategies for eosinophilic dermatoses. Curr Opin Pharmacol. 2019;46:29–33. doi:10.1016/j.coph.2019.01.002
10. Yang R, Wang L-Q, Yuan B-C, Liu Y. The pharmacological activities of licorice. Planta Med. 2015;81(18):1654–1669. doi:10.1055/s-0035-1557893
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

