Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
A Case of Erythrodermic Psoriasis Successfully Treated with Risankizumab
Authors Megna M, Ruggiero A , Salsano A, Lauletta G , Portarapillo A , Torta G, Martora F , Potestio L
Received 29 October 2023
Accepted for publication 3 December 2023
Published 5 December 2023 Volume 2023:16 Pages 3503—3507
DOI https://doi.org/10.2147/CCID.S447123
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Matteo Megna,* Angelo Ruggiero,* Antonia Salsano, Giuseppe Lauletta, Antonio Portarapillo, Ginevra Torta, Fabrizio Martora, Luca Potestio
Section of Dermatology, Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy
*These authors contributed equally to this work
Correspondence: Luca Potestio, Section of Dermatology, Department of Clinical Medicine and Surgery, University of Naples Federico II, Via Pansini, 5, Naples, 80131, Italy, Tel +39 - 081 -7462457, Fax +39 - 081 - 7462442, Email [email protected]
Abstract: Psoriasis is a chronic inflammatory cutaneous disease, affecting up to 3% of the worldwide population. Several clinical phenotypes can be distinguished. Among these, erythrodermic psoriasis (EP) is a rare and severe variant (less than 3% of cases), characterized by severe generalized erythema and scaling affecting at least 90% of the body surface area. EP is often a life-threatening condition, since several systemic symptoms (tachycardia, fever, fatigue, lymphadenopathy, dehydration, serum electrolyte disturbances) can be associated. Thus, a prompt and appropriate treatment is mandatory. Unfortunately, EP treatment is challenging. Indeed, the reduced prevalence of EP makes clinical trials feasibility difficult, leading to the absence of established guidelines. So, the treatment of EP is often derived from moderate-to-severe psoriasis management which relies on the use of conventional systemic drugs (cyclosporine, dimethyl fumarate, methotrexate, retinoids) and biologic agents. However, conventional systemic drugs are often contraindicated for patients’ comorbidities, or their use is characterized by reduced efficacy and various adverse events (AEs). The recent development of biologic drugs, which showed excellent results in terms of effectiveness and safety in plaque psoriasis, made these drugs an ideal weapon in EP management, despite their use in EP is still off-label. Among these, risankizumab, a humanized immunoglobulin G1 monoclonal antibody targeting the p19 subunit of the IL23, is one of the latest biologics approved for the management of moderate-to-severe psoriasis. Herein, we reported the first case of a caucasian patient affected by EP successfully treated with risankizumab, reaching PASI100 response after 16 weeks of treatment, without experiencing AEs.
Keywords: erythrodermic psoriasis, treatment, risankizumab
Introduction
Psoriasis is a chronic inflammatory cutaneous disease, affecting up to 3% of the worldwide population.1 Psoriasis may present at any age, and lead to a substantial burden for patients and society.2 Moreover, several medical conditions can be associated with psoriasis, including psoriatic arthritis, depression, and cardiometabolic syndrome, making this disease a systemic disorder.2,3 Several clinical phenotypes can be distinguished.2,3 Globally, plaque psoriasis is the commonest clinical presentation (>80% of cases), characterized by raised, red patches covered with silvery white scales.2,3 However, other psoriasis phenotypes exist.2,3 Among these, erythrodermic psoriasis (EP) is a rare and severe variant (less than 3% of cases), characterized by severe generalized erythema and scaling affecting at least 90% of the body surface area (BSA).4 EP is often a life-threatening condition, since several systemic symptoms (tachycardia, fever, fatigue, lymphadenopathy, dehydration, serum electrolyte disturbances) can be associated.4–7 Thus, a prompt and appropriate treatment is mandatory.8 As regards EP pathogenesis, it is still unknown.4–7 However, EP often develop in subjects with uncontrolled psoriatic disease, or it can be triggered by systemic infection, abrupt withdrawal of systemic treatments (mainly corticosteroids), and drugs (eg interferon, lithium).4–7 Unfortunately, EP treatment is challenging. Indeed, the reduced prevalence of EP makes clinical trials feasibility difficult, leading to the absence of established guidelines.4–7 In this context, the latest guidelines date back to 2010, before the approval of the majority of biologic drugs.4–7 So, the treatment of EP is often derived from moderate-to-severe psoriasis management which relies on the use of conventional systemic drugs (cyclosporine, dimethyl fumarate, methotrexate, retinoids), biologic agents targeting anti-tumor necrosis factor (TNF) α, and anti-interleukin (IL) 12/23, 23 and 17, as well as small molecules.9,10 However, conventional systemic drugs are often contraindicated for patients’ comorbidities, or their use is characterized by reduced efficacy and various adverse events (AEs).11,12 The recent development of biologic drugs, which showed excellent results in terms of effectiveness and safety in plaque psoriasis, made these drugs an ideal weapon in EP management, despite their use in EP is still off-label.13,14 Among these, risankizumab, a humanized immunoglobulin G1 monoclonal antibody targeting the p19 subunit of the IL23, is one of the latest biologics approved for the management of moderate-to-severe psoriasis.15 Its effectiveness and safety were reported both in clinical trials and in real-life experiences,16,17 making this drug a possible therapeutic option in EP.
Case Report
Herein, we describe the case of a 62-year-old man referring to our Department in April 2023 for the presence of psoriasis affecting the trunk, upper and lower limbs, and the scalp. At the anamnesis, the patient reported a sudden exacerbation of his long-term psoriasis (30 years) one month after the third dose of BNT162b2 vaccine. Moreover, the patients reported that its disease was previously well-controlled with topical drugs (calcipotriol/betamethasone dipropionate). Clinical examination revealed the presence of psoriasis affecting more than 90% of BSA, with a Psoriasis Area Severity Index (PASI) of 37 (Figure 1a–c). The patient also reported fatigue. Comorbidities included diabetes, advanced heart failure, obesity and irritable bowel syndrome. A diagnosis of EP was performed.
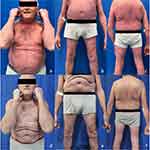 |
Figure 1 Patient at baseline (a–c) and after 16 weeks (d–f) of treatment with risankizumab. |
Because of the extension of the disease and the need for a rapid and safe treatment, also considering patient’s comorbidities, he was screened for biologic drug initiation. Risankizumab was chosen, also considering the patient’s need for a reduced number of administrations. The drug was scheduled at labelled dosage: 150mg at week 0, 4 and every 12 weeks thereafter.
After 16 weeks of treatment, a complete resolution of psoriasis (PASI 100) was observed, with a significant improvement in patient’s quality of life (Figure 1d–f). Of note, no AEs were collected.
Discussion
The introduction of biologic drugs completely changed the management of psoriasis.18 Moreover, their use showed faster and safer results for EP, as compared with conventional systemic treatments.4 Despite the rarity and the severity of this clinical condition do not allow the inclusion of EP patients in clinical trials, the current literature is enriched by evidence from real-world experiences.4 Indeed, positive treatment outcomes have been described in patients treated with different classes of biologics, from anti-TNF-α to anti-IL17 and anti-IL23.4–6 However, data on risankizumab use are limited. As regards other anti-IL23 agents, despite their effectiveness and safety were widely reported,19,20 cases of EP successfully treated with guselkumab and tildrakizumab are scant. The use of risankizumab in EP management has been reported in a primary analysis and 180-week follow-up results from the Phase 3, multicenter IMMspire study enrolling 9 Japanese patients with EP randomized to receive risankizumab 75mg (n=5; mean PASI and BSA at baseline: 46.7±16.1 and 91.0±7.7) or 150mg (n=4; mean PASI and BSA at baseline: 58.7±6.8 and 94.3±3.8) at week 0 and week 4 and every 12 weeks thereafter through week 160, with the aim of assessing the proportion of patients reaching clinical response of at least “minimally improved” in the overall improvement rating at week 16.21 This result was achieved by all of the patients, regardless risankizumab dosage.21 Furthermore, 3 (60.0%) and 4 (100%) patients receiving risankizumab 75mg and 150mg at week 16 achieved PASI90 response, as well as 4 (80.0%) and 4 (100%) subjects in these cohorts reached PASI90 at week 52.21 As regards the safety, 3 (60.0%) and 4 (100%) patients in risankizumab 75mg or 150mg group, reported at least one AE.21 Of these, one for each group was considered serious: a case of ischemic heart failure in the 75mg group and urinary calculus in the 150mg cohort; both of the AEs were not considered treatment related.21
Finally, a case of a 48-year-old Saudi man affected by EP successfully treated with risankizumab, achieving PASI100 response after 3 administrations, was described.22 However, the existing clinical trials only enrolled Japanese patients and the patient described by Alajlan et al has also received an injection of secukinumab 1 week before starting risankizumab.22 So, results are not generalizable, and more data are needed.
Herein, we reported the first case of a caucasian patient affected by EP successfully treated with risankizumab, reaching PASI100 response after 16 weeks of treatment, without experiencing AEs. Of interest, psoriasis exacerbation was probably related to COVID-19 vaccination, as described in literature.23–25 However, the casual temporal correlation cannot be ruled out.23,24
Despite our experience is limited to a single case of EP treated by risankizumab, further studies could widen the therapeutical horizons of biologics, to evaluate their efficacy and safety for EP, in order to offer patients a personalized approach.
Conclusion
EP management is challenging. The reduced prevalence of this condition as well as the exclusion of EP patients from clinical trials make difficult the establishment of guidelines. Thus, more data are needed. In this context, we reported a case of EP successfully treated with risankizumab. Despite limited, our experience is part of the literature reports from real-world evidence that could have a significant role to create the best therapeutic algorithm and tailored options for EP patients.
Data Sharing Statement
Data are reported in the current study.
Patient Consent
The authors obtained written consent from patients for their photographs and medical information to be published in print and online and with the understanding that this information may be publicly available. Patient consent forms were not provided to the journal but are retained by the authors.
Funding
Medical writing was funded through a donation by Abbvie Srl.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386(9997):983–994. doi:10.1016/S0140-6736(14)61909-7
2. Langley RGB, Krueger GG, Griffiths CEM. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(Suppl 2):
3. Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370(9583):263–271. doi:10.1016/S0140-6736(07)61128-3
4. Shao S, Wang G, Maverakis E, Gudjonsson JE. Targeted treatment for erythrodermic psoriasis: rationale and recent advances. Drugs. 2020;80(6):525–534. doi:10.1007/s40265-020-01283-2
5. Lo Y, Tsai TF. Updates on the Treatment of Erythrodermic Psoriasis. Psoriasis. 2021;11:59–73. doi:10.2147/PTT.S288345
6. Potestio L, Camela E, Cacciapuoti S, et al. Biologics for the management of erythrodermic psoriasis: an updated review. Clin Cosmet Investig Dermatol. 2023;16:2045–2059. doi:10.2147/CCID.S407813
7. Dogra S, Mehta H. Biological treatment for erythrodermic psoriasis. Expert Opin Biol Ther. 2022;22(12):1531–1543. doi:10.1080/14712598.2022.2128669
8. Camela E, Potestio L, Fabbrocini G, Pallotta S, Megna M. The holistic approach to psoriasis patients with comorbidities: the role of investigational drugs. Expert Opin Investig Drugs. 2023;1–16. doi:10.1080/13543784.2023.2219387
9. Megna M, Camela E, Battista T, et al. Efficacy and safety of biologics and small molecules for psoriasis in pediatric and geriatric populations. Part II: focus on elderly patients. Expert Opin Drug Saf. 2023:1–16. doi:10.1080/14740338.2023.2173171
10. Megna M, Camela E, Battista T, et al. Efficacy and safety of biologics and small molecules for psoriasis in pediatric and geriatric populations. Part I: focus on pediatric patients. Expert Opin Drug Saf. 2023;22(1):25–41. doi:10.1080/14740338.2023.2173170
11. Nast A, Smith C, Spuls PI, et al. EuroGuiDerm Guideline on the systemic treatment of Psoriasis vulgaris - Part 1: treatment and monitoring recommendations. J Eur Acad Dermatol Venereol. 2020;34(11):2461–2498. doi:10.1111/jdv.16915
12. Nast A, Smith C, Spuls PI, et al. EuroGuiDerm Guideline on the systemic treatment of Psoriasis vulgaris - Part 2: specific clinical and comorbid situations. J Eur Acad Dermatol Venereol. 2021;35(2):281–317. doi:10.1111/jdv.16926
13. Megna M, Battista T, Potestio L, et al. A case of erythrodermic psoriasis rapidly and successfully treated with Bimekizumab. J Cosmet Dermatol. 2023;22(3):1146–1148. doi:10.1111/jocd.15543
14. Megna M, Potestio L, Fabbrocini G, Cinelli E. Tildrakizumab: a new therapeutic option for erythrodermic psoriasis? Dermatol Ther. 2021;34(5):e15030. doi:10.1111/dth.15030
15. Gu C, Yang J. Risankizumab for the treatment of psoriasis. Expert Rev Clin Pharmacol. 2019;12(9):851–857. doi:10.1080/17512433.2019.1657829
16. Gordon KB, Lebwohl M, Papp KA, et al. Long-term safety of risankizumab from 17 clinical trials in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2022;186(3):466–475. doi:10.1111/bjd.20818
17. Megna M, Ruggiero A, Battista T, Marano L, Cacciapuoti S, Potestio L. Long-term efficacy and safety of risankizumab for moderate to severe psoriasis: a 2-year real-life retrospective study. J Clin Med. 2023;12(9):3233. doi:10.3390/jcm12093233
18. Jiang Y, Chen Y, Yu Q, Shi Y. Biologic and small-molecule therapies for moderate-to-severe psoriasis: focus on psoriasis comorbidities. BioDrugs. 2023;37(1):35–55. doi:10.1007/s40259-022-00569-z
19. Ruggiero A, Camela E, Potestio L, Fabbrocini G, Megna M. Drug safety evaluation of tildrakizumab for psoriasis: a review of the current knowledge. Expert Opin Drug Saf. 2022;21(12):1445–1451. doi:10.1080/14740338.2022.2160447
20. Ruggiero A, Potestio L, Cacciapuoti S, et al. Tildrakizumab for the treatment of moderate to severe psoriasis: results from a single center preliminary real-life study. Dermatol Ther. 2022;35(12):e15941. doi:10.1111/dth.15941
21. Yamanaka K, Okubo Y, Yasuda I, Saito N, Messina I, Morita A. Efficacy and safety of risankizumab in Japanese patients with generalized pustular psoriasis or erythrodermic psoriasis: primary analysis and 180-week follow-up results from the phase 3, multicenter IMMspire study. J Dermatol. 2023;50(2):195–202. doi:10.1111/1346-8138.16667
22. Alajlan A, Madani A, Qadoumi TA, Aljaloud A, Alessa M. Erythrodermic Psoriasis Managed with Risankizumab. Case Rep Dermatol. 2022;14(2):219–224. doi:10.1159/000525774
23. Martora F, Villani A, Battista T, Fabbrocini G, Potestio L. COVID-19 vaccination and inflammatory skin diseases. J Cosmet Dermatol. 2023;22(1):32–33. doi:10.1111/jocd.15414
24. Martora F, Villani A, Marasca C, Fabbrocini G, Potestio L. Skin reaction after SARS-CoV-2 vaccines Reply to “cutaneous adverse reactions following SARS-CoV-2 vaccine booster dose: a real-life multicentre experience”. J Eur Acad Dermatol Venereol. 2023;37(1):e43–e44. doi:10.1111/jdv.18531
25. Megna M, Potestio L, Battista T, et al. Immune response to Covid-19 mRNA vaccination in psoriasis patients undergoing treatment with biologics. Clin Exp Dermatol. 2022;47(12):2310–2312. doi:10.1111/ced.15395
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
