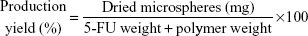Back to Journals » Drug Design, Development and Therapy » Volume 10
A 5-fluorouracil-loaded floating gastroretentive hollow microsphere: development, pharmacokinetic in rabbits, and biodistribution in tumor-bearing mice
Authors Huang Y, Wei Y, Yang H, Pi C, Liu H, Ye Y, Zhao L
Received 6 October 2015
Accepted for publication 25 November 2015
Published 3 March 2016 Volume 2016:10 Pages 997—1008
DOI https://doi.org/10.2147/DDDT.S97735
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Wei Duan
Yu Huang,1,* Yumeng Wei,1,* Hongru Yang,2 Chao Pi,1 Hao Liu,1 Yun Ye,1,3 Ling Zhao1
1Department of Pharmaceutical Sciences, College of Pharmacy, Sichuan Medical University, 2Department of Oncology, 3Department of Pharmacy, The First Affiliated Hospital of Sichuan Medical University, Luzhou, People’s Republic of China
*These authors contributed equally to this work
Abstract: 5-Fluorouracil (5-FU) was loaded in hollow microspheres to improve its oral bioavailability. 5-FU hollow microspheres were developed by a solvent diffusion–evaporation method. The effect of Span 80 concentration, ether/ethanol volume ratio, and polyvinyl pyrrolidone/ethyl cellulose weight ratio on physicochemical characteristics, floating, and in vitro release behaviors of 5-FU hollow microspheres was investigated and optimized. The formulation and technology composed of Span 80 (1.5%, w/v), ether/ethanol (1.0:10.0, v/v), and polyvinyl pyrrolidone/ethyl cellulose (1.0:10.0, w/w) were employed to develop three batch samples, which showed an excellent reproducibility. The microspheres were spherical with a hollow structure with high drug loading amount (28.4%±0.5%) and production yield (74.2%±0.6%); they exhibited excellent floating and sustained release characteristics in simulated gastric and intestinal fluid. Pharmacokinetic studies demonstrated that 5-FU hollow microspheres significantly enhanced oral bioavailability (area under curve, [AUC](0-t): 12.53±1.65 mg/L*h vs 7.80±0.83 and 5.82±0.83 mg/L*h) with longer elimination half-life (t1/2) (15.43±2.12 hours vs 2.25±0.22 and 1.43±0.18 hours) and mean residence time (7.65±0.97 hours vs 3.61±0.41 and 2.34±0.35 hours), in comparison with its solid microspheres and powder. In vivo distribution results from tumor-bearing nude mice demonstrated that the animals administered with 5-FU hollow microspheres had much higher drug content in tumor, plasma, and stomach at 1 and 8 hours except for 0.5 hours sample collection time point in comparison with those administered with 5-FU solid microspheres and its powder. These results suggested that the hollow microspheres would be a promising controlled drug delivery system for an oral chemotherapy agent like 5-FU.
Keywords: hollow microspheres, polyvinyl pyrrolidone, ethyl cellulose, pharmacokinetics, distribution, 5-fluorouracil
Introduction
5-Fluorouracil (5-FU) belongs to a family of antimetabolite antitumor drugs and interferes with nucleic acid and DNA synthesis, resulting in cancer cell death.1–3 It is one of the most widely used antitumor drugs for the treatment of several solid tumors such as colorectal, breast, liver, brain, and pancreas cancer, either alone or in combination with other drugs.4–9 Because of the poor absorption of 5-FU from gastrointestinal tract and greatly variable oral bioavailability, it was commonly used intravenously in clinic. Patients have to be continually administered high drug doses because of the short biological half-life (10–20 minutes) of 5-FU. At present, to obtain an ideal therapeutic effect in patients, 5-FU injection is frequently administered every day by intravenous bolus injection for 5 days and this treatment procedure is repeated every 28 days with three cycles.10 However, intravenous administration is commonly associated with hematological, neural, and cardiac severe systemic adverse effects. Therefore, oral delivery systems containing 5-FU, including gelatin/chitosan microspheres, poly (dl-lactic-co-glycolic acid) nanoparticles, alginate-chitosan/montmorillonite nanocomposite systems, and floating microspheres, have been developed in order to improve oral bioavailability with a continuous sustained release behavior.11–14
The oral sustained or controlled drug delivery system can release the drug slowly into the gastrointestinal tract and maintain a relative steady blood drug concentration for a longer time. However, such drug delivery systems have been affected by different gastric retention times resulting from gastric emptying.15 In general, an incomplete release and short residence time of the drug formulation in the upper gastrointestinal tract will result in lower oral bioavailability. Thus, it is particularly important to avoid these problems and prolong the gastric retention time of drug dosage forms, which contribute to retain the sustained or controlled drug delivery in stomach for a longer time and improve oral bioavailability of the drug which is preferentially absorbed in the upper site of the small intestine. During the past decades, many studies concerning the oral sustained or controlled release dosage forms possessing prolonged gastric emptying time have been done, such as bioadhesive systems, swelling type dosage forms, high density drug preparations, and floating dosage forms.16–24
In order to improve the gastric retention time of the drug, floating dosage forms that are less dense than the gastric fluid (1.004 g/cm3) have been extensively studied, including single-unit and multiunit dosage forms. The single-unit dosage forms, such as tablets, pills, and capsules, have a shortcoming in that drugs exhibit an “all-or-nothing” release in the emptying process.15,17 Hollow microspheres, as a multiunit floating drug delivery system, pass through the gastrointestinal tract, avoid gastric emptying, and then release the drug more uniformly. Furthermore, compared with single-unit dosage forms, the more uniform the distribution of these multiunit drug dosage forms is in the gastrointestinal tract, the more reproducible drug absorption and the less local irritation will be obtained.25–27
The objective of the study was to develop and evaluate multiple-unit hollow microspheres containing 5-FU in order to prolong the release of the drug and improve oral bioavailability and biodistribution in a tumor-bearing model animal. On the basis of our previous studies,25,26 solvent diffusion–evaporation method was used to prepare 5-FU hollow microspheres employing various ratios of polyvinyl pyrrolidone (PVP) and ethyl cellulose (EC) as drug controlled-release polymer blends. The morphology, particle size distribution, drug loading amount, floating properties, and in vitro drug release behavior of 5-FU loaded hollow microspheres were investigated. In addition, the pharmacokinetic behavior in rabbits and biodistribution in a tumor-bearing model animal of 5-FU hollow microspheres were compared with that of its nonfloating microspheres (solid microspheres) and its powder in rabbits to assess the usefulness of oral hollow microspheres drug delivery system.
Materials and methods
Cells and animals
MCF-7 cells were provided from the Department of Oncology, The First Affiliated Hospital of Sichuan Medical University (Luzhou, People’s Republic of China). The protocol for this study was approved by the ethics committee of Sichuan Medical University.
Albino rabbits (average weight of 1.35±0.10 kg) were obtained from the Laboratory Animal Center of Sichuan Medical University. Balb/c nude mice (5–6 weeks of age, ~17 g) were supplied by Jianyang Dashuo Biology Technology Co, Ltd. (Luzhou, People’s Republic of China). All animals used in this study were exposed to a 12-hour light/dark cycle and received food and water throughout the study. All the experimental procedures were approved (Approval number: 2013004) by the Sichuan Medical University Animal Ethical Experimentation Committee (Luzhou, People’s Republic of China).
Reagents
RPMI 1640 cell culture medium and fetal bovine serum were obtained from Luzhou Kelong Biology Technology Co. Ltd. (Luzhou, People’s Republic of China). 5-FU was purchased from Chongqing Laimei Pharmaceuticals Com., Ltd. (Chongqing, People’s Republic of China). PVP k-29/32 was a gift sample from ISP Technologies, Inc. (New Jersey, USA). EC (10 cp) was provided by Shanghai Colorcon Coating Technology Co. Limited (Shanghai, People’s Republic of China). Ethanol, ether, and other reagents (analytical grade) were purchased from Luzhou Juhe Chemical Regent Co. Ltd. (Luzhou, People’s Republic of China).
Preparation of hollow microspheres
Hollow microspheres containing 5-FU were prepared by a solvent diffusion–evaporation method described in our previous studies.26,28,29 Briefly, 5-FU powder and polymer blends of EC and PVP were firstly dissolved or dispersed in ethanol at room temperature by ultrasonication, followed by addition of ether. Under a constant stirring with a propeller type agitator at 300× g at 30°C in oil bath, the polymer–drug solution was introduced into liquid paraffin containing Span 80 (1.5%, w/v), forming an oil-in-oil (o/o) type emulsion. Then, the emulsion was constantly stirred until the evaporation of the solvent. After being filtered, washed with n-hexane, and dried overnight at 40°C, the hollow microsphere samples were stored in desiccators at room temperature.
Preparation of nonfloating microspheres (solid microspheres) was the same as the hollow microspheres and the formulation of organic solution was composed of 5-FU, EC, PVP, and ethanol.
Scanning electron microscopic observation
Scanning electron microscopy (Hitachi S-3000N, Hitachi Ltd., Tokyo, Japan) was used to confirm the characteristics of the hollow microspheres. Before scanning, hollow microspheres containing 5-FU were sputtered with gold to make the surface conductive. In addition, the microspheres were dissected with a knife to investigate the internal morphology.
Particle size distribution analysis
The particle size distribution of hollow microspheres containing 5-FU was measured by standard test sieves (Anping County Wire and Wire Mesh Factory, Hengshui, Hebei Province, People’s Republic of China). Particles that passed through one sieve but were retained on the other were collected and accurately weighed, then evaluated on the basis of the weight fraction on each sieve.
Drug loading amount and production yield determination
Drug loading amount in hollow microspheres containing 5-FU was performed by dissolving about 10 mg of hollow microsphere samples in 10 mL of ethanol at room temperature by ultrasonication. The drug concentration was determined using a UV-visible spectrophotometer (UV-2102; Shanghai Unico Instrument Co. Ltd, Shanghai, People’s Republic of China) at 265 nm.
The production yield of hollow microspheres containing 5-FU was calculated by dividing the weight of the dried microspheres by the weight of the drug and polymers. Drug loading amount and production yield of hollow microspheres were calculated as per Equations 1 and 2, respectively.
|
|
|
|
Buoyancy test
The buoyancy of 5-FU loaded microspheres was studied using the method described in previous literature.30 Briefly, 100 microspheres were dispersed in 900 mL of enzyme-free simulated gastric fluid (HCl/NaCl solution containing 0.02% Tween-80; pH 1.2) or enzyme-free simulated intestinal fluid (KH2PO4/NaOH solution containing 0.02% Tween-80; pH 7.4) at 37°C±0.5°C. The dispersing medium was stirred at 100× g with standard paddle method of Chinese Pharmacopoeia appendix Xc. No 2.31 Buoyancy capacity was evaluated based on the number of microspheres that remained buoyant on the test medium.
In vitro dissolution studies
The in vitro release of 5-FU loaded microspheres was investigated using rotating basket method of Chinese Pharmacopoeia appendix XC. No 1.31 An appropriate amount of microspheres that were equivalent to 10 mg of drug was placed in a rotating basket that had a smaller pore size than microspheres. Then the rotating basket containing 5-FU microspheres was soaked in 900 mL of enzyme-free simulated gastric fluid (HCl/NaCl solution containing 0.02% Tween-80; pH 1.2) or enzyme-free simulated intestinal fluid (KH2PO4/NaOH solution containing 0.02% Tween-80; pH 7.4) at 37°C±0.5°C as the release medium at a stirring speed of 100× g. At appropriate time intervals, a 5 mL sample solution was taken from medium and replaced with the same volume of blank release medium at the same temperature after each sampling to maintain an original volume. The sample solution was analyzed by UV-visible spectrophotometer (UV-2102) at 265 nm and calculated with a standard cure (A=0.055C+0.058 for simulated gastric fluid, A=0.058C−0.007 for simulated gastric fluid). According to drug quality standard of Chinese Pharmacopoeia for the validation of analytical methods,31 the selectivity, linearity, accuracy, precision, recovery, and stability of UV analytical methods were validated. As shown in Figure 1, no interference of the excipients including PVP, EC, and Tween-80 used in hollow microspheres was observed and the λmax value related to the absorption peak of 5-FU in the medium was 265 nm.
Pharmacokinetic study
The rabbits used in the present study were fasted overnight for 12 hours with free access to water. 5-FU hollow microspheres as test formulation and its solid microspheres and powder as controls in the sodium carboxymethy cellulose suspension (0.5%, w/w) form were respectively administered to six rabbits at a dose of 50 mg/kg body weight through the oral routes. An amount of 1.2 mL blood samples were collected from the marginal ear vein before administration and at 0.25, 0.5, 0.75, 1, 2, 3, 4, 6, 8, 10, 12, and 24 hours after oral administration. Each blood sample was centrifuged immediately at 5,000× g for 10 minutes and the supernatant plasma was collected and kept at −20°C for further analysis.
Frozen plasma sample was thawed at room temperature. Biosample was further carried out and analyzed by modification of our previous study.1 In brief, 0.5 mL of plasma sample was taken into a 5 mL centrifuge tube. And 100 μL ammonium acetate buffer (pH 3.5; 0.01 M) and 3 mL of isopropanol-ethyl acetate (15:85, v/v) were added. The mixture was vortexed for 3 minutes and centrifuged at 10,000× g for 10 minutes. The supernatant was decanted and evaporated to dryness at 50°C using N2 flow. The dry residues were reconstituted with 100 μL of mobile phase and centrifuged at 10,000× g for 10 minutes. Then, 20 μL sample of the clear supernatant was injected onto a high-performance liquid chromatography (HPLC) system.
The amount of 5-FU in rabbit plasma sample was determined by the HPLC system, which consisted of Dionex ultimate 3000 series including pump (LPG-3400SD), UV-vis detector (VWD-3100), auto injector (WPS-3000), and column oven (TCC-3000). Separation was performed on a reverse phase C18 column (Inertsil ODS-SP; 4.6×250 mm, 5 μm particle size, GL Sciences, Tokyo Japan) with a guard column (Phenomenex C18, 4.0 mm ×3.0 mm, 5 μm particle size, USA) at 25°C using a mobile phase of acetonitrile–ammonium acetate buffer (pH 3.5; 0.01 M) (1.0:99.0 v/v) at the flow rate of 0.8 mL/min. The sample was analyzed at a detection wavelength of 265 nm and the injection volume was 20 μL. According to the validation of bioanalytical methods of the US Food and Drug Administration guidelines, the method was validated in terms of selectivity, linearity, precision, accuracy, extraction efficiency, and stability in rabbit plasma. As shown in Figure 2, the HPLC assay resulted in good baseline resolution and rabbit plasma matrix components did not interfere with the analysis of 5-FU. The method was linear over the range of 0.020–20.0 μg/mL (r2=0.9986). The lower quantification limit was 0.020 μg/mL. The precision and accuracy for quality control plasma samples ranged from 4.57% to 8.87% and from 83.6% to 106.4%. Extraction efficiency at three concentrations was 91.5% to 96.4%. Therefore, the method was suitable for 5-FU quantification in rabbit plasma samples.
Biodistribution in tumor-bearing mice
Firstly, MCF-7 breast cancer cells were cultured in RPMI-1640 medium (GE Healthcare Life Science, Uppsala, Sweden) with 10% fetal bovine serum (GE Healthcare Life Science) at 37°C with 5% CO2. Then the aforementioned cancer cells (5×106) were subcutaneously inoculated in the right flanks of Balb/c nude mice to obtain a tumor-bearing animal model. When the tumor reached about 100 mm3 in size, the tumor-bearing mice were randomized into three groups (nine mice per group) and were fasted overnight for 12 hours with free access to water. Each group was orally administered with 5-FU hollow microspheres as test formulation and its solid microspheres and powder as controls in the sodium carboxymethyl cellulose suspension (0.5%, w/w) form at a dose of 100 mg/kg body weight. At 0.5, 1, and 8 hours after oral drug administration, three mice were sacrificed. Blood, tumor, heart, liver, spleen, lung, kidney, and brain samples were immediately collected. Blood was separated by centrifuging at 5,000× g for 5 minutes to obtain plasma sample. Tissues samples were washed with ice-cold 0.9% saline and dried with filter paper. Then these tissues were homogenized with 0.9% saline in appropriate concentration (tumor and liver 1:3; heart, spleen, lung, and brain 1:20; kidney 1:5, w/w).
Above 0.1 mL of plasma and tissue homogenates were mixed with 25 μL ammonium acetate buffer (pH 3.5; 0.01 M) and 1 mL of isopropanol-ethyl acetate (15:85, v/v). The mixtures were vortexed and extracted by ultrasonication, then centrifuged at 10,000× g for 10 minutes. After that, the organic layer was collected and evaporated to dryness under N2 flow at 50°C. The dry residues were redissolved with 100 μL of mobile phase. Then, 20 μL sample of the clear supernatant was injected into the above-mentioned HPLC system.
In order to investigate biodistribution in tumor-bearing mice after oral administration of 5-FU hollow microspheres as test formulation and its solid microspheres and powder as controls, the HPLC assay method was developed and validated through determination of specificity, linearity, quantification limit, precision, accuracy, recovery, and stability. The analysis method showed specificity since no significant interfering peaks at or near the retention time of 5-FU were detected from endogenous substances by comparing chromatograms of blank mice tissue homogenate of the tumor as a representative sample, blank mice tumor homogenate spiked with 5-FU, and mice tumor sample collected after oral administration of 5-FU hollow microspheres, respectively (Figure 3). The analysis method had proven to be simple, specific, sensitive, accurate, and precise and was suitable for 5-FU quantification in biosamples in tumor-bearing mice.
Data analysis
The plasma drug concentration over time data was used to calculate pharmacokinetic parameters including the area under the plasma drug concentration–time curve up to 24 hours postadministration, time to reach the maximum plasma drug concentration (Tmax), maximum plasma drug concentration (Cmax), t1/2, and MRT by DAS 2.0 pharmacokinetics software.
Student’s t-tests were used to evaluate the significant differences between the pharmacokinetic data of 5-FU hollow microspheres as test formulation and its powder as control. Values were reported as mean ± standard deviation and P<0.05 was considered the statistical significant difference.
Results and discussion
Effect of Span 80 concentration on particle size, drug loading amount, and production yield
In the present study, the solvent diffusion–evaporation method was employed to develop 5-FU hollow microspheres as a floating drug delivery system. From our previous studies,26,28,29 the process of o/o type emulsification is very important to form hollow microspheres. Thus, when the other conditions were equal, the effect of Span 80 concentration in the liquid paraffin on particle size, drug loading amount, and production yield of 5-FU hollow microspheres was investigated and the results are summarized in Table 1. It can be seen from particle size distribution that when Span 80 (as emulsifier) concentration in the liquid paraffin was increased from 0.5% (w/v) to 2.0% (w/v), the particle size of 5-FU hollow microspheres decreased, because of the decreasing interfacial tension between organic phase and liquid paraffin. In order to stabilize o/o emulsion, the main function of Span 80 is to form a thin film around the droplets to prevent their coalescence.11 In fact, when the concentration of Span 80 was 1.5%, a more uniform particle size distribution of 5-FU hollow microspheres was obtained. In other words, the percentage of a 5-FU hollow microsphere ranged from 450 to 600 μm in diameter (67.5%±1.6%). As shown in Table 1, drug loading amount and production yield increased with increasing concentration of the Span 80 up to 1.5%. However, when the concentration of the Span 80 was more than 1.5%, the drug loading amount and production yield decreased. Thus, 1.5% of Span 80 was chosen as an emulsifier for further study.
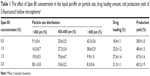  | Table 1 The effect of Span 80 concentration in the liquid paraffin on particle size, drug loading amount, and production yield of 5-fluorouracil hollow microspheresa |
Effect of ether/ethanol volume ratio on particle size, drug loading amount, and production yield
As shown in Table 2, the formation of 5-FU hollow microspheres was greatly affected by ether/ethanol volume ratio. When ethanol was employed alone as organic phase, no hollow microspheres were observed, except some nonfloating microspheres (solid microspheres). The reason was that ethanol cannot diffuse into the liquid paraffin: the polymer cannot aggregate between the continuation phase and dispersion phase.28,29 In comparison with ethanol alone, a mixture of ether and ethanol was used to obtain 5-FU hollow microspheres. Interestingly, after emulsification of about 5 minutes, the air bubble was observed in the microsphere droplets by using an optical microscope. This was because the ether can be quickly diffused into the liquid paraffin, resulting in polymer instantly solidifing into a thin film. When ether/ethanol volume ratio was increased from 0.5:10.0 to 1.0:10.0, drug loading amount and production yield increased, too. However, when the concentration of the ether/ethanol volume ratio was more than 1.0:10.0, the drug loading amount and production yield decreased. In addition, there was no difference in particle size distribution among three samples prepared by ether/ethanol volume ratio that ranged from 0.5:10.0 to 1.5:10.0 (P>0.05). Therefore, ether/ethanol (1.0:10.0, v/v) was used for further study.
Effect of PVP/EC weight ratio on particle size, drug loading amount, production yield, and floating and in vitro release behavior
On the basis of preliminary experiment, it was suggested that the drug release rate and cumulative release percentage of 5-FU hollow microspheres prepared by mixing polymer blends of hydrophilic PVP and hydrophobic EC were significantly improved.26 Therefore, the effect of PVP/EC weight ratio on particle size, drug loading amount, production yield, and floating and in vitro release behavior was investigated. As shown in Table 3, the average diameter and drug loading amount increased with the increased PVP/EC weight ratio from 0:10 to 1.0:10.0. The reason was that a higher viscosity of the internal phase with increasing PVP/EC weight ratio rendered a higher resistance to the shearing of emulsion, thereby increasing the particle size of 5-FU hollow microspheres.15 Another possibility is that the rapid polymer precipitation resulted in hardness avoiding further particle size reduction during solvent evaporation.26 Drug loading amount is usually associated with the particle size distribution of microspheres, and the larger the particle size, the higher the drug loading amount. In general, as the particle size of 5-FU hollow microspheres was increased, the thickness of the wall of microspheres increased. The result was in agreement with the results reported in literature.27 In addition, when the PVP/EC weight ratio was increased from 0:10.0 to 1.0:10.0, there was no significant difference in the production yield of 5-FU hollow microspheres (P>0.05). However, as the PVP/EC weight ratio was increased to 2.0:10.0, production yield decreased. A possible reason is that the viscosity of drug–polymer solution at PVP/EC weight ratio of 2.0:10.0 was too high to disperse in liquid paraffin containing Span 80 (1.5%, w/v), forming an o/o type emulsion.
As shown in Figure 4, 5-FU hollow microspheres in the case of PVP/EC weight ratio ranging from 0:10 to 1.0:10.0 possessed good buoyancy in gastric fluid and intestinal fluid containing Tween-80 that simulates the surface tension of gastrointestinal fluids.24 More specifically, the buoyancy rate of these hollow microspheres after 12 hours was more than 95%. Furthermore, no significant difference in the buoyancy rate among these formulations was observed (P>0.05). However, when 5-FU hollow microspheres were prepared using PVP/EC weight ratio of 2.0:10.0, the buoyancy rate decreased to 53.7%±2.9% and 51.5%±3.6% after 12 hours in simulated gastric fluid and intestinal fluid, respectively. The phenomenon could be explained in that the channels on the surface of 5-FU hollow microspheres, which resulted from PVP and 5-FU dissolution, became too large to be penetrated into the hollow cavities by the release medium with an increasing of PVP/EC weight ratio up to 2.0:10.0.
Drug release profiles from 5-FU hollow microspheres are presented in Figure 5. It was observed that drug release kinetics in simulated gastric fluid and intestinal fluid was significantly influenced by the PVP/EC weight ratio. Importantly, not only the slope but also the shape of the release curves was affected,26 which showed differences in the underlying drug release mechanisms. However, there was no difference in release behaviors in both the release mediums. When PVP/EC (0:10.0) was used to prepare 5-FU hollow microspheres, at the end of 24 hours the cumulative drug release amount in simulated gastric fluid and intestinal fluid was only 62.5%±2.0% and 60.2%±2.9%, respectively. When the PVP/EC weight ratio was increased to 1.0:10.0, it was observed that the hollow microspheres containing 5-FU slowly released the drug and at the end of 24 hours the cumulative drug release amount increased to 97.8%±3.4% and 96.9%±3.0%, respectively. In the case of PVP/EC weight ratio of 2.0:10.0, the hollow microspheres containing 5-FU released 100% of the drug within 8 hours. In order to prolong the release of 5-FU, the production yield and floating capability of 5-FU hollow microspheres with PVP/EC (2.0:10.0) were lower than those of the PVP/EC (1.0:10.0). Thus, PVP/EC (1.0:10.0) was chosen for further study.
Preparation and in vitro evaluation of 5-FU hollow microspheres
On the basis of the above experiments, when the amount of 5-FU remained constant, the formulation and technology composed of Span 80 (1.5%, w/v), ether/ethanol (1.0:10.0, v/v), and PVP/EC (1.0:10.0) were employed to develop three batches (20120401, 20120402, and 20120403) of 5-FU hollow microspheres. The particle size, morphology, drug loading amount, production yield, floating efficiency (12 hours), and in vitro release behavior of three batches samples were evaluated and the results are summarized in Table 4 and Figures 6 and 7. As shown in Table 4, there was no difference in particle size distribution, drug loading amount, production yield, and floating efficiency of 5-FU hollow microspheres. In vitro floating efficiency investigation indicated that, in spite of stirring the simulated gastric fluid for 12 hours, more than 95.0% 5-FU hollow microspheres still continued floating. As shown in Figure 4, 5-FU hollow microspheres were spherical with a cavity within a hard polymer shell and a lot of pores in internal walls of hollow microspheres, which resulted in lower density. It was suggested that 5-FU hollow microspheres possessed an excellent floating effect. As shown in Figure 7, dissolution profiles of in vitro release patterns indicated that similar drug release behaviors among the three batches of 5-FU hollow microspheres were observed. Therefore, the formulation and technology used to develop 5-FU hollow microspheres in this study have a good reproducibility.
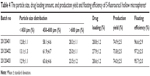  | Table 4 The particle size, drug loading amount, and production yield and floating efficiency of 5-fluorouracil hollow microspheresa |
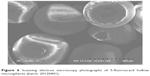  | Figure 6 Scanning electron microscopy photographs of 5-fluorouracil hollow microspheres (batch: 20120401). |
  | Figure 7 Percentage cumulative release of three batches of 5-fluorouracil hollow microspheres in simulated gastric fluid (A) and simulated intestinal fluid (B). |
Pharmacokinetic evaluation
In order to investigate further whether 5-FU hollow microspheres could enhance its oral bioavailability and improve pharmacokinetic behaviors, 5-FU hollow microspheres and its solid microspheres and powder as the control groups were orally administrated to rabbits at a dose of 50 mg/kg body weight. The mean plasma drug concentration–time curve is shown in Figure 8 and the relevant pharmacokinetic parameters calculated by DAS 2.0 software are summarized in Table 5. As shown in Figure 8 and Table 5, 5-FU hollow microspheres demonstrated the greatest absorption and the slowest elimination after gavage administration in comparison with its solid microspheres and powder. The Cmax and Tmax of 5-FU solid microspheres and powder were 2.25±0.22 mg/L and 1.00±0.15 hours, and 2.55±0.19 mg/L and 0.75±0.11 hours, respectively; the plasma drug concentration was detectable up to 12 and 8 hours, whereas the Cmax and Tmax of hollow microspheres were 1.64±0.21 mg/L and 2.00±0.22 hours, respectively. At 24 hours after gavage administration of 5-FU hollow microspheres, the drug concentration in plasma was still detectable at about 0.13±0.02 mg/L. These results suggested that 5-FU from hollow microspheres possessed slower drug release or absorption relatively.
The t1/2 and MRT of 5-FU hollow microspheres were 15.43±2.12 hours and 7.65±0.97 hours, respectively, which were longer than those of its solid microspheres at t1/2 of 2.25±0.22 hours and MRT of 3.61±0.41 hours, and powder at t1/2 of 1.43±0.18 hours and MRT of 2.34±0.35 hours, respectively. Furthermore, it was worth noting that 5-FU hollow microspheres exhibited higher area under curve (AUC)(0-t) than its powder (12.53±1.65 mg/L*h vs 7.80±0.83 mg/L*h and 5.82±0.83 mg/L*h, P<0.01). It was suggested that 5-FU hollow microspheres prolonged drug residence time in the gastrointestinal tract due to its good floating characteristic in gastric fluid, leading to a better and longer absorption effect. Li et al developed acyclovir-loaded spray-dried hollow microspheres and investigated its pharmacokinetic behavior in rabbits. The results showed it improved two to three folds in the oral bioavailability of acyclovir.32 Another study also indicated riboflavin-loaded microballoons as a floating controlled drug delivery system were very useful for improving oral drug bioavailability in healthy humans.33
Biodistribution evaluation in tumor-bearing mice
There are many studies related to oral dosage forms of 5-FU.11–14 However, there are very few studies on the biodistribution in a tumor-bearing animal model after oral administration. In the present study, drug biodistribution behaviors were carried out in tumor-bearing mice model to investigate the amount of 5-FU that is delivered to main organs and tumors. The content of 5-FU in tumor and main organs including heart, liver, spleen, lung, kidney, and stomach at 0.5, 1, and 8 hours was measured by the HPLC method and illustrated in Figure 9 after oral administration of 5-FU hollow microspheres as a test formulation and its solid microspheres and powder as controls in the sodium carboxymethyl cellulose suspension form at a dose of 72 mg/kg body weight. As shown in Figure 9, the elimination of 5-FU powder was the fastest among the three formulations in plasma, tumor, and main organs, while the drug concentration in biosamples at 8 hours after administration was not detectable. 5-FU hollow microspheres and its solid microspheres showed similar drug distribution trends in tumor-bearing mice. However, on the one hand, the level of plasma drug concentration was found to be gentler in the case of 5-FU hollow microspheres than that of its solid microspheres, which indicated that 5-FU hollow microspheres reduced rapid clearance from the circulation due to slower drug release rate. On the other, the animals administered with 5-FU hollow microspheres had a much higher drug content in tumor, plasma, and stomach at 1 and 8 hours except for 0.5 hours sample collection time point in comparison with those administered with 5-FU solid microspheres. This result indicated that 5-FU hollow microspheres had improved tissue distribution characteristics of 5-FU to some extent.
Conclusion
In the present study, the effect of Span 80 concentration, ether/ethanol volume ratio, and PVP/EC weight ratio on physicochemical characteristics and floating and in vitro release behaviors was investigated and optimized. As a multiple-unit floating drug delivery system, 5-FU hollow microspheres were developed successfully by a solvent diffusion–evaporation method. An excellent reproducibility of formulation and technology was obtained. The microspheres were spherical with hollow structures with high drug loading amount and production yield. 5-FU hollow microspheres exhibited excellent floating and sustained release characteristics. Pharmacokinetic studies demonstrated that 5-FU hollow microspheres showed significantly enhanced absorption and oral bioavailability with longer t1/2 and MRT, which contributed to prolonging drug residence time in the gastrointestinal tract due to its good floating characteristic in gastric fluid. In vivo distribution results from tumor-bearing nude mice indicated that 5-FU hollow microspheres could improve tissue distribution characteristics of 5-FU to some extent. It could be concluded that the hollow microspheres would be a promising sustained and controlled drug delivery system for an oral delivery chemotherapy agent like 5-FU.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (81101678 and 81341124), Science and Technology Support Project of Sichuan Province (2013SZZ006, 2014SZ0071 and 2014FZ0105), the Joint Fund of Sichuan Province, Luzhou City and Sichuan Medical University (14JC0134), the Key Program of the Scientific Research Foundation of the Education Department of Sichuan Province (12ZZ020; 12ZB066), the Scientific Research Foundation of the Administration of Traditional Chinese Medicine of Sichuan Province (2012-F-026), the Scientific Research Foundation of the Health Bureau of Sichuan Province (130270 and 130269), and the Key Program of the Scientific Research Foundation of Bureau of Science and Technology of Luzhou City (2013LZLY-K80, 2013-S-47 [17/20]).
Disclosure
The authors report no conflicts of interest in this work.
References
Pi C, Wei Y, Yang H, et al. Development of a HPLC method to determine 5-fluorouracil in plasma: application in pharmacokinetics and steady-state concentration monitoring. Int J Clin Pharmacol Ther. 2014;52(12):1093–1101. | ||
Muzzalupo R, Tavano L, Rossi CO, Picci N, Ranieri GA. Novel pH sensitive ferrogels as new approach in cancer treatment: effect of the magnetic field on swelling and drug delivery. Colloids Surf B Biointerfaces. 2015;134:273–278. | ||
Otsubo D, Yamashita K, Fujita M, et al. Early-phase treatment by low-dose 5-fluorouracil or primary tumor resection inhibits MDSC-mediated lung metastasis formation. Anticancer Res. 2015;35(8):4425–4431. | ||
Xu ZY, Tang JN, Xie HX, et al. 5-Fluorouracil chemotherapy of gastric cancer generates residual cells with properties of cancer stem cells. Int J Biol Sci. 2015;11(3):284–294. | ||
Zhang XQ, Zhang HM, Sun XE, Yuan ZJ, Feng YG. Inhibitory effects and mechanism of 5-fluorouracil combined with celecoxib on human gastric cancer xenografts in nude mice. Exp Ther Med. 2015;9(1): 105–111. | ||
Tummala S, Satish Kumar MN, Prakash A. Formulation and characterization of 5-fluorouracil enteric coated nanoparticles for sustained and localized release in treating colorectal cancer. Saudi Pharm J. 2015; 23(3):308–314. | ||
Wang T, Huang B, Guo R, et al. A let-7b binding site SNP in the 3′-UTR of the Bcl-xL gene enhances resistance to 5-fluorouracil and doxorubicin in breast cancer cells. Oncol Lett. 2015;9(4):1907–1911. | ||
Nouso K, Miyahara K, Uchida D, et al; Liver Cancer Study Group of Japan. Effect of hepatic arterial infusion chemotherapy of 5-fluorouracil and cisplatin for advanced hepatocellular carcinoma in the Nationwide Survey of Primary Liver Cancer in Japan. Br J Cancer. 2013; 109(7):1904–1907. | ||
Uchihara T, Yamashita Y, Hualin W, et al. Recurrence 11 years after complete response to gemcitabine, 5-Fluorouracil, and Cisplatin chemotherapy followed by radiotherapy in a patient with advanced pancreatic cancer: a case report. Anticancer Res. 2015;35(5):2867–2871. | ||
Shakeri-Zadeh A, Shiran MB, Khoee S, Sharifi AM, Ghaznavi H, Khoei S. A new magnetic nanocapsule containing 5-fluorouracil: in vivo drug release, anti-tumor, and pro-apoptotic effects on CT26 cells allograft model. J Biomater Appl. 2014;29(4):548–556. | ||
Zhou Z, Cao D, Liu L, et al. Fabrication and properties of gelatin/chitosan microspheres loaded with 5-fluorouracil. J Macromol Sci B. 2013;52(7/9):973–983. | ||
Li X, Xu Y, Chen G, et al. PLGA nanoparticles for the oral delivery of 5-fluorouracil using high pressure homogenization-emulsification as the preparation method and in vitro/in vivo studies. Drug Dev Ind Pharm. 2008;34(1):107–115. | ||
Farshi Azhar F, Olad A. A study on sustained release formulations for oral delivery of 5-fluorouracil based on alginate-chitosan/montmorillonite nanocomposite systems. Appl Clay Sci. 2014;101:288–296. | ||
Vaghani S, Vasanti S, Chaturvedi K, Satish CS, Jivani NP. Stomach-specific drug delivery of 5-fluorouracil using ethylcellulose floating microspheres. Pharm Dev Technol. 2010;15(2):154–161. | ||
Soppimath KS, Kulkarni AR, Aminabhavi TM. Development of hollow microspheres as floating controlled-release systems for cardiovascular drugs: preparation and release characteristics. Drug Dev Ind Pharm. 2001;27(6):507–515. | ||
Ruiz-Caro R, Gago-Guillan M, Otero-Espinar FJ, Veiga MD. Mucoadhesive tablets for controlled release of acyclovir. Chem Pharm Bull (Tokyo). 2012;60(10):1249–1257. | ||
Hou JY, Gao LN, Meng FY, Cui YL. Mucoadhesive microparticles for gastroretentive delivery: preparation, biodistribution and targeting evaluation. Mar Drugs. 2014;12(12):5764–5787. | ||
Streubel A, Siepmann J, Bodmeier R. Gastroretentive drug delivery systems. Expert Opin Drug Deliv. 2006;3(2):217–233. | ||
El-Zahaby SA, Kassem AA, El-Kamel AH. Formulation and in vitro evaluation of size expanding gastro-retentive systems of levofloxacin hemihydrate. Int J Pharm. 2014;464(1–2):10–18. | ||
Chen RN, Ho HO, Yu CY, Sheu MT. Development of swelling/floating gastroretentive drug delivery system based on a combination of hydroxyethyl cellulose and sodium carboxymethyl cellulose for Losartan and its clinical relevance in healthy volunteers with CYP2C9 polymorphism. Eur J Pharm Sci. 2010;39(1–3):82–89. | ||
Hao S, Wang B, Wang Y. Density-dependent gastroretentive microparticles motion in human gastric emptying studied using computer simulation. Eur J Pharm Sci. 2015;70:72–81. | ||
Svirskis D, Seyfoddin A, Chaplain S, et al. Development of mucoadhesive floating hollow beads of acyclovir with gastroretentive properties. Pharm Dev Technol. 2014;19(5):571–576. | ||
Kotreka UK, Adeyeye MC. Gastroretentive floating drug-delivery systems: a critical review. Crit Rev Ther Drug Carrier Syst. 2011;28(1): 47–99. | ||
El-Gibaly I. Development and in vitro evaluation of novel floating chitosan microcapsules for oral use: comparison with non-floating chitosan microspheres. Int J Pharm. 2002;249(1–2):7–21. | ||
Kawashima Y, Niwa T, Takeuchi H, Hino T, Itoh Y. Hollow microspheres for use as a floating controlled drug delivery system in the stomach. J Pharm Sci. 1992;81(2):135–140. | ||
Zhao L, Wei YM, Yu Y, Zheng WW. Polymer blends used to prepare nifedipine loaded hollow microspheres for a floating-type oral drug delivery system: in vitro evaluation. Arch Pharm Res. 2010;33(3): 443–450. | ||
Dube TS, Ranpise NS, Ranade AN. Formulation and evaluation of gastroretentive microballoons containing baclofen for a floating oral controlled drug delivery system. Curr Drug Deliv. 2014;11(6):805–816. | ||
Wei YM, Zhao L. In vitro and in vivo evaluation of ranitidine hydrochloride loaded hollow microspheres in rabbits. Arch Pharm Res. 2008; 31(10):1369–1377. | ||
Wei YM, Zhao L, Yu MA. Preparation of oral hollow microsphere carriers. Chinese Patent number: ZL200610095105.5. 2006 Sept 6. | ||
Stithit S, Chen W, Price JC. Development and characterization of buoyant theophylline microspheres with near zero order release kinetics. J Microencapsul. 1998;15(6):725–737. | ||
Pharmacopoeia of the People’s Republic of China. Beijing: Chemical Industry Publishing House; 2010. | ||
Bhosale UV, Devi K, Choudhary S. Multiunit floating drug delivery system of acyclovir: development, characterization and in vitro-in vivo evaluation of spray-dried hollow microspheres. J Drug Del Sci Tech. 2012;22(6):548–554. | ||
Sato Y, Kawashima Y, Takeuchi H, Yamamoto H, Fujibayashi Y. Pharmacoscintigraphic evaluation of riboflavin-containing microballoons for a floating controlled drug delivery system in healthy humans. J Control Release. 2004;98(1):75–85. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.