Back to Journals » Risk Management and Healthcare Policy » Volume 16
A Case of Comorbid Weber Syndrome Following Mechanical Thrombectomy for Middle Cerebral Artery Occlusion
Authors Li XB, Feng H, Dai Y, Liu W
Received 17 July 2023
Accepted for publication 29 August 2023
Published 13 September 2023 Volume 2023:16 Pages 1875—1880
DOI https://doi.org/10.2147/RMHP.S427893
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Xiao-Bing Li, Hao Feng, Yi Dai, Wei Liu
Department of Neurology, Beijing Haidian Hospital, Beijing, 100730, People’s Republic of China
Correspondence: Wei Liu; Yi Dai, Department of Neurology, Beijing Haidian Hospital, No. 29 Zhongguancun Street, Haidian District, Beijing, 100730, People’s Republic of China, Tel +86– 10– 82693198 ; +86– 10– 82693192, Email [email protected]; [email protected]
Background: In Weber syndrome, one side of the cerebral peduncle of the midbrain is infarcted due to the occlusion of the interpeduncular branch of the posterior cerebral artery and the posterior choroidal artery, resulting in ipsilateral oculomotor nerve palsy and contralateral hemiparesis. However, Weber syndrome induced by simple anterior choroidal artery lesions has rarely been reported.
Case Description: Computed tomographic angiography revealed occlusion of the left internal carotid artery in a 57-year-old male patient who was admitted to the Beijing Haidian Hospital with cerebral infarction. Thrombectomy to clear the occlusion of the left internal carotid artery and the middle cerebral artery was successfully performed in the emergency department. However, postoperative digital subtraction angiography indicated occlusion of the middle and distal segments of the left anterior choroidal artery. After recovery from anesthesia, the patient had left blepharoptosis, inability to abduct the left eye, limitation of the upward and downward gaze, left mydriasis, absence of response to light, and right hemiplegia. Complete head magnetic resonance imaging suggested left cerebral peduncle and basal ganglia infarction. Therefore, the diagnosis was that the patient had left Weber syndrome caused by a left anterior choroidal arterial embolism.
Conclusion: When the anterior choroidal artery is the dominant supplier of blood to the medial region of the ipsilateral cerebral peduncle, the occlusion of this artery may lead to ipsilateral Weber syndrome.
Keywords: cerebral infarction, mechanical thrombectomy, anterior choroidal artery, oculomotor nerve palsy, Weber syndrome
Introduction
Stroke is the main cause of death in China, approximately 1.94 million people died of stroke in 2018,1 and acute ischemic stroke accounts for approximately 80% of stroke. Mechanical thrombectomy treatment refers to mechanical techniques leading to cerebral vascular recanalization with retraction, aspiration, sonolysis, or use of a retrievable stent (stent-retriever). Emergency arterial thrombectomy is now the primary surgery for large-vessel occlusion stroke because of its remarkable clinical benefits. Endovascular thrombectomy is recommended for patients with acute anterior and posterior circulation large vessel occlusion within 24 h after clinical and imaging screening, when it is in line with existing evidence-based evidence.2 Nevertheless, thrombectomy can result in symptomatic cerebral hemorrhage and even brain herniation, which still seriously affects the prognosis of patients.2,3 Pupil size and reflex to light are the most direct clinical signs of brain herniation and directly affect the treatment regimen of patients. In Weber syndrome, occlusion of the interpeduncular branch of the posterior cerebral artery and the posterior choroidal artery leads to ipsilateral oculomotor nerve palsy and contralateral hemiparesis.4 Notably, clinical reports of Weber syndrome induced by an anterior choroidal artery lesion are rare. This article reports such a case that occurred after thrombectomy.
Case Report
A male patient aged 57 years was admitted to the Beijing Haidian Hospital with a complaint of “right-limb weakness for 4 h”. Four hours before admission (8:00), the family had found that the patient had weakness in the right limb in the morning (which was absent at 22:00 the previous night)—he could not lift his upper and lower limbs off his bed and nor could he speak or understand what others spoke to him. Therefore, the patient was brought to our emergency department. This was considered to be a case of acute ischemic cerebrovascular disease. Computed tomography angiography (CTA) + computed tomography perfusion (CTP) of the patient’s head and neck revealed occlusion of the left internal carotid artery and a large hypoperfusion area in the left hemisphere, consistent with the indication of arterial thrombectomy with ultra-extended time window. Accordingly, following the diagnosis of “cerebral infarction”, the patient was admitted to the hospital for further emergency thrombectomy.
The patient had a history of hypertension and hyperlipidemia. In addition, four months prior to this event, it had been diagnosed that the patient had atrial fibrillation but he had not been prescribed anticoagulation medicines. The patient did not have a history of smoking or alcohol consumption.
The physical examination on admission showed a temperature of 36.5°C, heart rate of 110–130 beats/min, atrial fibrillation rhythm, blood pressure of 132/68 mmHg, and respiration rate of 18 breaths/min. Neurological examination showed that the patient had developed drowsiness and aphasia; his pupils were equal in size and round (2.5 mm in diameter), and he had a sensitive reflex to light, a leftward gaze of both eyes, and a shallow nasolabial sulcus. The right-limb muscle strength was grade 0. The left limb was visibly retracted when pain stimulation occurred, and the patient refused to cooperate during the sensory ataxia examination. Bilateral pathologic signs were positive. Atrial fibrillation rhythm was observed with absolute arrhythmia. Respiratory sounds in both lungs were clear, and no apparent rhonchi and moist rales were heard. The abdomen was soft, without tenderness, and the liver and spleen were not palpable below the costal margin. No edema was found in both lower limbs. The patient’s score on the National Institutes of Health Stroke Scale was calculated to be 18.
The laboratory test results were as follows. The examination of D-dimer and four blood coagulation indexes revealed an international normalized ratio of 1.34 ↑ and a D-dimer concentration of 1.063 μg/mL ↑. The examination of six tumor indexes showed 14.2 U/mL carbohydrate antigen 724 ↑. The examination of electrolytes and all biochemical indexes demonstrated 52.6 U/L alanine transferase ↑, 34.6 g/L albumin ↓, 2.05 mmol/L total cholesterol ↓, and 6.59 mmol/L triglyceride ↑. The glycosylated hemoglobin, whole blood cell, routine urine, and routine stool tests displayed no significant abnormalities.
Further, an electrocardiogram showed atrial fibrillation rhythm. Head and neck CTA + CTP on November 13, 2021, revealed left internal carotid artery occlusion, 20 mL core infarct volume, and 76.3 mL hypoperfusion area volume. The results of a cranial magnetic resonance imaging (MRI) + magnetic resonance angiography (MRA) on November 24, 2021 were as follows: hematoma in the left basal ganglia region (early-stage and chronic), multiple old microhemorrhagic foci in the brain, and demyelinating changes in the cerebral white matter. No significant abnormalities were found in the MRA of the patient’s head.
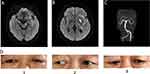
The diagnosis and treatment were as follows. The patient was admitted to the hospital for acute ischemic stroke due to large vessel occlusion. Whole brain arteriography was performed under emergency general anesthesia to confirm the occlusion of the left internal carotid artery (Figure 1A). Thrombectomy to clear the occlusion of this artery and the middle cerebral artery was performed simultaneously and was successful. The occlusion was present in the distal segment of the left anterior choroidal artery (Figure 1B and C).
The immediate postoperative physical examination revealed that the left pupil was enlarged and response to light was sluggish, whereas the right pupil was responsive to light (ratio of left pupil diameter to right pupil diameter = 3:2 mm). The right limb showed avoidance of pain stimulation. A small patchy high-density shadow in the left basal ganglia was detected during an immediate CT re-examination, but there were no symptoms of brain herniation. The patient was transferred to the intensive care unit after the operation. Another physical examination showed that the left pupil was dilated and response to light was now absent, whereas the right pupil was sensitive to light (ratio of left pupil diameter to right pupil diameter = 5:2 mm). The status of the right limb after pain stimulation was the same as that in the immediate postoperative period. Therefore, brain hemorrhage and herniation were not considered for the time being. The patient was treated for mannitol dehydration (for lowering cranial pressure), acid suppression, and lipid regulation and was given anti-inflammatory medicine. His systolic blood pressure was controlled at 90–100 mmHg. Four hours after the surgery, a CT scan was conducted to re-examine the head, which showed that the high-density sign in the left basal ganglia had reduced slightly. The remaining results were not different from those of the immediate postoperative CT. Considering the potential association between left mydriasis and oculomotor nerve injury, antithrombotic therapy through intravenous pumping of tirofiban at 0.2 mg/h was added. Twenty-four hours after the surgery, another head CT scan showed that the high-density sign in the left basal ganglia had disappeared. Meanwhile, the patient was found to be drowsy and with partially mixed aphasia as well as unequal bilateral pupils (ratio of left pupil diameter to right pupil diameter = 5:2.5 mm), sensitivity to light of the right eye, sluggish response of the left eye to light, and recovery of the muscle strength of the right limbs to grade. Aspirin (100 mg) was added for antiplatelet therapy, and tirofiban was discontinued after 2 h. Neurological symptoms gradually improved four days after the surgery, with consciousness, partially mixed aphasia, unequal bilateral pupils (ratio of left pupil diameter to right pupil diameter = 4:2 mm), sensitivity to light of the right eye, sluggish response of the left eye to light, left blepharoptosis, ratio of left eye fissure to right eye fissure = 2:7 mm, central facial-lingual palsy of the right eye, and recovery of muscle strength of the right limbs to grade 3. In addition, Babinski signs on the right side were positive. The patient was transferred to a general ward and given physical rehabilitation training. The patient’s right-limb muscle strength and speech gradually recovered and left mydriasis, response to light, and blepharoptosis gradually improved. At 10 days after surgery, a cranial MRI showed infarction in the left cerebral peduncle (Figure 2A) and left basal ganglia (Figure 2B), but no stenosis of the bilateral posterior cerebral arteries (Figure 2C).
The patient was discharged three weeks after surgery. The results of pre-discharge examinations were as follows: consciousness, partial motor aphasia, left blepharoptosis, correctable self-eye opening, ratio of left eye fissure to right eye fissure = 3:7 mm, round and equal-size bilateral pupils (ratio of left pupil diameter to right pupil diameter = 2:2 mm), sensitive reflex to light, abducent position of the left eye, 2 mm whiteness in the rightward gaze of the left eye, inadequate downward gaze of the left eye (Figure 2D), right central facial palsy, 5-grade right-limb muscle strength, diminished acupuncture sensation in the right limbs, and positive right Babinski sign. The patient’s score on the National Institutes of Health Stroke Scale was 3. One month after the surgery, the anticoagulation treatment was changed to 20 mg rivaroxaban (qd). Six months after the surgery, the patient was contacted via telephone to assess his capacity for self-care, occasional poor expressions, the disappearance of blepharoptosis, and bilateral muscle strength symmetry.
The final diagnosis for the patient was cerebral infarction, paroxysmal atrial fibrillation, hypertension, hyperlipidemia, atherosclerosis, pulmonary infection, stress ulcer, and cardiac insufficiency. Intracerebral hemorrhage is a serious complication that all the performers are extremely concerned about after thrombectomy. But a dilated pupil on one side after anterior circulation endovascular thrombectomy does not merely indicate the occurrence of a massive intracerebral hemorrhage, which can also manifest as Weber syndrome.
Discussion
Blood is supplied to the midbrain through five arteries: the superior cerebellar artery (primarily the medial branch), the collicular artery, the medial posterior choroidal artery, the middle branch of the interpeduncular fossa from the posterior cerebral artery, and the anterior choroidal artery from the carotid artery system.5 The midbrain is classified into four parts according to the region to which the perforating arteries supply blood: the anteromedial, anterolateral, lateral, and posterior parts.4 In detail, blood is supplied to the anteromedial part through the interpeduncular branch of the posterior cerebral artery, and to the anterolateral part through multiple perforating arteries of the posterior cerebral artery (collicular artery and posterior medial choroidal artery) or a branch of the anterior choroidal artery. Further, blood is supplied to the lateral part of the midbrain through the collicular artery, posterior medial choroidal artery, and posterior cerebral artery, and to the posterior part through the dorsal midbrain perforating artery of the superior cerebellar artery, the collicular artery, and the posterior medial choroidal artery. The anterior medial part is the most common site of infarction.6,7 In Weber syndrome, an occlusion of the interpeduncular branch of the posterior cerebral artery and the posterior choroidal artery leads to infarction of the cerebral peduncle of the midbrain on one side. This infarction damages the ipsilateral oculomotor nerve and the pyramidal tract located in the peduncle, thus causing the coexistence of oculomotor nerve palsy and contralateral hemiparesis. The disease manifests clinically as ipsilateral oculomotor nerve palsy (blepharoptosis; abducent position of the eyeballs; palsy of upward, abduction, and downward eye movements; mydriasis; and loss of light reflex) and contralateral hemiplegia (contralateral central facial palsy, tongue muscle paralysis, and upper and lower limb paralysis).4
The vessels responsible for the disease in this patient were the left internal carotid artery terminal and middle cerebral artery. However, the left pupil was dilated after the surgery, severely affecting the judgment of the postoperative condition. However, no signs of brain herniation due to large cerebral infarction or hemorrhage in the left hemisphere were seen on two consecutive reexaminations of the head CT. Therefore, it was considered that the lesion might involve the left oculomotor nerve. The complete head MRI suggested infarction of the blood-supplying region of the middle cerebral artery, such as the left basal ganglia area, and the combined infarction of the left cerebral peduncle, which was consistent with the manifestation of left oculomotor nerve injury. This lesion damaged the medial side of the left cerebral peduncle and presented with left oculomotor nerve palsy: paralysis of the upper eyelid caused blepharoptosis; the eyes were outward in downgaze due to predominance of the external rectus and superior oblique muscles; and pupil enlargement was caused by the palsy of parasympathetic nerve-innervated pupillary sphincter muscles observed on two consecutive reexaminations of the head CT. Therefore, it was considered that the lesion might involve the left oculomotor nerve. Meanwhile, contralateral central facial palsy and central spastic paralysis of the upper and lower extremities occurred because of damage to the pyramidal tract.
The blood supply to the cerebral peduncle is mainly from the posterior cerebral artery, the perforating arteries of the superior cerebellar artery, and an anterior choroidal artery.5 Atherosclerosis in the proximal posterior cerebral artery is a common pathogenesis of anterior midbrain lesions. Medial involvement is usually associated with the P1 segment of the posterior cerebral artery, and lateral involvement is usually associated with the P2 segment. In addition, embolism is a rare cause of midbrain infarction.4 No atherosclerotic changes in the posterior cerebral artery were observed on the CTA and digital subtraction angiography of this patient. Although the patient had a history of paroxysmal atrial fibrillation and experienced the occlusion of the left internal carotid and middle cerebral arteries due to cardioembolism, no new infarct lesions were seen in the distal blood-supply area of the left posterior cerebral artery. Thus, the possibility of the left cerebral peduncle infarction as a posterior cerebral artery lesion was excluded.
The blood-supply area of the anterior choroidal artery mainly includes (1) the posterior part of the posterior limb of the internal capsule and the middle and posterior regions around the parietal ventricle; (2) the medial part of the temporal lobe, hippocampus, optic tract, geniculate body, the middle part of the cerebral peduncle, a small part lateralis of the thalamus, and the junctional region of the medial part of the lenticular nucleus and the internal capsule;8,9 and (3) the distal segment into the ventricle that anastomoses extensively along the choroid plexus posteriorly with the posterior choroidal artery emanating from the posterior cerebral artery.10 The posterior cerebral artery and the perforating arteries of the superior cerebellar artery are the main vessels that supply blood to the cerebral peduncle.6 In addition, little is reported about cases of cerebral peduncle infarction due to the occlusion of the anterior choroidal artery. The post-thrombectomy angiogram for our patient showed that the middle and distal part of the left anterior choroidal artery was occluded. It was considered that the blood supply of the left medial cerebral peduncle was dominant through the anterior choroidal artery and that cardioembolism resulted in the occlusion of the distal part of the left anterior choroidal artery to cause infarction of the left medial cerebral peduncle, the posterior part of the left internal capsule, and the para-lateral ventricle area.
Weber syndrome is commonly seen in posterior circulation stroke. But previous studies have confirmed that endovascular therapy is effective for anterior circulation acute ischemic stroke, but not for posterior circulation stroke. Until the latest ATTENTION and BAOCHE study showed that the proportion of patients with basilar occlusive stroke who received intravascular therapy with good functional prognosis was higher than that of standard medical treatment in 0–12 hours and 6–24 hours of onset.11,12
Conclusion
This patient presented with ipsilateral mydriasis-like manifestations after mechanical thrombectomy of the carotid artery, and distal occlusion of the anterior choroidal artery is considered to be the possibility of cardiac embolus escaping to the distal end. Ultimately, left cerebral peduncle infarction was diagnosed through imaging, and the clinical outcome was excellent—a rare clinical report. In summary, given that thrombectomy has gradually been popularized recently, clinical attention is essential in order to differentiate Weber syndrome from signs of postoperative hemorrhage or brain herniation caused by massive infarction. The possibility of Weber syndrome should be considered that one of the pupils is abnormally dilated after anterior circulation thrombectomy with no bleeding on CT scan, and the normal postoperative treatment strategy should not be affected.
Ethics Approval and Consent to Participate
This study was conducted with approval from the Ethics Committee of Beijing Haidian Hospital. This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all patient guardians.
Consent for Publication
Consent for the publication of the case was obtained from the patient guardians.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Funding
This work was supported by Youth Foundation of Beijing Haidian Hospital (KYQ2022016) and High-level Talents Development Program of the Health System in Haidian District, Beijing, China(2022HDLJ002).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Report on stroke prevention and treatment in China writing Group. Brief report on stroke prevention and treatment in China, 2019. Chin J Cerebrovasc Dis. 2020;17(5):272–281. doi:10.3969/j.issn.1672-5921.2020.05.008
2. Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association [published correction appears in Stroke. 2021 Jul;52(7):e483–e484]. Stroke. 2021;52(7):e364–e467. doi:10.1161/STR.0000000000000375
3. Wu S, Wu B, Liu M, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019;18(4):394–405. doi:10.1016/S1474-4422(18)30500-3
4. Ortiz de Mendivil A, Alcalá-Galiano A, Ochoa M, Salvador E, Millán JM. Brainstem stroke: anatomy, clinical and radiological findings. Semin Ultrasound CT MR. 2013;34(2):131–141. doi:10.1053/j.sult.2013.01.004
5. Tatu L, Moulin T, Vuillier F, Bogousslavsky J. Arterial territories of the human brain. Front Neurol Neurosci. 2012;30:99–110. doi:10.1159/000333602
6. Kim JS, Kim J. Pure midbrain infarction: clinical, radiologic, and pathophysiologic findings. Neurology. 2005;64(7):1227–1232. doi:10.1212/01.WNL.0000156520.46056.6B
7. Ogawa K, Suzuki Y, Oishi M, Kamei S. Clinical study of twenty-one patients with pure midbrain infarction. Eur Neurol. 2012;67(2):81–89. doi:10.1159/000334105
8. Tanriover N, Kucukyuruk B, Ulu MO, et al. Microsurgical anatomy of the cisternal anterior choroidal artery with special emphasis on the preoptic and postoptic subdivisions. J Neurosurg. 2014;120(5):1217–1228. doi:10.3171/2014.1.JNS131325
9. Nomura M, Kida S, Kita D, Hasegawa M, Matsui O, Yamashita J. Anomalous origin of anterior choroidal artery associated with an aneurysm. Acta Neurochir. 2000;142(9):1067–1068. doi:10.1007/s007010070065
10. Choi CY, Lee CH. Transposition of anterior choroidal artery and posterior communicating artery origin. J Korean Neurosurg Soc. 2012;52(3):240–242. doi:10.3340/jkns.2012.52.3.240
11. Tao C, Nogueira RG, Zhu Y, et al. Trial of endovascular treatment of acute basilar-artery occlusion. N Engl J Med. 2022;387(15):1361–1372. doi:10.1056/NEJMoa2206317
12. Jovin TG, Li C, Wu L, et al. Trial of thrombectomy 6 to 24 hours after stroke due to basilar-artery occlusion. N Engl J Med. 2022;387(15):1373–1384. doi:10.1056/NEJMoa2207576
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.