Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
A Case of Gangrenous Herpes Zoster Complicated with Candida albicans Infection
Authors Wang D, Xu Z, Zeng L , Zhang J, Zhang G
Received 24 April 2023
Accepted for publication 23 June 2023
Published 3 July 2023 Volume 2023:16 Pages 1737—1740
DOI https://doi.org/10.2147/CCID.S415746
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Dongxue Wang,1,* Zhe Xu,2,* Linxi Zeng,1 Jinfang Zhang,1 Guoqiang Zhang1
1Department of Dermatology, The First Hospital of Hebei Medical University, Candidate Branch of National Clinical Research Center for Skin Diseases, Shijiazhuang, People’s Republic of China; 2Department of Dermatology, National Center for Children’s Health, Beijing Children’s Hospital, Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guoqiang Zhang, Department of Dermatology, the First Hospital of Hebei Medical University, Shijiazhuang, Hebei Province, 050031, People’s Republic of China, Tel +86 18633888122, Email [email protected]
Background: Herpes zoster is a disease caused by varicella-zoster virus infection, which is characterized by dense clusters of vesicles distributed along unilateral bands of nerves and accompanied by neuralgia. Although the disease is self-limited, some patients may develop neurological, ocular, skin, or visceral complications.
Case Presentation: We report a 65-year-old Chinese man with ulceration secondary to cutaneous blister rupture on the left lumbar abdomen, who was diagnosed with herpes zoster and did not respond to conventional treatment. Dermatological examination showed diffuse dark erythema with clear boundaries on his left waist and abdomen. Deep ulcers of different sizes were densely distributed with steep edges and relatively dry base, while yellow secretions and black scabs could be seen. Fungal microscopy showed a few pseudohyphae and clusters of spores. Meanwhile, the fungal culture of the secretions showed Candida albicans growth. Skin biopsy of the affected skin from the ulcer of the left abdomen revealed epidermal absence and clusters of spores in the superficial dermis. PAS staining was positive. The patient was diagnosed with gangrenous herpes zoster complicated with Candida albicans infection. After antifungal treatment based on the results of drug sensitivity, the patient’s condition was improved.
Conclusion: This case reveals the co-existence of herpes zoster and Candida albicans infection, expands our understanding of overlapping diseases, and provides value for clinical diagnosis and treatment.
Keywords: gangrenous herpes zoster, skin wound, fungal infection, Candida albicans
Introduction
Herpes zoster is an infectious disease affecting the nerves and skin caused by infection with the varicella-zoster virus.1 After the first infection with varicella-zoster virus, the human body usually appears varicella on the skin or presents an inapparent infection state, becoming a virus carrier. The virus is latent in the posterior root ganglion or trigeminal ganglion of the spinal cord for a long time. When the body’s cellular immune function is compromised by factors such as exertion, infection, or tumor, the virus in the body reactivates and spreads along sensory nerves to the dermatomes, resulting in herpes zoster. Herpes zoster is characterized by dense clusters of blisters that are spread in a unilateral ribbon along a nerve and connected to neuralgia. Its incidence ranges from 3 to 5 per 1000 person-years.2 Although the condition is self-limiting, some patients may develop neurologic, ophthalmic, cutaneous, or visceral complications.3 Herpes zoster and its complications are more common as people get older, especially after the age of 50, and this has a negative impact on patients’ physical, emotional, and overall quality of life. Here, we report a case of gangrenous herpes zoster combined with Candida albicans infection.
Case Presentation
A 65-year-old man presented with skin ulcers on his left waist and abdomen for four weeks secondary to burst blisters. Several patches of edematous erythema and clustered blisters appeared on the left side of his waist and abdomen five weeks ago. After being diagnosed with herpes zoster in the local hospital, he received intravenous Ganciclovir, Xinhuang tablet, and topical treatment with burn cream. As the disease progressed, the initial blisters turned into blood blisters, which burst and caused erosion four weeks ago, so he was given intravenous Cefonicid and traditional Chinese medicine topical treatment. However, the lesions gradually aggravated, forming ulcers of varying sizes, and the pain intensified, so the patient came to the hospital. He had a history of diabetes for 30 years, and his blood sugar was under reasonable control. No apparent abnormalities were found in the systematic physical examination. Dermatological examination showed diffuse dark erythema with clear boundaries on his left waist and abdomen. Deep ulcers of different sizes were densely distributed with steep edges and relatively dry base, while yellow secretions and black scabs could be seen (Figure 1). There was no obvious bleeding after touching.
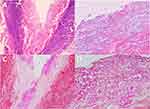
Laboratory tests revealed the erythrocyte sedimentation rate was 62 mm/h (normal 0–15 mm/h), and procalcitonin was 0.05 ng/mL (normal 0–0.05 ng/mL). Fungal dextran, bacterial endotoxin, and aspergillus did not show any abnormality. Bacterial cultures of secretions were negative several consecutive times. Fungal microscopy showed a few pseudohyphae and clusters of spores. Meanwhile, fungal culture of the secretions showed Candida albicans growth (Figure S1 and S2), and further drug sensitivity tests showed that the minimum inhibitory concentrations were voriconazole ≤0.125 μg/mL (S), itraconazole ≤0.125 μg/mL (S), amphotericin ≤1 μg/mL (S), flucytosine ≤4 μg/mL (S), fluconazole ≤8 μg/mL (S). Skin biopsy of the affected skin from the ulcer of the left abdomen revealed epidermal absence and clusters of spores in the superficial dermis. PAS staining was positive (Figure 2).
Finally, the diagnosis of gangrenous herpes zoster with Candida albicans infection was considered, and the patient was given voriconazole 200mg Q12 orally. The ulcer surface became significantly shinier after seven days and recovered after one month, so the drug was discontinued. The follow-up was conducted for five months without recurrence.
Discussion
Herpes zoster is a viral skin disease caused by the activation of the varicella-zoster virus latent in the ganglia when the immune function of the body is reduced. The virus moves along the nerve fibers to the skin, causing intense inflammation of the invaded nerves and skin. It is typical of unilateral erythema and cluster blisters with neuralgia. Because of the different immune states, the skin lesions of some patients are often atypical, manifesting as cadence, incomplete, hemorrhagic, bullous, gangrene, generalized, and so on. Although herpes zoster is usually mild in healthy young people, older people are at increased risk for complications, such as postherpetic neuralgia, the most common form, and cutaneous complications, including scarring, pigmentation, and secondary infections, as well as ocular or visceral complications.3 In this case, the patient’s rash evolved from blisters to blood blisters, which ruptured to form erosive surfaces and then developed into ulcers, consistent with the appearance of gangrenous herpes zoster. Gangrene herpes zoster occurs in the elderly and weak people and is characterized by deep skin lesions, slow healing, easy scarring, and neuralgia. The patient was treated with anti-virus, anti-infection, promoting skin repair, and other treatments, but the effect was not good. Studies have shown that local hypoxia, specific infection, radiation injury, long-term bedridden, malnutrition, diabetes, and advanced age can affect wound healing and make it a chronic refractory wound.4–6 The patient’s nutritional status was good, but the effect of conventional treatment was poor. Specific pathogenic bacteria infection was taken into consideration. Finally, the growth of Candida albicans colonies was confirmed through secretions fungal culture and skin biopsy, and the ulcer surface gradually healed after antifungal therapy.
Candida is an opportunistic pathogen that multiplies and becomes more virulent when the immune function of the body is reduced or the normal balance of the flora is disrupted and can invade local skin, mucous membranes, and all tissues and organs of the body, causing superficial or deep mycosis.7 Cutaneous candidiasis tends to occur in wrinkled and moist areas of the skin.8 With the increase of risk factors such as overuse of antibiotics, impaired skin barrier function, and immunodeficiency caused by various causes, the incidence of cutaneous candidiasis is gradually increasing,9 and the detection rate of fungi in skin ulcers and intractable wounds is also growing, among which Candida albicans is the most common.
Secondary infection with Candida albicans based on gangrenous herpes zoster is thought to be associated with local colonization by Candida due to an inadequate skin barrier in this case. The organism’s impaired immune function and altered local microenvironment (eg, low pH or nutrient utilization) also provide favorable conditions for Candida albicans growth and survival. Candida albicans invades infection via adhesion and invasion, hydrolytic enzyme secretion, cell morphology conversion, quorum sensing, and bacterial biofilm formation, and can evade host immune attack, affect the function of macrophage and release of cytokine, and worsen the infection.10 In addition to Candida albicans, there are a variety of conditionally pathogenic bacteria that can multiply in chronic skin wounds. In clinical practice, attention should be paid to asepsis, active treatment of injuries, rational use of antibiotics, raising awareness of fungal infections, and shortening wound healing time as much as possible.
Conclusion
This case reveals the co-existence of herpes zoster and Candida albicans infection, expands our understanding of overlapping diseases, and provides value for clinical diagnosis and treatment.
Disclosure
The authors report no conflicts of interest in this work. Written informed consent has been obtained from the patient to publish this paper. No institutional approval was required.
References
1. Cohen JI. Herpes Zoster. N Engl J Med. 2013;369(3):255–263. doi:10.1056/NEJMcp1302674
2. Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6):e004833–e004833. doi:10.1136/bmjopen-2014-004833
3. Sengupta S. Cutaneous Herpes Zoster. Curr Infect Dis Rep. 2013;15(5):432–439. doi:10.1007/s11908-013-0356-y
4. Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–229. doi:10.1177/0022034509359125
5. Beyene RT, Derryberry SL, Barbul A. The effect of comorbidities on wound healing. Surg Clin North Am. 2020;100(4):695–705. doi:10.1016/j.suc.2020.05.002
6. El-Ashram S, El-Samad LM, Basha AA, El Wakil A. Naturally-derived targeted therapy for wound healing: beyond classical strategies. Pharmacol Res. 2021;170:105749. doi:10.1016/j.phrs.2021.105749
7. Kashem SW, Kaplan DH. Skin immunity to Candida albicans. Trends Immunol. 2016;37(7):440–450. doi:10.1016/j.it.2016.04.007
8. Janniger CK, Szepietowski JC, Szepietowski JC, Reich A. Intertrigo and common secondary skin infections. Am Fam Physician. 2005;72(5):833–838.
9. Valenti L. Topical treatment of intertriginous candidal infection. Mycoses. 2008;51:44–45. doi:10.1111/j.1439-0507.2008.01617.x
10. Lopes JP, Lionakis MS. Pathogenesis and virulence of Candida albicans. Virulence. 2022;13(1):89–121. doi:10.1080/21505594.2021.2019950
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.