Back to Journals » Psychology Research and Behavior Management » Volume 16
A Behavioral and Event-Related Potentials Study of Food-Related Inhibitory Control in Probable Binge Eating Disorder
Authors Yan WS, Liu MM, Liu SJ
Received 25 September 2023
Accepted for publication 15 November 2023
Published 22 November 2023 Volume 2023:16 Pages 4737—4748
DOI https://doi.org/10.2147/PRBM.S441949
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Gabriela Topa
Wan-Sen Yan,1,2 Meng-Meng Liu,1 Su-Jiao Liu1
1Department of Psychology, School of Medical Humanitarians, Guizhou Medical University, Guiyang, People’s Republic of China; 2Guizhou Research Institute for Health Development, Guizhou Medical University, Guiyang, People’s Republic of China
Correspondence: Wan-Sen Yan, Department of Psychology, School of Medical Humanitarians, Guizhou Medical University, 9 Beijing Road, Yunyan District, Guiyang, 550004, People’s Republic of China, Tel +86-136-4850-4644, Email [email protected]
Background: Similar to addictive disorders, deficits on cognitive control might be involved in the onset and development of Binge Eating Disorder (BED). However, it remains unclear whether general or food-related inhibitory control impairments would be basically linked to overeating and binge eating behaviors. This study thus aimed to investigate behavioral performance and electrophysiological correlates of food-related inhibitory control among individuals with binge eating behavior.
Methods: Sixty individuals with probable BED (pBED) and 60 well-matched healthy controls (HCs) were assessed using the typical Stop-Signal Task, a revised Go/No Go Task, and a food-related Go/No Go Task. Besides, another separate sample, including 35 individuals with pBED and 35 HCs, completed the food-related Go/No Go Task when EEG signals were recorded with the event-related potentials (ERPs).
Results: The data revealed that the pBED group performed worse with a longer SSRT on the Stop-Signal Task compared with HCs (Cohen’s d = 0.58, p = 0.002). Moreover, on the food-related Go/No Go Task, the pBED group had a lower success rate of inhibition in no-go trials (Cohen’s d = 0.47, p = 0.012). The ERPs data showed that in comparison with HCs, the pBED group exhibited increased P300 latency (FC1, FC2, F3, F4, FZ) in the no-go trials of the food-related Go/No Go Task (Cohen’s d 0.56– 0.73, all p < 0.05).
Conclusion: These findings suggested that individuals with binge eating could be impaired in both non-specific and food-related inhibitory control aspects, and the impairments in food-related inhibitory control might be linked to P300 abnormalities, implying a behavioral-neurobiological dysfunction mechanism implicated in BED.
Keywords: binge eating disorder, inhibitory control, stop-signal, Go-No Go, ERPs
Introduction
Binge eating disorder (BED) has been recognized as a fully diagnostic category since the introduction of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). As a public health issue, BED is one of the most prevalent eating disorders, with a lifetime prevalence rate of 2.8% for women and 1.0% for men.1,2 The 12-month prevalence estimates of BED are 1.4% for women and 0.6% for men.1,3 Notably, youths and adolescents are at a high risk for BED, possibly due to their immature cognitive control abilities,4,5 with past-year prevalence estimates of 1.8–3.6% in girls, 0.2–1.2% in boys, and 1.5% in gender-diverse youths.6,7 BED is associated with significant psychiatric and physical complications, as well as an increased risk of obesity. It can also lead to impairments in quality of life, a substantial burden of disease, and excess mortality.8,9 Neurobiological dysfunctions in reward processing, emotion regulation, and inhibitory control are highlighted in individuals with BED, and these domains have been considered as potential targets for emerging treatment approaches.10–12 However, the pathogenesis of BED remains unclear, and ongoing efforts are necessary to gain a better understanding of its underlying causes and to improve prevention and treatment outcomes.13
Similar to addictive disorders, diminished control over behavior is a hallmark feature of BED, with the experience of losing control over eating behavior being an integral part of its core psychopathology.14 Impaired self-control, including impulsive and compulsive transdiagnostic features, may thus represent a key alteration characterizing BED.15 As an operational paradigm of self-control, inhibitory control or cognitive control reflects the ability of top-down control and recruits prefrontal networks to regulate behavior,16–18 and it has been widely studied in substance use disorders, behavioral addictions,19–21 as well as in BED.15,22–24
Based on cognitive tasks of inhibitory control such as the Stop-Signal Task and Go/No-Go Task, previous studies have provided substantial evidence linking poor inhibitory control to BED.22,25–28 However, recent systematic reviews have argued that the current literature on general inhibitory control in BED has yielded mixed findings and equivocal data (or a lack of available evidence), which seemingly does not support the notion that individuals with BED definitively and consistently exhibit impairments in inhibitory control compared to normal-weight healthy control groups.15,24,29,30 Heterogeneity in sample characteristics, task methodologies, and comorbidities may be important confounding factors that contribute to the limited power of conclusions.13,15,24 Nonetheless, inhibitory control abnormalities associated with BED continue to be a matter of concern, given that failed inhibitory control is indicated as a potential contributor to unhealthy food consumption and an early marker of disordered eating behaviors in adolescents,31,32 and inhibitory control may serve as a target for reducing overeating.10
Compared to general inhibitory control dysfunctions, impairments in food-related inhibitory control appear to be more likely involved in BED.24,29 Indeed, it has been suggested that a food-specific focus on inhibitory control and impulsivity might allude to processes closer to the core pathology of BED.33 One prior study compared behavioral performance on the Stop-Signal Task with both neutral and food stimuli between individuals with BED and overweight/obese individuals without BED. The findings indicated that individuals with BED exhibited greater difficulty in inhibiting responses triggered by food trials.34 Furthermore, food-related rash-spontaneous behaviors towards food were found to be increased in individuals with BED, as assessed by adapted inhibitory control tasks involving food pictures.35–37 In addition, incorporating food-specific inhibition training protocols has been suggested as a beneficial component in the treatment of BED.38,39 However, controversial evidence claimed that the inhibitory deficits associated with binge eating were not specific to food.40 By comparing overweight/obese subjects with and without BED on a Stop-Signal Task that contained food-specific stimuli, positive non-food stimuli, and neutral stimuli, this study exhibited poorer inhibitory control across different stimuli types in the BED group, but the deficits appeared not to be specific to food stimuli.40 Therefore, further replication research is still needed to clarify the inhibitory control related to food in BED.
In the current study, we aimed to further investigate the cognitive characteristics of general and food-related inhibitory control in a non-clinical sample of Chinese young adults at risk for BED, considering that the majority of studies in the literature have focused on clinical samples of BED and were primarily conducted in Western cultures.13 A large sample of 120 young adults, consisting of individuals with probable BED (pBED) and well-matched healthy controls, was assessed using the typical Stop-Signal Task, a revised Go/No Go Task, and a food-related Go/No Go Task. Furthermore, the electrophysiological correlates of food-related inhibitory control have been of great interest in the search for possible biological markers in BED.41–43 Therefore, we included an additional separate sample of 35 individuals with pBED and 35 healthy controls, who completed the food-related Go/No Go Task when electroencephalogram (EEG) signals were recorded with the event-related potentials (ERPs). It was hypothesized that the individuals with pBED would prominently exhibit impairments in food-related inhibitory control, which might be closely linked to abnormalities in certain neurobiological indicators (eg, N200 and P300).
Materials and Methods
Participants
The participants were recruited from a local university in Guiyang, China between September and November 2021. All of them were young adult students who attended three-year public psychology courses. Firstly, the students were invited to complete a short self-report screening questionnaire during a 45-min psychology class. This questionnaire consisted of demographic information (ie, age, gender, years of education, ethnicity, home locality, smoking and drinking behaviors) and a Chinese version of the Binge Eating Scale (BES),44,45 which has been properly used among Chinese college students in previous studies.46,47 A total of 862 students voluntarily responded to the screening questionnaire, 63 of whom were identified as individuals with pBED, with a total score ≥ 18 on the BES.45,48 All 63 students voluntarily participated in our study and were subsequently scheduled to complete a person-to-person interview in the laboratory. This interview included a brief checklist regarding physical diseases/conditions, current or past brain trauma, and history of psychoactive substance use/abuse, major psychiatric disorders, neurological diseases, and other mental disorders (see Supplementary Tables S1 for details). Afterwards, these students completed several cognitive tasks. Inclusion criteria included: (1) 18–25 years of age, and (2) willingness to participate in this study. Exclusion criteria included: (1) current or past severe psychiatric disorders (eg, schizophrenia, psychosis, bipolar disorder, major depressive disorder), neurological diseases, or other mental disorders; (2) a history of psychoactive substance use/abuse (eg, cocaine, heroin, ketamine, amphetamine); (3) current or past brain trauma; and (4) severe physical diseases or conditions that were inappropriate to complete the tasks, all of which were evaluated by self-reports. In total, three students were excluded based on one or more of these exclusion criteria. Finally, 60 individuals with pBED (aged 19.13 ± 0.89 years, from 18 to 23 years; 46 females, 76.7%) were included.
Sixty healthy control students (HCs) were recruited through advertisements and posters in the same university classes, matched with the pBED group on body mass index (BMI), age, gender, and educational level. The HCs were voluntarily enrolled in this study. They completed the self-report screening questionnaire, underwent the person-to-person interview, and performed the cognitive tasks in the laboratory. Inclusion criteria included: (1) 18–25 years of age, (2) willingness to participate in this study, and (3) a total score of 17 or less on the BES. Exclusion criteria included: (1) current or past severe psychiatric disorders, neurological diseases, or mental disorders; (2) current or past psychoactive substance abuse; (3) a history of brain trauma; and (4) severe physical diseases. The HCs were aged 19.37±1.18 years (18–23 years; 47 females, 78.3%).
In addition to the 60 individuals with pBED and 60 HCs who were only tested on cognitive tasks (ie, the Stop-Signal Task, Go/No Go Task, and food-related Go/No Go Task), another separate sample was recruited at the same university using a similar procedure, with the same inclusion and exclusion criteria. This sample included 35 students with pBED and 35 HCs, who were matched on BMI, age, gender, and educational level. They completed a food-related Go/No Go Task when EEG signals were recorded in the ERPs study. The two samples were comparable on demographics and task scores (Tables 1–3). All subjects were compensated with RMB ¥ 50.
 |
Table 1 Demographic Characteristics of the Groups in the Behavioral Study |
 |
Table 2 Inhibitory Control Performance of the Groups on the Cognitive Tasks (M±SD) |
 |
Table 3 Demographics and Task Scores of the Sample in the Event-Related Potentials (ERPs) Study |
Measures
Demographics
A brief self-report questionnaire was used to collect demographic data (eg, age, gender, years of education, ethnicity, and home locality). Standard procedures were used to measure weight and height, and then BMI was calculated by weight divided by the square of height (ie, kg/m2). Smoking and drinking behaviors were assessed using one single question each (ie, “Have you smoked at least one cigarette in the past 14 days” and “Did you take at least one drink in the past 14 days”, with 0 = No and 1 = Yes, respectively).
Binge Eating
The BES44,45 was designed to screen binge eating behavior. It is a 16-item self-report scale assessing behavioral, emotional, and cognitive symptoms of binge eating. Each item consists of four or three statements reflecting a range of severity (0 = no problem, 3 = severe problem), with a possible total score ranging from 0 to 46. A Chinese version of the BES46,47 was adopted. Higher total scores of the BES indicate more severe binge eating problems, with a total score ≧ 18 indicating pBED.45,48 The Cronbach’s α was 0.860 in the present study.
Stop-Signal Task (SST)
The SST49 was a typical measure of inhibitory control, which requires individuals to make rapid responses on Go-trials (ie, left button-press for a left-pointing arrow, right button-press for a right-pointing arrow), but to inhibit their responding on Stop-trials when an auditory stop signal (a 300-Hz tone) was presented. There were 5 blocks of 64 trials each. In the Stop-trials (25%), stop signals occurred after the Go stimulus with a variable delay (stop-signal delay, SSD). The SSD was initially 200 ms, and adjusted using a staircase procedure to identify a point at which the participants inhibited successfully on about 50% of the Stop-trials, thus allowing the estimation of the Stop-Signal Reaction Time (SSRT). After a successfully inhibited Stop-trial, the SSD increased by 50 ms (making the next Stop-trial more difficult), whereas the SSD decreased by 50 ms if the subject failed to stop (making the next Stop-trial easier). The SSRT estimates the time required for one subject to withhold responses, with a longer SSRT indicating poorer inhibitory control.
Go/No Go Task
A previously modified and validated Go/No Go Task50,51 was employed to investigate response inhibition. This task was essentially designed to separate cognitive processing of infrequent stimuli (stimulus-driven attention) from inhibitory processes by including three different types of colored circles: (a) frequent-go trials (frequent gray, n = 388, about 75%), (b) infrequent-go trials (rare yellow, n = 65, about 12.5%), and (c) no-go trials (rare blue, n = 65, about 12.5%). Contrast of infrequent-go trials versus no-go trials thus was expected to detect response inhibition (excluding stimulus-driven attention). In this task, a colored circle was presented on the black screen for 400 ms, with a 400-ms inter-stimulus interval, lasting for about 7 minutes. Participants were told to press a button as soon as possible in response to gray and yellow circles, but to refrain from responding to blue circles. The frequent-go, infrequent-go, and no-go trials were intermixed in a pseudo-random order. Prior to the formal experiments, participants practiced 30 filler trials (10 gray, 10 yellow, and 10 blue circles). This task was programmed using the E-prime Version 2.0. Response accuracy in three types of trials and reaction time (RT) in frequent-go and infrequent-go trials were recorded and analyzed.
Food-Related Go/No Go Task
A food version of the original Go/No Go Task50,51 was also adopted in this study. It was an analogue of the original task, but consisted of three different types of object squares/pictures instead of colored circles: (a) frequent-go trials (natural flowers, n = 388, about 75%), (b) infrequent-go trials (daily-use articles, such as teacups, n = 65, about 12.5%), and (c) no-go trials (delicious high-calorie foods, such as pizza, n = 65, about 12.5%). In this task, the object square was similarly presented on the black screen for 400 ms, with a 400-ms inter-stimulus interval. Participants have to quickly press a button in response to flowers and daily-use articles, but to withhold a response to the foods. These three types of trials were also intermixed in a pseudo-random order. This task was programmed using the E-prime Version 2.0. Response accuracy in all trials and RT in frequent-go and infrequent-go trials were analyzed.
EEG Recording and Preprocessing
ERPs Experimental Design
In the food-related Go/No Go Task, an object square/picture (ie, either natural flowers, daily-use articles, or high-calorie foods) was randomly presented on the central visual area of a black computer screen for 400 ms, followed by an inter-trial interval of 400 ms. Participants were instructed to press a button in response to flowers (left key) and daily-use articles (right key) as rapidly as possible, but to withhold a response to foods (no responses). This task consisted of 388 frequent-go trials (natural flowers, 75%), 65 infrequent-go trials (daily-use articles, 12.5%) and 65 no-go trials (high-calorie foods, 12.5%), lasting for about 7 minutes. All stimulus pictures were collected from the image database on the Internet and edited to be homogeneous with respect to color, brightness, contrast, viewing distance, and visual complexity. The task was programmed by the E-prime 2.0. Accuracy and RT were recorded and analyzed.
EEG Recording
EEG signals were recorded, using the EEG system (Brain Amp MR plus, Gilching, Germany), from a 32 electrode cap (Easy-Cap, Herrsching-Breitbrunn, Germany) positioned according to electrode montage of the international 10/20 system. All the leads were referenced to the linked earlobes. A ground electrode was placed on the forehead. The vertical electro-oculogram (VEOG) was recorded using an electrode placed approximately 1 cm below the left eye. The horizontal electro-oculogram (HEOG) was recorded using an electro-oculogram signal from one electrode placed approximately 1 cm from outer canthi of the left eye. The impedance was kept below 5 kΩ during EEG recording. Signals were recorded using a sampling rate of 500 Hz, with a band-pass from 0.1 to 70 Hz.
EEG Data Preprocessing
The EEG data were processed offline using the Brain Vision Analyzer software (Brain Products, Munich, Germany). Firstly, the VEOG and HEOG were deleted, and the data were then re-referenced to TP9 and TP10 (bilateral mastoid electrodes). The remaining EEG data were filtered with a band-pass of 0.01–40 Hz and were divided into epochs of 700 ms duration (−800 to 2000 ms). Baseline corrections were made based on the time interval of −800 to 0 ms. Bad-segment interpolation and rejection were conducted for each channel and each subject. The eye artefacts, head movement, muscle artefacts, frequency interference, and electrocardiographic activity were then removed from the data using an independent component analysis (ICA). All channels were subjected to additional correction, and trials with drifts larger than ±100 μV in any scalp electrode were rejected. The data were averaged for each trial condition (ie, frequent-go trials, infrequent-go trials, and no-go trials). Two subjects (one from pBED group, another from HCs) were excluded from analysis due to the number of usable no-go trials less than 32 (ie, 50% of no-go trials), resulting in a final sample of 68 subjects (n = 34 in each group). The peaks of N200 (150–300 ms) and P300 (250–450 ms) were automatically marked using the “peak finder” function of the Brain Analyzer. Since N200 and P300 were both located mainly at the fronto-central electrode sites, amplitude and latency of them were analyzed from these electrodes (ie, FC1, FC2, F3, F4, CZ, PZ, FZ, FCz), as suggested before.52
Statistical Analyses
Data analysis was conducted with the Statistical Package for the Social Sciences for Windows, Version 22.0 (SPSS Inc., Chicago, IL, USA). Chi-square tests were performed to test between-group differences on categorical variables (ie, gender, ethnicity, home locality, smoking and drinking status). T-tests were used to analyze group differences on age, years of education, BMI, and BES scores. Multivariate analysis of variance (mANOVA) models were used to compare task scores between the two groups. If mANOVA models indicated significant effects, additional pairwise comparisons on the task scores were performed using t-tests. Differences on the amplitude and latency of N200 and P300 were tested by a 2 (group: pBED, HCs) × 3 (trial type: frequent-go trials, infrequent-go trials, no-go trials) × 8 (electrode: FC1, FC2, F3, F4, CZ, PZ, FZ, FCz) design, with trial type and electrode as the within-subject factors and group as the between-subject factor. Simple contrasts were then conducted using t-tests on each electrode if group effects were significant. Statistical significance was set as p < 0.05, two-tailed.
Results
Demographic Characteristics
As seen in Table 1, no between-group differences were found on BMI, age, years of education, gender, ethnicity, home locality, smoking and drinking status (all p > 0.05).
Inhibitory Control Task Performance
On the Stop-Signal Task, the mANOVA models revealed significant group differences on the SSRT (F(1, 117) = 11.296, p = 0.001, ηp2 = 0.088), but not on the correct accuracy in Go-trials (F(1, 117) = 0.111, p = 0.740), correct accuracy in Stop-trials (F(1, 117) = 0.022, p = 0.883), or RT in Go-trials (F(1, 117) = 0.085, p = 0.772). Pairwise comparisons (Table 2) indicated that the pBED group performed worse with a longer SSRT than HCs (t = 3.168, p = 0.002, Cohen’s d = 0.58).
On the Go/No Go Task, the mANOVA models revealed no significant group differences on the correct accuracy in frequent-go trials (F(1, 117) = 0.037, p = 0.847), correct accuracy in rare-go trials (F(1, 117)=0.627, p=0.430), correct accuracy in no-go trials (F(1, 117) = 0.187, p = 0.666), RT in frequent-go trials (F(1, 117) = 0.156, p = 0.693), or RT in rare-go trials (F(1, 117) = 0.013, p = 0.910).
On the food-related Go/No Go Task, the mANOVA models revealed significant group differences on the correct accuracy in no-go trials (F(1, 117) = 6.947, p = 0.010, ηp2 = 0.056), but not on the correct accuracy in frequent-go trials (F(1, 117) = 0.120, p = 0.730), correct accuracy in rare-go trials (F(1, 117) = 0.071, p = 0.790), RT in frequent-go trials (F(1, 117) = 0.283, p = 0.595), or RT in rare-go trials (F(1, 117) = 0.234, p = 0.629). Pairwise comparisons (Table 2) indicated that pBED group had a lower correct accuracy in no-go trials than HCs (t = −2.555, p = 0.012, Cohen’s d = 0.47).
Event-Related Potentials (ERPs) Study Outcomes
As seen in Table 3, there were no between-group differences on BMI, age, gender, years of education, ethnicity, home locality, smoking and drinking status (all p > 0.05).
Behavioral Data
The mANOVA models revealed significant group differences on the correct accuracy in no-go trials (F(1, 67) = 5.895, p = 0.018, ηp2 = 0.081), but not on the correct accuracy in frequent-go trials (F(1, 67) = 0.106, p = 0.745), correct accuracy in rare-go trials (F(1, 67) = 0.002, p = 0.961), RT in frequent-go trials (F(1, 67) = 0.096, p = 0.758), or RT in rare-go trials (F(1, 67) = 0.768, p = 0.384). Pairwise comparisons (Table 3) showed that the pBED group had a lower correct accuracy in no-go trials than HCs (t = −2.581, p = 0.012, Cohen’s d = 0.62).
EEG Data
Regarding N200, the 2 (group: pBED, HCs) × 3 (trial type: frequent-go trials, infrequent-go trials, no-go trials) × 8 (electrode: FC1, FC2, F3, F4, CZ, PZ, FZ, FCz) between-within models revealed no significant main effects of the group, trial, and electrode on N200 amplitude or N200 latency (all p > 0.05). The interaction effects of trial × group, electrode × group, trial × electrode, and group × trial × electrode were also not significant (all p > 0.05). See more details of N200 amplitude and latency in Supplementary Tables S2 and S3, respectively. Regarding P300, the 2×3 × 8 between-within models revealed no significant main effects of group, trial, and electrode on P300 amplitude (all p > 0.05), without significant interaction effects of trial × group, electrode × group, trial × electrode, and group × trial × electrode (all p > 0.05). See more details of P300 amplitude in Supplementary Tables S4. Instead, the 2×3 × 8 between-within models revealed significant main effects of group on P300 latency (F(1, 65) = 15.048, p < 0.001, ηp2 = 0.188), although without main effects of trial and electrode or interaction effects of trial×group, electrode×group, trial×electrode, and group×trial×electrode (all p > 0.05). Simple contrasts at each electrode point (Table 4) revealed significant increases in P300 latency (FC1, FC2, F3, F4, FZ) during the no-go trials for the pBED group compared to HCs (Cohen’s d = 0.56–0.73, ps < 0.05). This suggests that impairments in food-related inhibitory control may be closely linked to increased P300 latency. We further tested the correlations between correct accuracy in no-go trials (ie, food-related inhibitory control) and P300 latency (FC1, FC2, F3, F4, FZ) using Pearson correlation. Data showed that there were significant negative correlations between P300 latency (FC1, FC2, F3, F4, FZ) and correct accuracy in no-go trials (r(68) = −0.257 to −0.309, ps < 0.05).
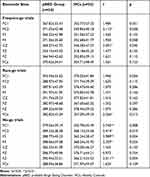 |
Table 4 Comparisons of P300 Latency (Ms) on the Food-Related Go/No Go Task (M±SD) |
Discussion
In this study, we investigated aspects of general and food-related inhibitory control in a non-clinical sample of young adult students with pBED, and linked food-related inhibitory control to potential electrophysiological correlates. Our data revealed that the individuals with pBED were compromised in both non-specific and food-related inhibitory control aspects, with a longer SSRT on the Stop-Signal Task and a lower successful inhibition rate on a food-related Go/No Go Task, compared with well-matched healthy controls. Additionally, impaired food-related inhibitory control in the pBED individuals was associated with biological abnormalities (increased P300 latency) in fronto-central sites (FC1, FC2, F3, F4, FZ). These findings implied a behavioral-neurobiological dysfunction mechanism of inhibitory control implicated in BED.
Inhibitory control is a crucial neurocognitive construct, underlying both impulsivity and compulsivity mechanisms in various psychiatric disorders such as addictive behaviors53,54 and eating disorders.55,56 With reference to BED, a loss of control over eating behavior has been included in the core psychopathology,13 and accordingly, deficient inhibitory control is believed to stand at the core of the pathogenesis of BED.15 However, despite a large number of related studies, the literature on inhibitory control in BED remains inconclusive. A meta-analysis (up to December 2019) identified global cognitive impairments among individuals with BED, including inhibitory control, cognitive flexibility, attention and planning.22 Conversely, other systematic reviews did not draw definitive impairments of inhibitory control in BED populations compared to normal-weight control groups.15,24,30 Insufficient data from small studies and heterogeneity in methodologies might account for the mixed conclusions.13,30 In our study, non-clinical samples of BED exhibited worse inhibitory control performance on the Stop-Signal Task compared to well-matched healthy controls, potentially indicating a significant deficit in non-specific inhibitory control for BED.22,23,40 In addition, dysfunctional inhibitory control is considered an early marker of eating problems in adolescents31 and one treatment target for BED.10 Therefore, our findings of impairments in inhibitory control in a non-clinical sample of individuals with BED should provide further firsthand evidence for the literature, and support the pivotal role of general aspects of inhibitory control implicated in the development of BED.13 Interestingly, patients with BED exhibited an impaired ability to estimate time, which positively correlated with compulsive self-monitoring and impulsivity,57 thus suggesting a possible link between impulsivity, compulsivity, and self-awareness in BED. Impaired inhibitory control has been considered a common neurocognitive mechanism underlying compulsivity and impulsivity,16 hence it might also play a role in self-awareness in BED, which should be studied in future.
Comparatively speaking, food-specific inhibitory control domains have been supposed to be consistently compromised in BED,24,34–37 in spite of the limited research. However, previous studies also indicated that overweight/obese individuals with BED had poorer inhibitory control on tasks across food-specific and non-food stimuli, suggesting that inhibitory deficits associated with binge eating were not food-specific.40 In our study, we utilized both a typical Go/No Go Task and an analogue with food stimuli (ie, the food-related Go/No Go Task), affording a direct comparison between food-related and non-food-related inhibitory control with the same task paradigm among the pBED group and HCs. Interestingly, our data revealed that individuals with pBED had a lower successful inhibition rate (ie, a lower correct accuracy in no-go trials) on the food-related Go/No Go Task with a moderate effect size (Cohen’s d = 0.47), whereas they had a normal performance equivalent to the HCs on the typical Go/No Go Task (Table 2). Similar findings were detected in the smaller separate sample (Table 3), indicating that individuals with pBED exhibited poorer inhibitory control performance on the food-related Go/No Go Task compared to HCs, with a large effect size (Cohen’s d = 0.62). These convergent data pointed to an explicit food-related inhibitory control deficit in BED (assessed with the Go/No Go paradigm), in addition to the impairments of general/non-specific inhibitory control (evaluated by the Stop-Signal Task). Our results thus provided more evidence for the proposals that emerging treatments targeting food-specific inhibition training might serve as effective novel approaches to treating BED.13,38,39 Specifically, in a recent randomized controlled trial, food-related inhibitory control assessed using the antisaccade paradigm was increased in BED patients who attended an impulsivity-focused group intervention, indicating that food-related inhibitory control/impulsivity could be effectively modified due to the training effects of certain precise treatments such as the IMPULS.58 In this respect, our findings may suggest a potential target for impulsivity-focused neurocognitive training programs in the treatment of BED, such as SSRT or correct accuracy in no-go trials. This is because both the Go/No Go and Stop-Signal tasks are typical paradigms of impulsivity (inhibitory control). Nevertheless, future randomized controlled trials are warranted.
In the present study, to link food-related inhibitory control with potential neurobiological markers in BED, we further investigated the electrophysiological correlates of dysfunctions in food-related inhibitory control in a separate sample of individuals with pBED and HCs. Previous neuroimaging studies suggested a generalized dysfunction in the fronto-striatal areas (eg, orbitofrontal cortex) during inhibitory control processes in BED.59,60 Besides, significant electrophysiological differences in the N200, P200, P300, and LPP components were found in participants with binge-purge eating disorders compared to healthy controls.43,61 Importantly, inhibitory control-related ERP components (ie, N200 and P300) increased during food-specific no-go trials in eating/weight disorders.42 In our study, the ERPs data showed that impaired food-related inhibitory control in the pBED group could be associated with a longer P300 latency at the fronto-central sites (FC1, FC2, F3, F4, FZ), suggesting increased recruitment (or a blocked functioning) of inhibitory control in response to food-specific stimuli in the BED subjects.62 Moreover, previous studies found that overweight individuals with BED did not show an increased N200 latency related to conflict processing on a food-related antisaccade task.36 Similarly, our pBED group did not display abnormalities in N200 components (ie, latency and amplitude) on the food-related No-Go trials or non-food Go trials compared to HCs, although we used a revised food-related Go/No Go Task. Nonetheless, future similar studies should overcome methodological shortcomings with consistent tasks. Our ERPs results, together with previous findings, indicated that P300, rather than N200 (at least in the current samples), might represent a potential neurophysiological marker for food-related inhibitory control in binge eating.41,63
There were several limitations that should be noted in the present study. First, this study was essentially a cross-sectional design, and thus could not draw a causal conclusion between the inhibitory control processes and BED. Future longitudinal or follow-up studies are needed. Second, our samples mainly consisted of non-clinical young adult college students who were not diagnosed with clinical criteria for BED. In particular, the students were those who have attended three-year public psychology courses. Such a population might be likely to possess an advanced awareness of their behaviors, emotional states, and cognitive symptoms, which could introduce certain subjective biases into the findings. As a result, our findings could not be generalized to clinical samples with serious binge-eating problems, and the results should be explained carefully. In addition, although we included two separate samples in this study, the sample size in the ERPs design was not extremely large and the electrophysiological findings might be limited partially by the sample characteristics. Finally, despite its temporal precision, the ERPs technology could not offer an exact spatial orientation related to the inhibitory control areas. Therefore, more intensive studies using neuroimaging methods are warranted to uncover underlying neural bases of general and food-related inhibitory control in BED, for a better understanding of its pathogenesis.
Conclusions
Despite these limitations, our study suggested that individuals with pBED might be compromised in both non-specific and food-related inhibitory control aspects. Furthermore, their impaired food-related inhibitory control was associated with an increased fronto-central P300 latency. Our study might contribute to a better understanding of the pathology of binge eating.
Data Sharing Statement
Data could be obtained by contacting the corresponding author.
Ethics Approval and Informed Consent
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Human Research Ethics Committee of the Guizhou Medical University (2020LS05). Informed consent was obtained from all subjects involved in the study.
Acknowledgments
The authors are thankful for all the participants in this study. We also thank Dr. Richard Tossell for his proofreading on our manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National Natural Science Foundation of China (Nos: 32060195 and 31560284) to Dr. Wan-Sen Yan.
Disclosure
The authors declare no conflict of interest.
References
1. Galmiche M, Déchelotte P, Lambert G, Tavolacci MP. Prevalence of eating disorders over the 2000-2018 period: a systematic literature review. Am J Clin Nutr. 2019;109(5):1402–1413. doi:10.1093/ajcn/nqy342
2. Treasure J, Hübel C, Himmerich H. The evolving epidemiology and differential etiopathogenesis of eating disorders: implications for prevention and treatment. World Psychiatry. 2022;21(1):147–148. doi:10.1002/wps.20935
3. Keski-Rahkonen A, Mustelin L. Epidemiology of eating disorders in Europe: prevalence, incidence, comorbidity, course, consequences, and risk factors. Curr Opin Psychiatry. 2016;29(6):340–345. doi:10.1097/YCO.0000000000000278
4. Hornberger LL, Lane MA, Committee on Adolescence. Identification and management of eating disorders in children and adolescents. Pediatrics. 2021;147(1):e2020040279. doi:10.1542/peds.2020-040279
5. Marzilli E, Cerniglia L, Cimino S. A narrative review of binge eating disorder in adolescence: prevalence, impact, and psychological treatment strategies. Adolesc Health Med Ther. 2018;9:17–30. doi:10.2147/AHMT.S148050
6. Erskine HE, Whiteford HA. Epidemiology of binge eating disorder. Curr Opin Psychiatry. 2018;31(6):462–470. doi:10.1097/YCO.0000000000000449
7. Olsen EM, Koch SV, Skovgaard AM, Strandberg-Larsen K. Self-reported symptoms of binge-eating disorder among adolescents in a community-based Danish cohort-A study of prevalence, correlates, and impact. Int J Eat Disorders. 2021;54(4):492–505. doi:10.1002/eat.23458
8. Udo T, Grilo CM. Psychiatric and medical correlates of DSM-5 eating disorders in a nationally representative sample of adults in the United States. Int J Eat Disorders. 2019;52(1):42–50. doi:10.1002/eat.23004
9. Santomauro DF, Melen S, Mitchison D, Vos T, Whiteford H, Ferrari AJ. The hidden burden of eating disorders: an extension of estimates from the Global Burden of Disease Study 2019. Lancet Psychiatry. 2021;8(4):320–328. doi:10.1016/S2215-0366(21)00040-7
10. Chami R, Cardi V, Lawrence N, et al. Targeting binge eating in bulimia nervosa and binge eating disorder using inhibitory control training and implementation intentions: a feasibility trial. Psychol Med. 2022;52(5):874–883. doi:10.1017/S0033291720002494
11. Mestre-Bach G, Potenza MN. Potential biological markers and treatment implications for Binge eating disorder and behavioral addictions. Nutrients. 2023;15(4):827. doi:10.3390/nu15040827
12. Preuss H, Pinnow M, Schnicker K, Legenbauer T. Improving inhibitory control abilities (ImpulsE)-A promising approach to treat impulsive eating?. Eur Eat Disord Rev. 2017;25(6):533–543. doi:10.1002/erv.2544
13. Giel KE, Bulik CM, Fernandez-Aranda F, et al. Binge eating disorder. Nat Rev Dis Primers. 2022;8(1):16. doi:10.1038/s41572-022-00344-y
14. Colles SL, Dixon JB, O’Brien PE. Loss of control is central to psychological disturbance associated with binge eating disorder. Obesity. 2008;16(3):608–614. doi:10.1038/oby.2007.99
15. Carr MM, Wiedemann AA, Macdonald-Gagnon G, Potenza MN. Impulsivity and compulsivity in binge eating disorder: a systematic review of behavioral studies. Prog Neuro Psychopharmacol Biol Psychiatry. 2021;110:110318. doi:10.1016/j.pnpbp.2021.110318
16. Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69(4):680–694. doi:10.1016/j.neuron.2011.01.020
17. Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335(6068):601–604. doi:10.1126/science.1214463
18. Friedman NP, Robbins TW. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology. 2022;47(1):72–89. doi:10.1038/s41386-021-01132-0
19. Antons S, Brand M, Potenza MN. Neurobiology of cue-reactivity, craving, and inhibitory control in non-substance addictive behaviors. J Neurol Sci. 2020;415:116952. doi:10.1016/j.jns.2020.116952
20. Le TM, Potvin S, Zhornitsky S, Li CR. Distinct patterns of prefrontal cortical disengagement during inhibitory control in addiction: a meta-analysis based on population characteristics. Neurosci Biobehav Rev. 2021;127:255–269. doi:10.1016/j.neubiorev.2021.04.028
21. von Deneen KM, Hussain H, Waheed J, Xinwen W, Yu D, Yuan K. Comparison of frontostriatal circuits in adolescent nicotine addiction and internet gaming disorder. J Behav Addict. 2022;11(1):26–39. doi:10.1556/2006.2021.00086
22. Iceta S, Rodrigue C, Legendre M, et al. Cognitive function in binge eating disorder and food addiction: a systematic review and three-level meta-analysis. Prog Neuro Psychopharmacol Biol Psychiatry. 2021;111:110400. doi:10.1016/j.pnpbp.2021.110400
23. Kessler RM, Hutson PH, Herman BK, Potenza MN. The neurobiological basis of binge-eating disorder. Neurosci Biobehav Rev. 2016;63:223–238. doi:10.1016/j.neubiorev.2016.01.013
24. Smith KE, Mason TB, Johnson JS, Lavender JM, Wonderlich SA. A systematic review of reviews of neurocognitive functioning in eating disorders: the state-of-the-literature and future directions. Int J Eat Disorders. 2018;51(8):798–821. doi:10.1002/eat.22929
25. Boeka AG, Lokken KL. Prefrontal systems involvement in binge eating. Eat Weight Disord. 2011;16(2):e121–e126. doi:10.1007/BF03325317
26. Claes L, Mitchell JE, Vandereycken W. Out of control? Inhibition processes in eating disorders from a personality and cognitive perspective. Int J Eat Disorders. 2012;45(3):407–414. doi:10.1002/eat.20966
27. Grant JE, Chamberlain SR. Neurocognitive findings in young adults with binge eating disorder. Int J Psychiatry Clin Pract. 2020;24(1):71–76. doi:10.1080/13651501.2019.1687724
28. Kober H, Boswell RG. Potential psychological & neural mechanisms in binge eating disorder: implications for treatment. Clin Psychol Rev. 2018;60:32–44. doi:10.1016/j.cpr.2017.12.004
29. Bartholdy S, Dalton B, O’Daly OG, Campbell IC, Schmidt U. A systematic review of the relationship between eating, weight and inhibitory control using the stop signal task. Neurosci Biobehav Rev. 2016;64:35–62. doi:10.1016/j.neubiorev.2016.02.010
30. Cury MEG, Berberian A, Scarpato BS, Kerr-Gaffney J, Santos FH, Claudino AM. Scrutinizing domains of executive function in Binge eating disorder: a systematic review and meta-analysis. Front Psychiatry. 2020;11:288. doi:10.3389/fpsyt.2020.00288
31. Bartholdy S, O’Daly OG, Campbell IC, et al., IMAGEN Consortium. Neural correlates of failed inhibitory control as an early marker of disordered eating in adolescents. Biol Psychiatry. 2019;85(11):956–965. doi:10.1016/j.biopsych.2019.01.027
32. McGreen J, Kemps E, Tiggemann M. The relationship between inhibitory control and food consumption or choice: a systematic review and meta-analysis. Appetite. 2023;183:106466. doi:10.1016/j.appet.2023.106466
33. Dawe S, Loxton NJ. The role of impulsivity in the development of substance use and eating disorders. Neurosci Biobehav Rev. 2004;28(3):343–351. doi:10.1016/j.neubiorev.2004.03.007
34. Svaldi J, Naumann E, Trentowska M, Schmitz F. General and food-specific inhibitory deficits in binge eating disorder. Int J Eat Disorders. 2014;47(5):534–542. doi:10.1002/eat.22260
35. Giel KE, Teufel M, Junne F, Zipfel S, Schag K. Food-related impulsivity in obesity and Binge eating disorder-a systematic update of the evidence. Nutrients. 2017;9(11):1170. doi:10.3390/nu9111170
36. Leehr EJ, Schag K, Dresler T, et al. Food specific inhibitory control under negative mood in binge-eating disorder: evidence from a multimethod approach. Int J Eat Disorders. 2018;51(2):112–123. doi:10.1002/eat.22818
37. Schag K, Schönleber J, Teufel M, Zipfel S, Giel KE. Food-related impulsivity in obesity and binge eating disorder--a systematic review. Obesity Rev. 2013;14(6):477–495. doi:10.1111/obr.12017
38. Giel KE, Speer E, Schag K, Leehr EJ, Zipfel S. Effects of a food-specific inhibition training in individuals with binge eating disorder-findings from a randomized controlled proof-of-concept study. Eat Weight Disord. 2017;22(2):345–351. doi:10.1007/s40519-017-0371-3
39. Keeler JL, Chami R, Cardi V, et al. App-based food-specific inhibitory control training as an adjunct to treatment as usual in binge-type eating disorders: a feasibility trial. Appetite. 2022;168:105788. doi:10.1016/j.appet.2021.105788
40. Manasse SM, Goldstein SP, Wyckoff E, et al. Slowing down and taking a second look: inhibitory deficits associated with binge eating are not food-specific. Appetite. 2016;96:555–559. doi:10.1016/j.appet.2015.10.025
41. Berchio C, Cambi S, Pappaianni E, Micali N. EEG biomarkers in children and adolescents with feeding and eating disorders: current evidence and future directions. Front Psychiatry. 2022;13:882358. doi:10.3389/fpsyt.2022.882358
42. Chami R, Cardi V, Lautarescu A, Mallorquí-Bagué N, McLoughlin G. Neural responses to food stimuli among individuals with eating and weight disorders: a systematic review of event-related potentials. Int Rev Psychiatry. 2019;31(4):318–331. doi:10.1080/09540261.2019.1622515
43. Hiluy JC, David IA, Daquer AFC, Duchesne M, Volchan E, Appolinario JC. A systematic review of electrophysiological findings in Binge-Purge eating disorders: a window into brain dynamics. Front Psychiatry. 2021;12:619780. doi:10.3389/fpsyg.2021.619780
44. Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addict Behav. 1982;7(1):47–55. doi:10.1016/0306-4603(82)90024-7
45. Greeno CG, Marcus MD, Wing RR. Diagnosis of binge eating disorder: discrepancies between a questionnaire and clinical interview. Int J Eat Disorders. 1995;17(2):153–160. doi:10.1002/1098-108x(199503)17:2<153::aid-eat2260170208>3.0.co;2-v
46. Yan WS, Zhang RR, Lan Y, Li ZM, Li YH. Questionnaire-based Maladaptive decision- coping patterns involved in binge eating among 1013 college students. Front Psychiatry. 2018;9:609. doi:10.3389/fpsyg.2018.00609
47. Yan WS, Zheng DH, Liu MM. Trait impulsivity and choice impulsivity in young adult students with probable binge eating disorder. Front Psychiatry. 2022;13:838700. doi:10.3389/fpsyt.2022.838700
48. Ricca V, Mannucci E, Moretti S, et al. Screening for binge eating disorder in obese outpatients. Compr Psychiatry. 2000;41(2):111–115. doi:10.1016/s0010-440x(00)90143-3
49. Logan GD, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psychol Sci. 1997;8(1):60–64. doi:10.1111/j.1467-9280.1997.tb00545.x
50. Chikazoe J, Jimura K, Asari T, et al. Functional dissociation in right inferior frontal cortex during performance of go/no-go task. Cereb Cortex. 2009;19(1):146–152. doi:10.1093/cercor/bhn065
51. Froeliger B, McConnell PA, Bell S, et al. Association between baseline corticothalamic-mediated inhibitory control and smoking relapse vulnerability. JAMA psychiatry. 2017;74(4):379–386. doi:10.1001/jamapsychiatry.2017.0017
52. Pires L, Leitão J, Guerrini C, Simões MR. Event-related brain potentials in the study of inhibition: cognitive control, source localization and age-related modulations. Neuropsychol Rev. 2014;24(4):461–490. doi:10.1007/s11065-014-9275-4
53. Lee RSC, Hoppenbrouwers S, Franken I. A systematic meta-review of impulsivity and compulsivity in addictive behaviors. Neuropsychol Rev. 2019;29(1):14–26. doi:10.1007/s11065-019-09402-x
54. Yücel M, Fontenelle LF, Chamberlain SR. Introduction to the special issue on the utility of transdiagnostic approaches for developing novel interventions for substance and behavioural addictions. Neuropsychol Rev. 2019;29(1):1–3. doi:10.1007/s11065-019-09403-w
55. Bartholdy S, Rennalls SJ, Jacques C, et al. Proactive and reactive inhibitory control in eating disorders. Psychiatry Res. 2017;255:432–440. doi:10.1016/j.psychres.2017.06.073
56. Wu M, Hartmann M, Skunde M, Herzog W, Friederich HC. Inhibitory control in bulimic-type eating disorders: a systematic review and meta-analysis. PLoS One. 2013;8(12):e83412. doi:10.1371/journal.pone.0083412
57. Meneguzzo P, Mancini C, Ormitti A, Bonello E, Todisco P. Time evaluation and its accuracy in eating disorders: differences in relation to interoceptive awareness. Eat Weight Disord. 2022;27(7):2551–2560. doi:10.1007/s40519-022-01394-7
58. Schag K, Leehr EJ, Meneguzzo P, Martus P, Zipfel S, Giel KE. Food-related impulsivity assessed by longitudinal laboratory tasks is reduced in patients with binge eating disorder in a randomized controlled trial. Sci Rep. 2021;11(1):8225. doi:10.1038/s41598-021-87231-w
59. Balodis IM, Grilo CM, Potenza MN. Neurobiological features of binge eating disorder. CNS Spectrums. 2015;20(6):557–565. doi:10.1017/S1092852915000814
60. Balodis IM, Molina ND, Kober H, et al. Divergent neural substrates of inhibitory control in binge eating disorder relative to other manifestations of obesity. Obesity. 2013;21(2):367–377. doi:10.1002/oby.20068
61. Werle D, Schroeder PA, Wolz I, Svaldi J. Incentive sensitization in binge behaviors: a mini review on electrophysiological evidence. Addict Behav Rep. 2021;13:100344. doi:10.1016/j.abrep.2021.100344
62. Carbine KA, Rodeback R, Modersitzki E, Miner M, LeCheminant JD, Larson MJ. The utility of event-related potentials (ERPs) in understanding food-related cognition: a systematic review and recommendations. Appetite. 2018;128:58–78. doi:10.1016/j.appet.2018.05.135
63. İnce B, Max SM, Plewnia C, et al. A pilot event-related potentials study on mechanisms underlying a tDCS-enhanced food-specific response inhibition task for patients with binge eating disorder. Front Psychiatry. 2021;12:721672. doi:10.3389/fpsyg.2021.721672
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
