Back to Journals » International Journal of General Medicine » Volume 15
A Bioinformatic Analysis: The Overexpression and Prognostic Potential of GPX7 in Lower-Grade Glioma
Authors Zhao Q, Zhang L, Wang Y, Sun Y, Wang T, Cao J, Qi M, Du X, Xia Z, Zhang R, Yang Y
Received 14 January 2022
Accepted for publication 1 April 2022
Published 21 April 2022 Volume 2022:15 Pages 4321—4337
DOI https://doi.org/10.2147/IJGM.S356850
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Qianqian Zhao,1 Luyu Zhang,1 Yingying Wang,2 Ye Sun,1 Tianpei Wang,1 Jingjing Cao,1 Meng Qi,3 Xiaoping Du,3 Zengrun Xia,3 Rongqiang Zhang,4 Yin Yang1,5
1School of Nursing, Shaanxi University of Chinese Medicine, Xianyang, Shaanxi, People’s Republic of China; 2The Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou, Henan, People’s Republic of China; 3Ankang R&D Center of Se-Enriched Products, Ankang, Shaanxi, People’s Republic of China; 4School of Public Health, Shaanxi University of Chinese Medicine, Xianyang, Shaanxi, People’s Republic of China; 5The Second Department of Orthopedics, Xi’an Central Hospital, Xi’an, Shaanxi, People’s Republic of China
Correspondence: Rongqiang Zhang, School of Public Health, Shaanxi University of Chinese Medicine, No.1 Middle Section of Century Avenue, Xianyang, Shaanxi, 712046, People’s Republic of China, Tel/Fax +86-029-38185219 Email [email protected] Yin Yang, The Second Department of Orthopedics, Xi’an Central Hospital, No. 161, West Fifth Road, Xincheng District, Xi’an, Shaanxi, 710003, People’s Republic of China, Email [email protected]
Purpose: Glutathione peroxidase-7 (GPX7) is a newly discovered non-selenium-containing protein with glutathione peroxidase activity, which mainly protects the organism from oxidative damage and is very important for basic biology studies. This study aims to reveal the expression pattern of GPX7 and its prognosis potential from a pan-cancer perspective.
Methods: Expression levels of GPX7 in human tumor tissues and normal tissues were evaluated using Human Protein Atlas (HPA), the Cancer Genome Atlas (TCGA), Genotype-Tissue Expression (GTEx) and UALCAN databases. The prognostic potential of GPX7 for 33 TCGA tumors was evaluated by Kaplan–Meier analysis and Cox regression analysis. Subsequently, the Chinese Glioma Genome Atlas (CGGA) dataset was used to further verify the expression of GPX7 and its prognostic potential in glioma. We explored the correlation between GPX7 and immune infiltration, tumor mutational burden (TMB) and microsatellite instability (MSI). Furthermore, a nomogram lower-grade glioma (LGG) was constructed to verify the prognostic outcome of patients. Finally, the relationship between GPX7 and treatment regimens for LGG was also explored.
Results: GPX7 was overexpressed in multiple tumors. Elevated expression of GPX7 was associated with poor prognosis of LGG patients (OS hazard ratio (HR) = 1.044, P < 0.0001; DFS HR = 1.035, P < 0.0001; PFS HR = 1.045, P < 0.0001). GPX7 was proved to be an independent prognostic factor of LGG through univariate and multivariate Cox analysis. The nomogram confirmed a better predictability (Concordance index (C-index): 0.845; 95% CI, 0.825– 0.865). GPX7 was positively correlated with TMB in LGG. GPX7 expression was negatively correlated with half-maximal inhibitory concentration (IC50) of temozolomide (TMZ) (spearman= − 0.59, P =1.3e-48).
Conclusion: GPX7 was upregulated in multiple tumors, and it was a potential prognostic biomarker in LGG. High-expressed GPX7 can predict the sensitivity of TMZ in LGG patients.
Keywords: GPX7, pan-cancer, gene expression, prognosis, biomarker
Introduction
Globally, cancer has become a major public health concern due to the increasing morbidity and mortality. According to statistics from the latest edition of the Global Cancer Report released by the International Agency for Research on Cancer (IARC), there were approximately 19.3 million new cancer cases and 10 million deaths worldwide in 2020.1 Cancer is associated with a heavy economic and social burden, and seriously threatens human health and safety. Malignant tumor is a complex multi-step disease with extremely complex biological characteristics. Due to the concealment of early clinical symptoms, early diagnosis is still challenging and the prognosis is poor. Gliomas is the most common and aggressive primary central nervous system tumors, which accounted for 80.8% of patients. The prognosis of glioma is unfavorable.2 Glioma are classified into grades I–IV by the World Health Organization (WHO) based on histopathological characteristics. Grades II/III are categorized as low-grade glioma (LGG) and grade IV is categorized glioblastoma (GBM).3 Due to the highly invasive nature and incomplete surgical resection, tumor recurrence and progression to GBM are common in LGG, which present a therapeutic challenge for doctors.4 Therefore, identifying effective biomarkers of LGG is essential to guide comprehensive treatment.
The glutathione peroxidase (GPXs) family is an important member of the selenoprotein family. It is one of the important antioxidant enzymes, and an important reactive oxygen species (ROS) free radical scavenger in an organism. Studies have proved that GPXs usually use glutathione as a reducing substrate to catalyze the reduction of H2O2 or organic hydroperoxide to water or the corresponding alcohol, respectively.5,6 There are 8 types of GPXs, namely GPX1–GPX8, of which GPX1–GPX4 and GPX6 are GPXs containing selenocysteine, while GPX5, GPX7 and GPX8 are non-selenocysteine GPXs.5–8 Mammalian glutathione peroxidase-7 (GPX7) is a non-selenocysteine containing phospholipid hydroperoxide glutathione peroxidase.7 It plays an important role in maintaining the redox state.9,10 When cells are exposed to oxidative stress, the unique pressure sensor function of GPX7 can effectively detect redox levels and transmit ROS signals to redox sensitive cells and thiol-containing proteins, further promoting protein folding and releasing non-targeted short interfering RNAs (siRNAs) related stress.11 The dysregulation of GPX7 may lead to some diseases and even cancer. Previous studies have shown that the differential expression of GPX7 is closely related to the occurrence and progression of multiple tumors. Experiments showed that GPX7 protects esophageal epithelia from bile acids-induced oxidative DNA damage, double strand breaks and apoptosis in oesophagus cancer.12 GPX7 was overexpressed in hepatocellular carcinoma tissues.13 GPX7 had tumor suppression function in gastric cancer and was silenced by promoter DNA methylation.14 The biological function of GPX7 is quite important for basic biology studies. Although GPX7 gene expression and its transcription characteristics have been explored in certain specific tumors, expression pattern of GPX7 as well as its prognostic value have not been studied through the pan-cancer perspective.
Pan-cancer analysis does not refer to a specific tumor, instead, data from different tumor types and multiple sets of platforms are integrated, analyzed, and interpreted to identify and analyze different genetic changes in malignancies.15 Through pan-cancer analysis, similarities and differences between genomes and cell changes for different malignant tumor types can be screened. In this study, taking advantages of the Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEx) databases, we comprehensively explored the expression pattern of GPX7, its value of prognostic, and its relationship with immune infiltration, tumor mutational burden (TMB) and microsatellite instability (MSI) through pan-cancer analysis. The prognostic potential of GPX7 in LGG was fully explored and a nomogram was established to predict the survival outcome of LGG patients. Especially, the biological significance of GPX7 for the possibility to determine sensitivity of chemoradiotherapy in LGG also explore in our present study.
Materials and Methods
Raw Data Collection
RNA-seq data from the Human Protein Atlas (HPA) (https://www.proteinatlas.org/) was used to explore the expression pattern of GPX7 in human normal tissues and cell lines. The mRNA expression data of GPX7 in tumor tissues were downloaded from TCGA database (http://cancergenome.nih.gov/). TCGA performed molecular profiling of more than 20,000 primary cancers, covering 33 tumor types and matching normal samples from 33 cancer types. In order to explore the expression differences of GPX7 in different cancer types and corresponding normal tissues, we obtained normal tissue samples matching 33 TCGA cancer types from the GTEx (https://www.gtexportal.org/home/) database, and then combined GTEx data with TCGA data. The transcriptome and clinical data of glioma patients were obtained from the Chinese Glioma Genome Atlas (CGGA) database (http://www.cgga.org.cn/) to verify the expression level of GPX7 in LGG and the prognostic outcome of LGG patients. The information about temozolomide (TMZ) sensitivity in LGG was collected from the Genomics of Drug Sensitivity in Cancer (GDSC, https://www.cancerrxgene.org/) database.
GPX7 Expression Profiles in Human Normal Tissues and Cell Lines
The HPA database was a comprehensive resource website that provided the expression and distribution of more than 17,000 individual proteins in all kinds of cells, tissues and organs.16 In the present study, GPX7 mRNA expression and distribution in human normal tissues (n=55) and all cell lines (n=69) used the HPA database to evaluate.
GPX7 Expression Pattern in Human Tumor Tissues
The RNA-seq expression data of 33 tumors with matched normal tissues were obtained from the TCGA database.17 The mRNA expression data of normal tissues were downloaded from the GTEx database.18 The expression profile of GPX7 was analyzed through the integrated data of GTEx and TCGA. The difference between the tumor tissues and the normal tissues was analyzed by rank sum test, and the P < 0.05 was considered statistically significant. The R software V 4.0.3 was used for statistical analysis. Data were visualized as violin plots.
UALCAN database (http://ualcan.path.uab.edu/analysis-prot.html) was an interactive web-portal for analyzing cancer OMICS data of TCGA.19 In the present study, the UALCAN database was used to explore gene expression at the protein level. The expression profile of GPX7 protein in 10 different tumors was retrieved used the CPTAC module, including: breast invasive carcinoma (BRCA), ovarian serous cystadenocarcinoma (OV), colon adenocarcinoma (COAD), kidney renal clear cell carcinoma (KIRC), uterine corpus endometrial carcinoma (UCEC), lung adenocarcinoma (LUAD), head and neck squamous cell carcinoma (HNSC), pancreatic adenocarcinoma (PAAD), GBM and liver hepatocellular carcinoma (LIHC). The expression profile of GPX7 protein was presented by box plots. Z-values represented standard deviations (SD) from the median (M) across samples for a particular cancer type.
Association Between GPX7 Protein Expression Level and Clinicopathological Parameters
Analysis data of GPX7-relevant proteomic characteristics, such as tumor stage, age, race and gender were downloaded from the “CPTAC Analysis” module of UALCAN. Z-values represent standard deviations (SD) from the median (M) across samples of a certain cancer and they were used to evaluate association between GPX7 protein expression level and clinicopathological parameters.
Survival and Prognosis Analysis
The “Survival map” module of the Gene Expression Profiling Interactive Analysis (GEPIA2)20 (http://gepia.cancer-pku.cn/index.html) was used to obtain overall survival (OS) and disease-free survival (DFS) significant survival curves of GPX7 in 33 TCGA tumors. The cutoff-high (50%) and cutoff-low (50%) values were defined as thresholds to group the whole cohort into high and low expression groups respectively. Kaplan-Meier survival curves were established by the “survival analysis” module of GEPIA2. Log rank test was used to perform hypothesis testing, and determine differences between Log rank test curves. Univariate Cox regression was used to calculate hazard ratios (HR) and 95% confidence interval (95% CI). The significance level was set at 0.05. Survival analysis (OS, DFS, progression-free survival (PFS) and disease specific survival (DSS)) used the “ggforest” in the R-package “survminer” to form forest plots to further evaluate the prognostic potential of GPX7 expression in patients with different tumors.
The CGGA was an open-access platform that collected a multi-dimensional functional genomic dataset of nearly 2000 glioma samples from Chinese cohorts.21 In this study, the CGGA database was used to further evaluate and verify the relationship between GPX7 expression and prognostic potential in glioma.
Correlation Between GPX7 and Infiltration of Immune Cells
Exploring the correlation between GPX7 and immune infiltration is of great significance for further basic biological research. RNA-seq data from the TCGA database were analyzed by Immunedeconv to explore the correlation between GPX7 and immune infiltration.22 Immunedeconv R package can be obtained from github (https://github.com/icbi-lab/immunedeconv/issues). It is often used to evaluate the immune cell components in tumor tissue and it integrates six of the latest algorithms. The EPIC algorithm was often used to explore the level of immune infiltration in most solid tumors. It can explain non-characteristic cells and the mRNA content in each cell type.23 In the present study, EPIC algorithm was used to evaluate the potential correlation between immune cell (CD8+T cell, CD4+T cell, NK cell, Macrophage, Endothelial cell, and B cell) infiltration levels and expression levels of GPX7. R software V4.0.3 was used for statistical analysis, and P < 0.05 was considered statistically significant.
TIMER 2.0 (http://timer.cistrome.org/) was an interactive web application that was used to comprehensively and flexibly analyze the richness of tumor infiltrating immune cells.24 In this study, the “Immune-gene” module of TIMER 2.0 was used to determine the association between GPX7 expression and cancer-associated fibroblasts (CAFs) infiltration level of TCGA tumors based on four algorithms including EPIC, MCPCOUNTER, XCELL and TIDE. When the results of four algorithms were all statistically significant, a scatter plot with the highest correlation coefficient was given. R software V 4.0.3 was used for statistical analysis. P < 0.05 was considered statistically significant.
Gene Mutation Analysis
TMB can predict the immune checkpoint inhibitor response.25 MSI, a genetic hypermutation state caused by the inactivation of mismatch repair genes, is a known immunotherapy biomarker.26 In this study, the somatic mutation profiles of all tumor patients were extracted from the Genomic Data Commons (GDC) (https://portal.gdc.cancer.gov/)27 data portal website of TCGA database to calculate the TMB score and MSI score. The association between the expression of GPX7 and TMB/ MSI was further explored. Statistical analysis was performed using the R software V 4.0.3, and the difference of the two groups were determined by rank sum test. P < 0.05 was considered statistically significant.
Prognostic Nomogram for LGG
We used univariate and multivariate Cox regression analysis to screen independent prognostic factors to further explore the association between GPX7 expression and prognosis in LGG. Based on the results of multivariate Cox regression analysis, the nomogram was established using the “rms” R package to predict the OS of LGG patients. Perform ROC analysis on the risk score of the multivariate Cox model to obtain the Concordance index (C-index), which was used to quantify the efficacy of the nomogram for prognostic evaluation. The closer the C-index was to 1, the better the nomogram predictive ability. The calibration plots were a comparison between the risk predicted by the nomogram and the actual risk of patient. The closer the predicted curve was to the standard curve, the better the predictability of the model.
The Effect of the Interaction of GPX7 with Chemoradiotherapy on Prognosis in LGG
Based on GPX7 mRNA data from TCGA LGG patients, we explored the effect of the interaction of GPX7 with chemoradiotherapy on the prognosis of the patients. The difference between the different groups was analyzed by rank sum test. The correlation between GPX7 expression and TMZ sensitivity in LGG was also analyzed by TCGA and GDSC database.28 The half-maximal inhibitory concentration (IC50) of TMZ was calculated by ridge regression. All the above analysis process were performed by R 4.0.3. P < 0.05 was considered statistically significant.
Results
GPX7 mRNA Expression and Distribution in Human Normal Tissues and Cell Lines
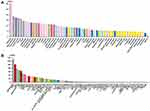
The tissue atlas from the HPA database was used to analyze the mRNA expression of GPX7 in human tissues and cell lines (Figure 1). Analysis showed that GPX7 mRNA was widely distributed in normal human tissues (Figure 1A) and cell lines (Figure 1B). These findings show that there is no specificity in mRNA expression of GPX7 in the human body, including tissues and cell lines.
The Expression Levels of GPX7 in Human Tumor Tissues
The expression levels of GPX7 mRNA in 33 tumor tissues and matched normal tissues were compared using TCGA and GTEx datasets (Figure 2). The results showed that with the exception of the 3 types of tumors, the expression difference between tumor tissues and normal tissues was statistically significant (P < 0.05). Analysis showed that compared to normal tissues, mRNA expression of GPX7 were over-expressed in 22 human tumor tissues, including BRCA, esophageal carcinoma (ESCA), GBM, LGG, LIHC. In contrast, GPX7 mRNA expression were under-expressed in kidney chromophobe (KICH) and prostate adenocarcinoma (PRAD).
 |
Figure 2 mRNA expression level of GPX7 in human tumor tissues and normal tissues from TCGA database and GTEx database. *p<0.05; **p< 0.01; ***p<0.001; “-” insignificant. |
The expression of GPX7 protein levels were analyzed using the CPTAC database (Figure 3). The results showed that GPX7 protein is highly expressed in 9 types of tumors, including BRCA, COAD, KIRC, UCEC, LUAD and HNSC (P < 0.05).
 |
Figure 3 Expression level of GPX7 protein in the normal tissues and primary tumors tissues from CPTAC database. |
Association Between GPX7 Protein Expression and Pathological Parameters
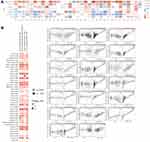
The data related to the expression of GPX7 protein and age, gender, race and cancer stage was obtained from the UALCAN dataset (Figure 4). Results showed that expression level of GPX7 protein exhibited a gradually increasing trend in COAD, OV, KIRC and UCEC. That is, the highest expression was found in stage IV (Figure 4A). Expression levels of the GPX7 protein in OV, COAD, KIRC, UCEC and LUAD were generally elevated in Caucasian patients than in other races (Figure 4B). In terms of age, elevated expression levels of the GPX7 protein were increased in people aged 20 to 40 years in BRCA, OV, COAD and KIRC (Figure 4C). In terms of gender, expression levels of the GPX7 protein were increased in males than in females in COAD and LUAD (Figure 4D).
Prognostic Value of GPX7 in Human Tumors
The association between the expression of GPX7 and prognosis of human tumors was analyzed using TCGA database (Figure 5). Kaplan-Meier survival curves showed that GPX7 overexpression was correlated with poor OS in LGG (P = 4.4e-08), LIHC (P = 0.022), mesothelioma (MESO) (P = 4e-04), sarcoma (SARC) (P = 0.015) and stomach adenocarcinoma (STAD) (P = 0.0096) (Figure 5A). DFS analysis showed that GPX7 was correlated with poor prognosis with high expression levels in LGG (P = 3.6e-05), SARC (P = 0.015) and STAD (P = 0.0096) (Figure 5B). These results indicate that GPX7 overexpression is a risk factor for poor prognosis in LGG patients regarding OS and DFS.
 |
Figure 5 Kaplan–Meier survival curves of high and low expression of GPX7 in different types of tumors. (A) OS in LGG, LIHC, MESO, SARC, STAD. (B) DFS in LGG, SARC, STAD, THCA. |
We used Cox regression model to further verify prognostic value of GPX7 on the survival outcomes (OS, PFS, DFS, DSS) of patients with different tumors (Figure 6). Elevated expression levels of GPX7 were significantly associated with poor prognosis in bladder urothelial carcinoma (BLCA), KICH, LGG, SARC and STAD regarding OS, DFS and PFS. In adrenocortical carcinoma (ACC) and MESO, elevated expression levels of GPX7 were associated with poor OS and PFS. In contrast, elevated expression levels of GPX7 in thymoma (THYM) were correlated with better OS and PFS outcomes (Figure 6A–C). In kidney renal papillary cell carcinoma (KIRP) and PAAD, elevated expression levels of GPX7 were associated with poor DSS (Figure 6D). The results of integrating Kaplan-Meier survival curves and Cox regression forest plot suggested that GPX7 was significantly associated with poor prognosis of OS, DFS and PFS in LGG, SARC and STAD. In general, elevated GPX7 expression levels were associated with poor clinical outcomes in most tumors.
 |
Figure 6 Forest plot for association between GPX7 and OS (A), DFS (B), PFS (C), DSS (D) in patients with different tumor types from TCGA database. P < 0.05 was considered statistically significant. |
The above survival analysis results indicated that the high expression of GPX7 was closely related to the poor prognosis of LGG patients. Therefore, we downloaded the glioma dataset from the CGGA database to further verify the prognostic value of GPX7 in LGG. The cohort consisted of 182 LGG cases and 139 GBM cases (Figure 7). Analysis showed that the expression levels of GPX7 gradually increased as the disease progresses (P = 2.4e-34, Figure 7A). Survival analysis showed that overexpression of GPX7 was correlated with poor prognosis in LGG (P < 0.0001, Figure 7B), which is consistent with the results of TCGA analysis.
Relationship Between GPX7 Expression and Immune Infiltration
The tumor microenvironment is crucial to the occurrence and development of tumors as well as to basic biological research. In this study, the EPIC algorithm was used to explore the correlation between GPX7 and immune infiltration (Figure 8A). Results showed that there was an overall positive correlation between GPX7 expression and endothelial cell, macrophage in most tumors. Expression of GPX7 was positively correlated with macrophage, endothelial cell and B cell infiltrations in BRCA, COAD, HNSC, PAAD and STAD. In LGG, GPX7 expression was negatively correlated with CD4+T cell, CD8+T cell and B cell infiltrations and it was positively correlated with macrophage infiltrations.
The TIMER 2.0 interactive web tool was used to explore the relationship between GPX7 expression and CAFs infiltration in different tumors based on four different algorithms (Figure 8B). Analysis showed that GPX7 expression and CAFs abundances were positively correlated in 20 tumors, including BLCA, BRCA, BRCA-LumA, BRCA-LumB, Cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC).
Relationships Between GPX7 and TMB/MSI
The relationship between GPX7 and TMB is shown in Figure 9A. The expression of GPX7 was positively correlated with TMB in 8 tumors, including LGG (R = 0.366, P = 1.15e-17) and OV (R = 0.148, P = 0.019). In contrast, the expression of GPX7 was negatively correlated with TMB in 24 tumors, including PRAD (R = −0.336, P = 4.31e-14), STAD (R = −0.284, P = 3.26e-08), uveal melanoma (UVM) (R = −0.249, P = 0.026), ESCA (R = −0.24, P = 0.002), THYM (R = −0.223, P = 0.016), skin cutaneous melanoma (SKCM)(R = −0.196, P = 2.26e-05), LIHC (R = −0.171, P = 0.002), PAAD (R = −0.16, P = 0.043), HNSC (R = −0.14, P = 0.001), COAD (R = −0.11, P = 0.039) and thyroid carcinoma (THCA)(R = −0.109, P = 0.017). The relationship between GPX7 and MSI is shown in Figure 9B. Analysis showed that there was a positive correlation between GPX7 and MSI in 14 tumors, including testicular germ cell tumors (TGCT) (R = 0.18, P= 0.038). On the other hand, the expression level of GPX7 was negatively correlated between GPX7 and MSI in 19 tumors, including lymphoid neoplasm diffuse large B-cell lymphoma (DLBC) (R = −0.296, P = 0.041), STAD (R = −0.221, P = 1.58e-05) and LUAD (R = −0.191, P = 1.35e-05). It is worth noting that the correlation coefficients between GPX7 and TMB was the highest in the LGG cohort.
Development and Validation of Prognostic Nomogram for LGG
The above results strongly support that there was a significant correlation between the expression of GPX7 and the prognosis of LGG. Therefore, a nomogram was developed and verified the value of GPX7 mRNA expression level on the prognosis potential of LGG patients. The TCGA cohort was used to perform univariate and multivariate Cox regression analysis on LGG patients to determine the independent prognostic factors of LGG patients. GPX7 and age were significantly correlated with OS in LGG patients through univariate Cox regression analysis (P < 0.0001, Figure 10A). Multivariate Cox regression analysis showed that age and GPX7 were identified as independent prognostic factors for LGG (P < 0.0001, Figure 10B). Based on the results of multivariate Cox regression analysis, the nomogram was established predict the 1-year, 2-year, 3-year, and 5-year OS of LGG patients (Figure 10C). The C-index of the nomogram model was 0.845 (95% CI, 0.825 to 0.865; P < 0.001), indicated that this model has a high predictability. The Calibration plots curve showed that the predicted survival outcome of LGG nomogram matches the observed actual survival outcome well (Figure 10D). These results indicated that this nomogram model was a good predictor of the OS of LGG patients, which strongly supports that GPX7 was a prognostic indicator for LGG patients.
The Effect of the Interaction of GPX7 with Chemoradiotherapy on Prognosis in LGG
The effect of the interaction of GPX7 with chemoradiotherapy on prognosis in LGG was explored by TCGA dataset (n=510). In radiotherapy (RT) group (n=142), high-expression of GPX7 with a log2(x+0.001) value bigger than 4.31 was correlated with poor overall survival (P < 0.05, Figure 11A). In chemotherapy group (n=223), high-expression of GPX7 with a log2(x+0.001) value higher than 4.37 was also associated with poor clinical outcomes (P < 0.05, Figure 11B). Subsequently, the patients with GPX7 high-expression (n=126) were divided into RT group (n=87) and non-RT group (n=39). There was no statistical difference in OS between the two groups, but a trend of shorter OS was found in RT group (P > 0.05, Figure 11C).
In order to explore potential significance of elevated GPX7 for the sensitivity of chemoradiotherapy in LGG, the correlation between GPX7 expression and TMZ sensitivity in LGG patients was analyzed. The results showed that GPX7 expression was negatively correlated with TMZ IC50 (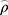 spearman = −0.59, P =1.3e-48, Figure 11D), which suggested that high-expressed GPX7 can predict the sensitivity of TMZ in LGG patients.
spearman = −0.59, P =1.3e-48, Figure 11D), which suggested that high-expressed GPX7 can predict the sensitivity of TMZ in LGG patients.
Discussion
GPXs family is an important member of the selenoprotein family. GPXs is one of the most important enzyme families that involve in ROS removing process. GPXs plays an important role in the antioxidant system by scavenging H2O2, organic peroxides and lipid peroxides.29 GPX7, a newly discovered non-selenium-containing member of the GPXs family, is free near the endoplasmic reticulum and is one of the proteins with lower glutathione peroxidase activity.8,30 It has a high amino acid sequence homology with other family members.5,7 The sequence of GPX7 encodes cysteine in its conserved catalytic motifs.11 GPX7 maintains the redox state and participates in protein folding in the body.6,7 Exploring the biological function of GPX7 is of great significance for further basic biological research. Emerging evidence indicates that differences in GPX7 expression are related to the occurrence and development of certain tumors, such as breast cancer,7 gastric cancer14 and hepatocellular adenocarcinoma.13 However, the expression profile and prognostic value of GPX7 has not been reported from a pan-cancer perspective. Therefore, this study integrates data from multiple online databases to comprehensively explore the expression pattern and prognostic value of GPX7.
The expression of GPX7 in human normal tissues does not show specificity, which provides a basis for further basic biological research. Multiple studies have explored the relationship between GPX7 and specific tumors.14,31,32 Our study revealed that the expression of GPX7 was elevated in multiple tumor tissues, including LGG, GBM, BRCA, ESCA, LIHC. Yao et al reported that the expression of GPX7 was higher in glioma tissue compared with normal brain tissue.32 Previous studies have shown that GPX7 has potential tumor suppressor effects in gastric cancer and esophageal adenocarcinoma.12,14,33 Down-regulation and dysfunction of GPX7 expression in esophageal cells increase ROS level and oxidative DNA damage.33 In hepatocellular carcinoma GPX7 expression was also elevated. Moreover, GPX7 expression in stage III hepatocellular carcinoma tissues were significantly higher than that in stage I–II hepatocellular carcinoma tissues.13 These findings were consistent with ours, which revealed that GPX7 function is active in these tumors. In addition, we confirmed that GPX7 expression was correlated with tumor stage. As the disease progressed, mRNA and protein expression level of GPX7 exhibited an upward trend.
Elevated expression levels of GPX7 were also associated with poor prognosis in certain tumors, such as LGG, SARC and STAD. It has also been reported the expression of GPX7 in glioma tissues was higher than those in normal brain tissues. Elevated expression of GPX7 led to poor prognosis of glioma patients.32 These findings are consistent with ours. In GBM and LGG tissues, we found that expression of GPX7 was significantly increased using TCGA and CGGA databases. Univariate and multivariate Cox regression analysis showed that GPX7 were significantly correlated with OS in LGG patients. In addition, in order to verify the above findings, we tried to use another independent analysis method to further verify. Nomogram is a powerful tool that has been widely used to predict an individual’s prognosis.34,35 Therefore, relevant prognostic factors were used to establish a nomogram to verify the correctness of the above results. Calibration plots revealed a good agreement between the nomogram prediction and actual observations in terms of the 1,2,3 and 5-year survival rates. These findings suggested that GPX7 played a vital role in the progression of LGG. GPX7 is identified as a novel prognostic indicator for LGG.
Exploring the relationship between GPX7 and immune infiltration is of great significance for further basic biological research. Previous studies have found that GPX-related genes play a regulatory role in the immune process.36 The GPX7 expression level and redox homeostasis of GPX7 are closely related to the body’s autoimmunity.11 ROS regulates the activity of immune cells. Once redox is unbalanced, it can easily lead to excessive activation of immune cells.37,38 In this study, GPX7 was positively correlated with macrophage, endothelial cell and B cell infiltrations in digestive tract tumors, including COAD, PAAD, rectum adenocarcinoma (READ) and STAD. Therefore, we speculate that the overexpression of GPX7 is closely related to the over-oxidation of digestive system tumors. Studies have shown that ROS levels are higher in digestive system tumors, such as colorectal cancer,39 pancreatic cancer40 and gastric cancer.41 A certain threshold of GPX7 in tumor tissues has a strong anti-inflammatory antioxidant role, which may be in the form of increased compensatory participate in tumor immune process. GPX7 protects cells from ROS production, oxidative stress and oxidative DNA damage. When this threshold is exceeded, it may be involved in tumor development with other tumor cells. On the other hand, the role of GPX7 in human tumors is dependent on tumor types and stages, GPX7 may be hijacked by immune cells during tumor progression. In addition, it has been reported that the immune system has a dual role, it antagonizes and promotes tumor development and progression,42 which also supports our hypothesis. CAFs were reported with fibroblast heterogeneity and plasticity in tumor microenvironment, and have been shown to play multiple roles in tumor development of a tumor. CAFs may also inhibit tumor progression in some circumstances.43,44 In the present study, we found that GPX7 was positively correlated with CAFs in 18 tumor types, including LGG, COAD, ESCA, HNSC, PAAD. These findings on GPX7-related immune infiltration are of great value for further basic biological research.
TMB is an emerging biomarker that predicts prognosis and the response of cancer patients to immunotherapy. It has been proven effective for some tumors, such as lung cancer,45 melanoma.46 Studies have shown that high TMB may promote cancer-testicular antigen expression and inflammation. Patients with high TMB can get a more favorable prognosis after receiving immunotherapy.47,48 In LGG, TMB is negatively correlated with OS, high TMB may inhibit the immune infiltration of LGG.49 TMB is an independent prognostic factor for glioma.50 These research findings were in agreement with the results of this study. Immune checkpoint inhibitor therapy is increasingly being considered as a potential treatment for gliomas. Currently, most immune checkpoint inhibitors are in the Phase I, II and III clinical trial stage in the field of neurosurgery, and the research objects are mostly focused on glioblastoma patients.51,52 The ideal therapeutic effect of immune checkpoint inhibitors may be more beneficial and more effective for patients with high TMB. This study revealed that the expression of GPX7 was positively correlated with TMB in LGG. We believe that immune checkpoint inhibitor was expected to be effective on LGG with high expresser of GPX7.
Although great efforts have been made in neurosurgery, the prognosis of LGG patients is still very poor. This study explored the effect of GPX7 combined with chemoradiotherapy on the prognosis of LGG, and revealed that RT may not be a recommended treatment for LGG patients with high GPX7 expression. And this study reported that high-expressed GPX7 could predict the sensitivity of TMZ in LGG patients. The above evidence suggests that chemotherapy may be an effective and beneficial treatment for LGG patients with high GPX7 expression. These findings will be further evaluated and optimized in clinical practice in order to improve the survival prognosis of more LGG patients.
The mechanism of GPX7 involved in the occurrence and development of glioma is quite complex. Elevated GPX7 increased the level of glutathione and inhibited lipid peroxidation. A recent study suggested that GPX7 may be relevant to ferroptosis in glioma. GPX7 silencing enhances ferroptosis related oxidative stress in glioma cells. Furthermore, it reported that GPX7 was a direct target of the miR-29 family. GPX7 restoration can reverse miR-29b mediated enhancement of ferroptosis related oxidative stress.53 In addition, a bioinformatics study revealed that elevated GPX7 is involved in glioma progression through several enriched pathways, including cell cycle pathway, ECM pathway, focal adhesion pathway and toll-like receptor pathway.32 These findings provided key clues to further study the basic biology of GPX7 in glioma.
This study has some limitations: First, all parameters and information were obtained from databases, which are limited and incomplete. Second, analytical data on GPX7 expression were based on mRNA levels, and conclusions of this study were derived from bioinformatics analysis, which lacks experimental data support. Therefore, more studies are needed to verify our results and to investigate the biological functions of GPX7, which will make our conclusions to be reliable and generalizable.
Conclusion
In summary, we elucidate on the expression of GPX7 across human tumors. GPX7 is upregulated in multiple tumors. Overexpression of GPX7 is closely related to poor prognosis in LGG patients. Therefore, GPX7 is a potential prognostic biomarker for LGG. High-expressed GPX7 can predict the sensitivity of TMZ in LGG patients. Chemotherapy is a potentially recommended treatment option for LGG patients with high GPX7 expression. Therefore, this study provided a basis for further evaluation of the role of GPX7 in clinical tumors.
Abbreviations
GPX7, Glutathione peroxidase-7; TCGA, The Cancer Genome Atlas; GTEx, Genotype-Tissue Expression; GDC, Genomic Data Commons; GEPIA, Gene Expression Profiling Interactive Analysis; HPA, Human Protein Atlas; CGGA, Chinese Glioma Genome Atlas; ROS, Reactive Oxygen Species; OS, overall survival; DFS, disease-free survival; PFS, progression-free survival; DSS, disease specific survival; HR, hazard ratio; 95% CI, 95% confidence intervals; ACC, adrenocortical carcinoma; BLCA, bladder urothelial Carcinoma; BRCA, breast invasive carcinoma; CESC, cervical squamous cell carcinoma and endocervical adenocarcinoma; CHOL, cholangiocarcinoma; COAD, colon adenocarcinoma; DLBC, lymphoid neoplasm diffuse large B-cell lymphoma; ESCA, esophageal carcinoma; GBM, glioblastoma multiforme; HNSC, head and neck squamous cell carcinoma; KICH, kidney chromophobe; KIRC, kidney renal clear cell carcinoma; KIRP, kidney renal papillary cell carcinoma; LGG, brain lower grade glioma; LIHC, liver hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; MESO, mesothelioma; OV, ovarian serous cystadenocarcinoma; PAAD, pancreatic adenocarcinoma; PCPG, pheochromocytoma and paraganglioma; PRAD, prostate adenocarcinoma; READ, rectum adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous melanoma; STAD, stomach adenocarcinoma; TGCT, testicular germ cell tumors; THCA, thyroid carcinoma; THYM, thymoma; UCEC, uterine corpus endometrial carcinoma; UCS, uterine carcinosarcoma; UVM, uveal melanoma; TMB, tumor mutational burden; MSI, microsatellite instability; EPIC, Estimating the Proportions of Immune and Cancer cells; CAFs, cancer-associated fibroblasts; TMZ, temozolomide; RT, radiotherapy; IC50, half-maximal inhibitory concentration.
Data Sharing Statement
The raw data analyzed during the current study are available in the TCGA database (http://cancergenome.nih.gov) and the GTEx database (https://gtexportal.org/home).
Ethics Approval and Informed Consent
All data in this study were obtained from public databases, and this study was approved by the Medical Ethics Committee of Xi’an Central Hospital.
Consent for Publication
All authors gave final approval to submit the manuscript for publication.
Acknowledgments
We thank those who participated in the preparation and maintenance of the databases used in this study, it is these databases that made our analysis possible. We thank the School of Nursing, Shaanxi University of Chinese Medicine, and Ankang R & D Center of Se-enriched Products for assistance.
Author Contributions
Qianqian Zhao and Luyu Zhang: the writing of the original manuscript; Yingying Wang, Tianpei Wang, Ye Sun and Jingjing Cao: data collection; Meng Qi, Xiaoping Du and Zengrun Xia: software and data analysis; Rongqaing Zhang and Yin Yang: revision of the original manuscript. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Special R&D Program Project of Chinese Academy of Se-enriched Industry (2020FXZX05-01), Key Research and Development Program of Shaanxi Province (2020SF-076), Research Project from Health Commission of Shaanxi Provincial Government (2018A017), Education Department of Shaanxi Provincial Government (19JS015) and Subject Invovation Team of Shaanxi University of Chinese Medicine(132041933).
Disclosure
The authors report no conflicts of interest in this work.
References
1. International Agency for Research on Cancer [homepage on the Internet]. Latest global cancer data: cancer burden rises to 19.3 million new cases and 10.0 million cancer deaths in 2020; 2020. Available from: https://www.iarc.who.int/news-events/latestglobal-cancer-data-cancer-burden-rises-to-19-3-million-new-casesand-10-0-million-cancer-deaths-in-2020/.
2. Meng X, Zhao Y, Han B, et al. Dual functionalized brain-targeting nanoinhibitors restrain temozolomide-resistant glioma via attenuating EGFR and MET signaling pathways. Nat Commun. 2020;11(1):594. doi:10.1038/s41467-019-14036-x
3. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–1251. doi:10.1093/neuonc/noab106
4. Claus EB, Walsh KM, Wiencke JK, et al. Survival and low-grade glioma: the emergence of genetic information. Neurosurg Focus. 2015;38(1):E6. doi:10.3171/2014.10.FOCUS12367
5. Toppo S, Vanin S, Bosello V, Tosatto SC. Evolutionary and structural insights into the multifaceted glutathione peroxidase (Gpx) superfamily. Antioxid Redox Signal. 2008;10(9):1501–1514. doi:10.1089/ars.2008.2057
6. Brigelius-Flohe R, Maiorino M. Glutathione peroxidases. Biochim Biophys Acta. 2013;1830(5):3289–3303. doi:10.1016/j.bbagen.2012.11.020
7. Utomo A, Jiang X, Furuta S, et al. Identification of a novel putative non-selenocysteine containing phospholipid hydroperoxide glutathione peroxidase (NPGPx) essential for alleviating oxidative stress generated from polyunsaturated fatty acids in breast cancer cells. J Biol Chem. 2004;279(42):43522–43529. doi:10.1074/jbc.M407141200
8. Nguyen VD, Saaranen MJ, Karala AR, et al. Two endoplasmic reticulum PDI peroxidases increase the efficiency of the use of peroxide during disulfide bond formation. J Mol Biol. 2011;406(3):503–515. doi:10.1016/j.jmb.2010.12.039
9. Chang YC, Yu YH, Shew JY, et al. Deficiency of NPGPx, an oxidative stress sensor, leads to obesity in mice and human. EMBO Mol Med. 2013;5(8):1165–1179. doi:10.1002/emmm.201302679
10. Bosello-Travain V, Conrad M, Cozza G, et al. Protein disulfide isomerase and glutathione are alternative substrates in the one Cys catalytic cycle of glutathione peroxidase 7. Biochim Biophys Acta. 2013;1830(6):3846–3857. doi:10.1016/j.bbagen.2013.02.017
11. Chen YI, Wei PC, Hsu JL, Su FY, Lee WH. NPGPx (GPx7): a novel oxidative stress sensor/transmitter with multiple roles in redox homeostasis. Am J Transl Res. 2016;8(4):1626–1640.
12. Peppelenbosch MP, Spaander MC, Bruno MJ. Glutathione peroxidase 7 prevents cancer in the oesophagus. Gut. 2014;63(4):537–538. doi:10.1136/gutjnl-2013-304906
13. Guerriero E, Capone F, Accardo M, et al. GPX4 and GPX7 over-expression in human hepatocellular carcinoma tissues. Eur J Histochem. 2015;59(4):2540. doi:10.4081/ejh.2015.2540
14. Chen Z, Hu T, Zhu S, Mukaisho K, El-Rifai W, Peng DF. Glutathione peroxidase 7 suppresses cancer cell growth and is hypermethylated in gastric cancer. Oncotarget. 2017;8(33):54345–54356. doi:10.18632/oncotarget.17527
15. Clough E, Barrett T. The gene expression omnibus database. Methods Mol Biol. 2016;1418:93–110. doi:10.1007/978-1-4939-3578-9_5
16. Digre A, Lindskog C. The human protein atlas-spatial localization of the human proteome in health and disease. Protein Sci. 2021;30(1):218–233. doi:10.1002/pro.3987
17. Tomczak K, Czerwinska P, Wiznerowicz M. The Cancer Genome Atlas (TCGA): an immeasurable source of knowledge. Contemp Oncol. 2015;19(1A):A68–77. doi:10.5114/wo.2014.47136
18. Carithers LJ, Moore HM. The Genotype-Tissue Expression (GTEx) project. Biopreserv Biobank. 2015;13(5):307–308. doi:10.1089/bio.2015.29031.hmm
19. Chandrashekar DS, Bashel B, Balasubramanya SAH, et al. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19(8):649–658. doi:10.1016/j.neo.2017.05.002
20. Tang Z, Kang B, Li C, Chen T, Zhang Z. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019;47(W1):W556–W560. doi:10.1093/nar/gkz430
21. Zhao Z, Zhang KN, Wang Q, et al. Chinese Glioma Genome Atlas (CGGA): a comprehensive resource with functional genomic data from Chinese glioma patients. Genomics Proteomics Bioinformatics. 2021;19(1):1–12. doi:10.1016/j.gpb.2020.10.005
22. Sturm G, Finotello F, List M. Immunedeconv: an R package for unified access to computational methods for estimating immune cell fractions from bulk RNA-sequencing data. Methods Mol Biol. 2020;2120:223–232. doi:10.1007/978-1-0716-0327-7_16
23. Racle J, Gfeller D. EPIC: a tool to estimate the proportions of different cell types from bulk gene expression data. Methods Mol Biol. 2020;2120:233–248. doi:10.1007/978-1-0716-0327-7_17
24. Li T, Fan J, Wang B, et al. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017;77(21):e108–e110. doi:10.1158/0008-5472.CAN-17-0307
25. Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9(1):34. doi:10.1186/s13073-017-0424-2
26. Yang G, Zheng RY, Jin ZS. Correlations between microsatellite instability and the biological behaviour of tumours. J Cancer Res Clin Oncol. 2019;145(12):2891–2899. doi:10.1007/s00432-019-03053-4
27. Jensen MA, Ferretti V, Grossman RL, Staudt LM. The NCI Genomic Data Commons as an engine for precision medicine. Blood. 2017;130(4):453–459. doi:10.1182/blood-2017-03-735654
28. Yang W, Soares J, Greninger P, et al. Genomics of Drug Sensitivity in Cancer (GDSC): a resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Res. 2013;41(Databaseissue):D955–61. doi:10.1093/nar/gks1111
29. Brigelius-Flohe R, Kipp A. Glutathione peroxidases in different stages of carcinogenesis. Biochim Biophys Acta. 2009;1790(11):1555–1568. doi:10.1016/j.bbagen.2009.03.006
30. Wei PC, Hsieh YH, Su MI, et al. Loss of the oxidative stress sensor NPGPx compromises GRP78 chaperone activity and induces systemic disease. Mol Cell. 2012;48(5):747–759. doi:10.1016/j.molcel.2012.10.007
31. Peng D, Belkhiri A, Hu T, et al. Glutathione peroxidase 7 protects against oxidative DNA damage in oesophageal cells. Gut. 2012;61(9):1250–1260. doi:10.1136/gutjnl-2011-301078
32. Yao J, Chen X, Liu Z, et al. The increasing expression of GPX7 related to the malignant clinical features leading to poor prognosis of glioma patients. Chin Neurosurg J. 2021;7(1):21. doi:10.1186/s41016-021-00235-3
33. Peng DF, Hu TL, Soutto M, Belkhiri A, El-Rifai W. Glutathione peroxidase 7 suppresses bile salt-induced expression of pro-inflammatory cytokines in Barrett’s carcinogenesis. J Cancer. 2014;5(7):510–517. doi:10.7150/jca.9215
34. Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol. 2015;33(8):861–869. doi:10.1200/JCO.2014.56.6661
35. Han DS, Suh YS, Kong SH, et al. Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer. J Clin Oncol. 2012;30(31):3834–3840. doi:10.1200/JCO.2012.41.8343
36. Huang Z, Rose AH, Hoffmann PR. The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2012;16(7):705–743. doi:10.1089/ars.2011.4145
37. Hultqvist M, Olsson LM, Gelderman KA, Holmdahl R. The protective role of ROS in autoimmune disease. Trends Immunol. 2009;30(5):201–208. doi:10.1016/j.it.2009.03.004
38. Ortona E, Maselli A, Delunardo F, Colasanti T, Giovannetti A, Pierdominici M. Relationship between redox status and cell fate in immunity and autoimmunity. Antioxid Redox Signal. 2014;21(1):103–122. doi:10.1089/ars.2013.5752
39. Lin S, Li Y, Zamyatnin AA
40. Durand N, Storz P. Targeting reactive oxygen species in development and progression of pancreatic cancer. Expert Rev Anticancer Ther. 2017;17(1):19–31. doi:10.1080/14737140.2017.1261017
41. Huang T, Zhou F, Yuan X, et al. Reactive oxygen species are involved in the development of gastric cancer and gastric cancer-related depression through ABL1-mediated inflammation signaling pathway. Oxid Med Cell Longev. 2019;2019:5813985. doi:10.1155/2019/5813985
42. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
43. Santi A, Kugeratski FG, Zanivan S. Cancer associated fibroblasts: the architects of stroma remodeling. Proteomics. 2018;18(5–6):e1700167. doi:10.1002/pmic.201700167
44. Biffi G, Tuveson DA. Diversity and biology of cancer-associated fibroblasts. Physiol Rev. 2021;101(1):147–176. doi:10.1152/physrev.00048.2019
45. Nan Z, Guoqing W, Xiaoxu Y, et al. The predictive efficacy of Tumor Mutation Burden (TMB) on nonsmall cell lung cancer treated by immune checkpoint inhibitors: a systematic review and meta-analysis. Biomed Res Int. 2021;2021:1780860. doi:10.1155/2021/1780860
46. Lin J, Lin Y, Huang Z, Li X. Identification of prognostic biomarkers of cutaneous melanoma based on analysis of tumor mutation burden. Comput Math Methods Med. 2020;2020:8836493. doi:10.1155/2020/8836493
47. Samstein RM, Lee CH, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51(2):202–206. doi:10.1038/s41588-018-0312-8
48. Wang X, Li M. Correlate tumor mutation burden with immune signatures in human cancers. BMC Immunol. 2019;20(1):4. doi:10.1186/s12865-018-0285-5
49. Yin W, Jiang X, Tan J, et al. Development and validation of a tumor mutation burden-related immune prognostic model for lower-grade glioma. Front Oncol. 2020;10:1409. doi:10.3389/fonc.2020.01409
50. Wang L, Ge J, Lan Y, et al. Tumor mutational burden is associated with poor outcomes in diffuse glioma. BMC Cancer. 2020;20(1):213. doi:10.1186/s12885-020-6658-1
51. Omuro A, Vlahovic G, Lim M, et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory Phase I cohorts of CheckMate 143. Neuro Oncol. 2018;20(5):674–686. doi:10.1093/neuonc/nox208
52. Reardon DA, Brandes AA, Omuro A, et al. Effect of Nivolumab vs Bevacizumab in patients with recurrent glioblastoma: the checkmate 143 Phase 3 randomized clinical trial. JAMA Oncol. 2020;6(7):1003–1010. doi:10.1001/jamaoncol.2020.1024
53. Zhou Y, Wu H, Wang F, et al. GPX7 is targeted by miR-29b and GPX7 knockdown enhances ferroptosis induced by erastin in glioma. Front Oncol. 2021;11:802124. doi:10.3389/fonc.2021.802124
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.