Back to Journals » Clinical, Cosmetic and Investigational Dentistry » Volume 12
A Case of Cheilitis Granulomatosa/Orofacial Granulomatosis
Authors Brown R , Farquharson A , Cherry-Peppers G, Lawrence L, Grant-Mills D
Received 2 April 2020
Accepted for publication 28 May 2020
Published 23 June 2020 Volume 2020:12 Pages 219—224
DOI https://doi.org/10.2147/CCIDE.S254899
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Christopher E. Okunseri
Ronald Brown,1 Andre Farquharson,1 Gail Cherry-Peppers,2 Leslie Lawrence,3 Donna Grant-Mills2
1Department of Oral Diagnosis & Radiology, Howard University College of Dentistry, Washington, DC, USA; 2Department of Restorative Dentistry, Howard University College of Dentistry, Washington, DC, USA; 3Department of Pediatric Dentistry, Howard University College of Dentistry, Washington, DC, USA
Correspondence: Ronald Brown
Department of Oral Diagnosis & Radiology, Howard University College of Dentistry, 600 W Street, NW, Washington, DC 20059, USA
Tel +1 202-806-0020
Email [email protected]
Abstract: A case of a 19-year-old female patient is presented to a private practice dental clinician with swelling of the lower lip and inflammation of the anterior dorsal tongue. The patient presented with moderate oral pain as well as abdominal pain. The lesions were biopsied and noted for a granulomatous histopathologic appearance. The patient reported a history of using cinnamon as a flavoring agent. The lesions resolved within two weeks after the biopsy procedures and topical steroid therapy. The lesions were diagnosed as cheilitis granulomatosa/orofacial granulomatosis. The patient has remained lesion free as of the three-year follow-up. Etiologic, diagnostic and therapeutic issues related to this relatively rare condition of cheilitis granulomatosa/orofacial granulomatosis are discussed.
Keywords: cheilitis granulomatosa, orofacial granulomatosis, cinnamon
Cheilitis granulomatosa (CG) was initially reported in 1945 by Miescher, and is a rare idiopathic (possibly immune-related) inflammatory condition described generally as a usually painless enlargement of one or both lips. CG is regarded as a subset of Orofacial Granulomatosis (OFG). The swelling may be episodic or persistent. The histopathologic presentations of CG are variable, and not necessarily specific or present, and therefore, not absolutely necessary for the establishment of a diagnosis of CG. The pathohistologic findings are non-necrotizing granulomas, edema, lymphangiectasia, and perivascular lymphocytic infiltration.1–3
OFG is a relatively uncommon chronic oral mucosal inflammatory condition of unknown causation. Subsets of OFG include Melkersson-Rosenthal syndrome (MRS), and CG. OFG is an immune related granulomatous condition limited to oral mucosal tissues, or a group of conditions such as extra-oral autoimmune or infectious granulomatous conditions which present intra-orally. The differential diagnosis consists of OFG, includes oral Crohn’s disease (CD), Sarcoidosis, infectious and other immune-related granulomatous conditions, contact lesions, and foreign body reactions.4–12 There is disagreement with respect to OFG and gender predilection, Meist et al,13 and Al-Hamad et al,5 reported no gender predilection. However, Mignogna et al12 evaluated an adult OFG study population consisting of seven females and two males, and Lazzerini et al,7 evaluated pediatric OFG and reported a male to female ratio of two to one. OFG occurs predominantly in young adults but may occur at any age.13
The prototypical presentation is chronic swelling of the lips. OFG lesions are known to have variable presentations as well, which may occur on various oral mucosal anatomical locations and include such oral findings as hyperplastic gingivitis, lingual plica (fissuring), and persistent deep aphthous-like oral ulcers. OFG is regarded as a diagnosis of exclusion, as several OFG-like oral lesions such as CD, Sarcoidosis, Irritable Bowel syndrome (IBS), and infectious granulomatous conditions may prove out to be the correct diagnostic categories in time. OFG lesions range from relatively mild presentations, to very painful disfiguring ulcerative lesions. The condition may present with neurologic manifestations of the head and neck region. The histopathology of OFG is noted for non-caseating granulomas. The OFG prognosis is typically favorable and spontaneous remissions are possible. Topical, intra-lesional, and systemic corticosteroid therapies are the most often employed therapies but noted to produce only mixed results.4–13 OFG can produce unsightly permanent face swelling and is associated with potentially life-threatening systemic diseases, early diagnosis is important with respect to prevent significant permanent cosmetic orofacial disfigurement and to aid in avoiding progression of systemic OFG-related systemic conditions.12
Sciubba and Said-Al-Naief,10 Mignogna et al,12 reported concerning the history of OFG, noting that OFG was first described by Mart in 1859, and later by Hubschumann in 1894, Rossolimo in 1901, Luscher in 1949, and Weisenfled in 1985. Melkersson reported similar findings in 1928, and reported a case which established a connection between facial edema, and facial palsy. Rosenthal in 1931, confirmed Melkersson’s findings in reporting several cases noting combined clinical findings of fissured tongue, facial paralysis, and facial edema which eventually became known as MRS.
OFG is used as a descriptive term with respect to describing granulomatous oral mucosal lesions. Due to the variability of the presentations and the relatively large differential diagnoses, an elimination diagnostic process is necessary. For example, the sometimes similar clinical and histologic appearance of oral CD, complicates the diagnostic process. Furthermore, several authors have proposed that CG may be a subset of MRS. The difficulty of the diagnostic processes and the seriousness of several of the differential diagnoses underline the importance of knowledge of OFG and CG for pediatric dentists, general dentists and hygienists.5,10,13
We present a singular case regarding a patient with lower lip swelling, and anterior tongue granulomatous condition in which we have a presumptive diagnosis of CG and OFG.
Case Report
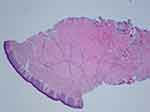
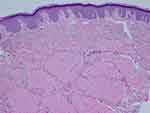
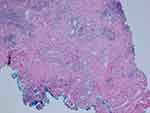
A 19-year-old female patient reported to a private practice oral medicine clinician in January of 2017, with a chief complaint of, “Canker sores, sores on tongue.” The patient reported that there was pain after eating acidic foods. The patient reported a past history of oral thrush this past September. The diagnosis of thrush by her general dentist was determined due to a whitish and reddish central dorsal tongue and the utilization of a steroid rinse for her oral recurrent canker sores. The patient reported oral bumps which she thought were canker sores, but the new (several days ago) tip of the tongue sore seemed to be different. She has been seeing a gastroenterologist due to abdominal pain, and the patient has been avoiding gluten and diary, and also some vegetables and fruits. The gastroenterologist reported a tentative diagnosis of some type of gastric inflammatory condition, and advised the patient to refrain from acidic foods and beverages. The medical history was noted for a history of Hashimoto's disease and acid reflux. The patient was taking lisdexamfetamine for attention deficit hyperactivity disorder. The patient reported no known drug allergies. The clinical examination revealed minor lymphadenopathy of the angle of the jaws bilaterally. Erythema was noted of the anterior tongue (Figure 1), approximately two cm in diameter. Also, the clinician noted a raised area of the right vermilion of the lip approximately 1.5 cm in diameter of normal coloration and texture (Figure 2). The lip lesion was asymptomatic. The clinician reported that the remaining oral tissues appeared to be within normal limits. The clinician performed a biopsy of both the anterior tongue and right lower lip with one cartridge of 2% lidocaine with 1:100,000 epinephrine, removing two specimens with a #3 punch. A prescription was written for a 340 mL bottle of dexamethasone elixir 0.5 mg/5 mL in a rinse and spit topical regimen, as well as clotrimazole 10 mg troches to be used several times a day while using the steroid rinse. The laboratory pathology report noted benign squamous mucosa showing ulceration, acute and chronic inflammation with granulation tissue formation, negative for dysplasia and malignancy. The histopathologic appearance of the lip biopsy noted dilated blood vessels in the superficial lamina propria surrounded by a scattered sparse lymphocytic infiltrate. There were numerous large and small nests of non-caseating granulomatous infiltrate consisting of epithelioid histiocytes and scattered inflammatory cells within the deep reticular zone. (Figures 3 and 4) The histologic appearance of the anterior tongue biopsy revealed numerous and extensive lymphocytic inflammation surrounding blood vessels and interlaced between were nests of non-caseating granulomatous infiltration of the reticular zone. (Figure 5) Giant cells were not evident in either site. On the initial follow-up two weeks after the biopsy, the patient reported that her oral lesions had cleared, and that she was scheduled to see the gastroenterologist in the near future.
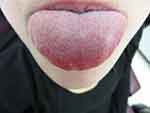 |
Figure 1 Inflammation of the anterior dorsal tongue is evident. |
 |
Figure 2 A lesion of the lower right lip is evident. |
The patient was alerted to the possibility that her condition could be related to food intolerance related to exposure to cinnamon and benzoates and admitted to a food preference for cinnamon. She was asked to consider eliminating cinnamon and benzoates from her diet as a trial. On four-month follow-up, there was no recurrence of oral lesions, nor abdominal pain. The patient had maintained a cinnamon and benzoate (mostly) free diet and appeared to be free of previous oral and gastrointestinal symptoms. At sixteen month follow-up, the patient reported that there had been multiple occurrences of oral lesions and abdominal pain. She reported that her gastroenterologist had determined a diagnosis of IBS. At three-year follow-up, the patient reported no new recurrences and noted that she occasionally uses cinnamon as a flavoring agent.
The patient provided informed consent (both written and verbal) for the case details and images to be published. With respect to publication of this case, institutional approval was not required by Howard University College of Dentistry.
Discussion
As noted by Mignogna, et al,12 with respect to OFG, variable patterns of onset are typical. The location of the granulomatous tongue lesion is certainly an atypical anatomic cite for OFG, but OFG lesions have previously been reported within the gingiva, buccal mucosa, chin, and palate.
Van der Waal et al,1 noted that in 13 patients with CG, that five had lingual plicata. They also noted CG lesions of the facial nerve (three), cheeks (two), nose (two), eyelids (one), gingiva (one), and philtrum (one). Van der Waal et al,1 classified three of their 13 subjects with MRS. They noted that subjects with CG could be regarded as mono-symptomatic variants of MRS. They noted that CG is most often found after the diagnosis of CD has been established or temporally associated with gastrointestinal signs of CD. They reported successful treatment with topical steroids with minor oral lesions and with systemic or injectable steroids for major oral lesions, and suggested surgical therapies only for the most disfiguring cases. Due to the rare nature of this condition, it is extremely difficult to enact prospective controlled studies.
With respect to the present case, the histopathologic presentation is not consistent with a diagnosis of CD and the gastroenterologist’s diagnosis of IBS appears to be supported by Sanderson’s et al,14 The patient reported utilizing cinnamon as a flavoring agent. The clinical presentation was very much unusual particularly with respect to the granulomatous anterior tongue lesion. Furthermore, the quick resolution of the oral lesions (to either the surgical biopsy response or to the topical steroid response or both) was noted. In that the etiology of CG and OFG is unknown, and various presentations have been noted, we believe that this case fits somewhere within the diagnostic categories of CG and OFG.
As noted previously in the introduction, Miescher was the first to describe GC/CG in 1945. CG was described as chronic swelling of one or both lips secondary to granulomatous inflammation. OFG and CG are often described as essentially the same entity. CG is found in only about 0.05% of CD patients. GC is a rare disorder first described by Miescher in 1945. In 1985, Wiesenfeld invented the term OFG as encompassing both Melkersson Rosenthal syndrome and CG. Histologically, OFG was described as characterized by the presence of non-caseating epithelioid cell granulomas, and lymphedema and indistinguishable from CD or Sarcoidosis.3 Histologically, there are various inflammatory conditions as CD, Sarcoidosis and Wegener’s granulomatosis which may be confused with CG. The clinical history and presentations are important with respect to differentiating between these various diagnostic entities.2 Van der Waal et al,1 reported that with respect to the histologic appearance of CG, that the more typical histopathologic findings, noting non-necrotizing granulomas, edema, lymphangiectasia, and perivascular lymphocytic infiltration, are not always present or specific and are not a necessary prerequisite for the establishment of the diagnostic category of CG. Al-Hamed et al,5 described the histology of OFG as dilated lymphatics, edema of corium, slight fibrosis, with/without multiple non-caseating granulomas with Langerhans giant cells and lymphocytes. Mentzer et al,9 evaluated variants of the clinical phenotypes of OFG with respect to the coding regions of the NOD2 gene and screened for rare and potentially pathogenic variants of OFG. They gathered 201 subjects with a diagnosis of OFG with and without intestinal symptoms of CD. Patients were classified into two phenotypic groups based upon clinical findings:
1) OFG without any intestinal symptoms, and 2) OFG with endoscopic and/or radiologic evidence of intestinal CD. The DNA from these two groups was compared with DNA from a UK birth cohort of 1023 population controls. They concluded that their findings suggested that genetic variants in NOD2 were associated only in patients with combined OFG and intestinal disease.
Campbell et al,15 evaluated the results from several studies which evaluated OFG patients who were treated with a cinnamon and benzoate free diet. They noted that 1) White et al,16 reported that 18 out of 25 patients reported benefitted, 2) Nunes et al,17 reported that 39 out of 57 OFG patients benefitted, 3) Kiparissi et al,18 reported that 10 out of 57 OFG patients reported complete remission, and 4) Campbell et al,15 noted that 172 out of 199 demonstrated some benefit and 45 out of these 199 OFG patients required no adjunctive therapies. Campbell et al19 reported through a comprehensive literature search with respect to the diagnosis of OFG such common sensitivities as determined through patch testing as benzoic acid (38%), food additives (33%), perfumes and flavorings (28%), cinnamaldehyde (27%), cinnamon (17%), and chocolate (11%). The mechanism of cinnamon and benzoate relationship with OFG is presently not understood. Data taken from studies related to benzoates in food additives implicates a type IV hypersensitivity reaction mediated by lymphocytes and an activated T cell response to antigens.19 The patient was informed of the potential therapeutic value of a cinnamon and benzoate free diet and was provided several of the articles which reported the success of such a diet.
The potential drug side-effect of abdominal pain secondary to the administration of lisdexamfetamine was overlooked. A drug lisdexamfetamine holiday should have been considered to elucidate whether or not the abdominal pain was secondary to a drug side-effect.20
Sanderson et al,14 questioned whether or not OFG is merely a localized disorder, as they reported that Ileocolonoscopy performed on what appeared to be 35 OFG subjects revealed that 19 of these 35 subjects had macroscopic and microscopic intestinal abnormalities. They surmised that OFG may be a different form of CD with an attenuated intestinal phenotype and a more aggressive oral phenotype. Furthermore, they noted that the histopathologic features of OFG oral only presentations differed from the characteristic features of CD which suggested that OFG may represent a novel form of inflammatory bowel disease. In another study, Campbell et al,21 reported that OFG mainly presents in young adults with lip and buccal-sulcal involvement. CD-associated predictive features include abnormalities in inflammatory markers, hematology and oral features of ulceration, and buccal-sulcal involvement. While with respect to OFG, the initial presentation with such features is not necessarily predictive with respect to the development of CD in young adults.
In conclusion, we have presented a case with both unilateral lower lip and anterior tongue lesions with histopathologic evidence of non-caseating granulomatous disease, along with a history of transient abdominal pain. We believe that a diagnosis of cheilitis granulomatosa/orofacial granulomatosis is appropriate. It is important for dentists and physicians to be aware of these relatively rare conditions.
Disclosure
The authors report no conflicts of interest in this work.
References
1. van der Waal RIF, Schulten AJM, van der Meij EH, et al. Cheilitis granulomatosa: overview of 13 patients with long-term follow-up - results of management. Int J Dermatol. 2002;41:225–229. doi:10.1046/j.1365-4362.2002.01466.x
2. Critchlow WA, David Chang D. Cheilitis granulomatosa: a review. Head Neck Pathol. 2014;8(2):209–213. doi:10.1007/s12105-013-0488-2
3. Wehl G, Rauchenzauner M. A systemic review of the literature of the three related disease entities cheilitis granulomatosa, orofacial granulomatosis and Melkersson-Rosenthal syndrome. Curr Pediatr Rev. 2018;14(3):196–203. doi:10.2174/1573396314666180515113941
4. Troiano G, Dioguardi M, Giannatempo G, et al. Orofacial granulomatosis: clinical signs of different pathologies. Med Princ Pract. 2015;24(2):117–122. doi:10.1159/000369810
5. Al-Hamad A, Porter S, Fedele S. Orofacial granulomatosis. Dermatol Clin. 2015;33(3):433–446. doi:10.1016/j.det.2015.03.008
6. Shams MG, Motamedi MHK. Orofacial granulomatosis of the lower lip and cheek: report of a case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:e42–44.
7. Lazzerini M, Bramuzzo M, Ventura A. Association between orofacial granulomatosis and Crohn’s disease in children: systemic review. World J Gastroenterol. 2014;20(23):7497–7504. doi:10.3748/wjg.v20.i23.7497
8. Zbar AP, Ben-Horin S, Beer-Gabel M, Eliakim R. Oral Crohn’s disease: is it a separable disease from orofacial granulomatosis? A review. J Crohn’s Colitis. 2012;6(2):135–142. doi:10.1016/j.crohns.2011.07.001
9. Mentzer A, Goel R, Elliott T. Azathioprine is effective for oral involvement in Crohn’s disease but not for orofacial granulomatosis alone. Oral Pathol Med. 2016;45:312–318.
10. Sciubba JJ, Said-Al-Naief N. Orofacial granulomatosis: presentation, pathology and management of 13 cases. J Oral Pathol Med. 2003;32:576–585. doi:10.1034/j.1600-0714.2003.t01-1-00056.x
11. El-Hakim M, Chauvin P. Orofacial granulomatosis presenting as persistent lip swelling: review of 6 new cases. J Oral Maxillofac Surg. 2004;62(9):1114–1117. doi:10.1016/j.joms.2003.11.013
12. Mignogna MD, Fedele S, Russo L, Lo Muzio L. The multiform and variable patterns of onset of orofacial granulomatosis. J Oral Pathol Med. 2003;32(4):200–205. doi:10.1034/j.1600-0714.2003.00106.x
13. Miest R, Bruce A, Rogers RS. Orofacial granulomatosis. Clin Dermatol. 2016;34(4):505–513. doi:10.1016/j.clindermatol.2016.02.024
14. Sanderson J, Nunes C, Escudier M, et al. Oro-facial granulomatosis: crohn’s disease or a new inflammatory bowel disease? Inflamm Bowel Dis. 2005;11(9):840–846. doi:10.1097/01.MIB.0000178261.88356.67
15. Campbell HE, Escudier MP, Milligan P, Challacombe SJ, Sanderson JD, Lomer MC. Development of a low phenolic acid diet for the management of orofacial granulomatosis. J Hum Nutr Diet. 2013;26(6):527–537. doi:10.1111/jhn.12046
16. White A, Nunes C, Escudier M, et al. Improvement in orofacial granulomatosis on a cinnamon- and benzoate-free diet. Inflamm Bowel Dis. 2006;12(6):508–514. doi:10.1097/00054725-200606000-00011
17. Nunes C, Lomer MC, Escudier M, Shirlaw P, Challacombe S, Sanderson JD. The dietary management of orofacial granulomatosis. Gut. 2008;56:A117.
18. Kiparissi F, Lindley K, Hill S, Milla P, Shah N, Elawad M. Orofacial granulomatosis is a separate entity of Crohn’s disease comprising and allergic component. J Pediatr Gastroenterol Nutr. 2006;42:E3.
19. Campbell HE, Escudier MP, Patel P, Challacombe SJ, Sanderson JD, Lomer MCE. Review article: cinnamon- and benzoate-free diet as a primary treatment for orofacial granulomatosis. Aliment Pharmacol Ther. 2011;34(7):687–701. doi:10.1111/j.1365-2036.2011.04792.x
20. Cowles BJ. Update on the management of attention-deficit/hyperactivity disorder in children and adults: patient considerations and the role of lisdexamfetamine. Ther Clin Risk Manag. 2009;5:943–948.
21. Campbell H, Escudier M, Patel P. Distinguishing orofacial granulomatosis from Crohn’s disease: two separate disease entities? Inflamm Bowel Dis. 2011;17(10):2109–2115. doi:10.1002/ibd.21599
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.