Back to Journals » Clinical Ophthalmology » Volume 10
A 10-year review of pediatric uveitis at a Hispanic-dominated tertiary pediatric ophthalmic clinic
Authors Dajee K, Rossen J, Bratton M, Whitson J , He Y
Received 14 September 2015
Accepted for publication 31 December 2015
Published 22 August 2016 Volume 2016:10 Pages 1607—1612
DOI https://doi.org/10.2147/OPTH.S96323
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Kruti P Dajee,1,2 Jennifer Landau Rossen,1 Monica L Bratton,1,2 Jess T Whitson,1 Yu-Guang He1,2
1Department of Ophthalmology, UT Southwestern Medical Center, 2Department of Ophthalmology, Children’s Medical Center of Dallas, Dallas, TX, USA
Purpose: The aim of this study was to evaluate the characteristics and outcomes of pediatric uveitis cases at a large tertiary referral center in Dallas, TX, USA.
Materials and methods: The authors performed a retrospective chart review between 2001 and 2011 to identify children with uveitis.
Results: A total of 46 children (68 eyes) with uveitis were identified. Sixty-seven percent were Hispanic, and the mean age was 9.2 years. The majority of cases were idiopathic (74%). Anterior uveitis accounted for 42% of cases followed by intermediate uveitis/pars planitis (33%), posterior uveitis/retinitis (7%), and panuveitis (20%). Most patients were treated with corticosteroids (98% topical), 52% with systemic immunosuppression therapy, and 30% with surgery. Complications occurred in 74% of patients, with the most common complication being cataract development (26%), followed by posterior synechiae (24%). Twenty-four percent of patients had recurrences. Hispanic patients had worse visual acuities at presentation (P-value =0.073) and follow-up (P-value =0.057), compared to non-Hispanic patients.
Conclusion: Pediatric uveitis cases seen in a large center in Dallas were largely idiopathic, had commonly developed complications, and were associated with worse visual outcomes in Hispanic patients.
Keywords: pediatric ophthalmology, uveitis, outcomes, Dallas, Hispanic
Introduction
Approximately 4.3%–6.9% out of 100,000 children in North America develop uveitis annually.1 Pediatric uveitis is a complex disease that can have a variety of etiologies and presentations. The condition often has an insidious onset.2 Symptoms, if present, may include vision loss, erythema, leukocoria, or strabismus.3 In particular, intermediate uveitis, posterior uveitis, and panuveitis are difficult to control, rarely respond to topical therapy alone, and have potential to cause significant visual loss.2,4 The current treatment for pediatric uveitis includes corticosteroids (topical, intravitreal, periorbital, and systemic) and immunomodulatory agents.2–4 For infectious etiologies, antibiotics and antiviral medications are needed.2 Although often idiopathic, pediatric uveitis may be associated with specific etiologies including infection and autoimmune conditions such as juvenile idiopathic arthritis (JIA), juvenile spondyloarthropathy, sarcoidosis, Adamantiades–Behçet disease, Vogt–Koyanagi–Harada syndrome, reactive arthritis, pars planitis, and sympathetic ophthalmia.1,3–5 Children are particularly at risk for complications secondary to uveitis, including vision loss, posterior synechiae, cataracts, increased intraocular pressure, amblyopia, band keratopathy, cystoid macular edema, and complications secondary to surgery.1–4 In this study, we evaluated the characteristics and outcomes of pediatric uveitis at a large tertiary referral center in Dallas, TX, USA.
Materials and methods
Children’s Medical Center in Dallas is a large tertiary referral center, serving a diverse population of children, majority of whom are Hispanic. Our study describes the disease characteristics, severity, complications, and outcomes of pediatric uveitis in children treated over a 10-year period. Several different providers including a mix of general pediatric ophthalmologists, retina specialists, and ophthalmology residents evaluated the patients and managed their care. After approval by the Institutional Review Board at the University of Texas Southwestern and Children’s Medical Center, we performed a retrospective chart review between 2001 and 2011 and identified 46 children (68 eyes) with a diagnosis of uveitis. Patient consent was deemed unnecessary by the University of Texas Southwestern and Children’s Medical Center Institutional Review Board because this was a retrospective review over a 10 year period.
The following demographic information was collected on each patient: sex, race, ethnicity, age at diagnosis, and length of follow-up. Uveitis was delineated by clinical description, anatomic location, affected eye/laterality, and etiology. We evaluated visual acuity and graded the severity of inflammation at presentation and at most recent follow-up. Anterior chamber cell, flare, and vitreous cell scores were documented according to the Standardization of Uveitis Nomenclature grading scheme.6 Complications and any surgical intervention were noted. Statistical analysis was performed by Mann–Whitney rank sum and paired t-test.
Results
Patient demographics are described in Table 1. There were 46 children (68 eyes) diagnosed with uveitis: 57% were male and 43% female. The ethnicities of patients included the following: Hispanic (67%) and non-Hispanic (33%), which were further classified per race: African American (17%), Caucasian (11%), and Asian (5%). The median age at diagnosis was 9.2 years (range 2.2–16.5 years). Uveitis was bilateral in 48% and unilateral in 52%. Patients’ follow-up was a mean of 21.3 months (median: 13.5 months) after diagnosis. Non-Hispanic patients had a mean of 11.6-month (median: 9.7 months) follow-up, and Hispanic patients had a mean of 25.9-month (median: 17.0 months) follow-up.
  | Table 1 Patient demographics |
The majority of cases were determined to be idiopathic (74%); other etiologies of uveitis seen in our patients are described in Figure 1 and include HLA B27-associated (9%), toxoplasmosis (5%), cytomegalovirus (2%), sympathetic ophthalmia (2%), herpes simplex virus (2%), JIA (2%), irritable bowel disease (2%), and juvenile spondyloarthropathy (2%). The findings of examination were based on Standardization of Uveitis Nomenclature criteria and were documented as follows: anterior uveitis (42%), intermediate uveitis/pars planitis (33%), posterior uveitis/retinitis (7%), and panuveitis (20%), which are shown in Figure 2. The average anterior chamber cell score at diagnosis was 2.26 and improved to 0.60 (Δ 1.66) at most recent follow-up (Table 2). The average anterior vitreous cell score at diagnosis was 1.32 and improved to 0.52 (Δ 0.8) at most recent follow-up.
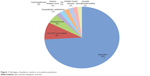  | Figure 1 Etiologies of pediatric uveitis in our patient population. |
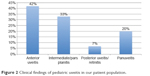  | Figure 2 Clinical findings of pediatric uveitis in our patient population. |
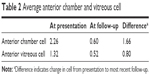  | Table 2 Average anterior chamber and vitreous cell |
Forty-five patients (98%) were treated with topical corticosteroids (prednisolone, difluprednate, fluorometholone), 17 patients (37%) with subtenons corticosteroids, eight patients (17%) with oral corticosteroids, two patients (4%) with intravitreal corticosteroids, and two patients (4%) with intravenous or intramuscular corticosteroids (Table 3). Patients could have been treated with more than one form of steroids. Eight patients (17%) were treated with antibiotics or antivirals. Twenty-four patients (52%) were treated with systemic immunosuppressants, including methotrexate, mycophenolate mofetil, adalimumab, and sulfasalazine.
  | Table 3 Treatments in our patient population |
Complications were defined as any adverse ocular event that occurred as a direct result of the primary disease process (uveitis) or as a result of the treatment. In our cohort, complications were common and were found in 74% of our patients (Figure 3). For the total 68 eyes, complications included cataract (18 eyes, 26%), posterior synechiae (16 eyes, 24%), ocular hypertension/glaucoma (eleven eyes, 16%), band keratopathy (nine eyes, 13%), retinal detachment (three eyes, 4%), strabismus (three eyes, 4%), cystoid macular edema (two eyes, 3%), vitreous hemorrhage (one eye, 1%), and retinal neovascularization (one eye, 1%). Recurrence of uveitis was documented in 24% of patients, and this was most commonly secondary to noncompliance with medical therapy. Surgical intervention in 30% of patients included cataract extraction, glaucoma filter, retinal laser, and vitrectomy.
  | Figure 3 Complications in our patient population. |
Due to the large majority of Hispanic patients, we divided our study into Hispanic group (H) and non-Hispanic group (NH) in order to compare the outcomes. There was a trend toward Hispanic patients presenting with worse visual acuities (P-value =0.073) and worse visual acuities at most recent follow-up (P-value =0.057) when compared to their non-Hispanic counterparts (Table 4). We found that there were no statistically significant differences between the two groups when comparing diagnostic description (P-value =0.485), etiology (P-value =0.658), or location (P-value =0.343).
  | Table 4 Visual acuity in Hispanic vs non-Hispanic patients |
Discussion
There have been several studies on pediatric uveitis describing population-specific cases of pediatric uveitis in various regions of the world. Some of the data from the more recent studies are included here for comparison to our study population.1,4,7–13 The mean age of our patients was 9.2 years, which fell within the range of the averages found in other studies (6.7–12.4 years).1,4,9–13 The majority of our patients were diagnosed with idiopathic pediatric uveitis (74%), while other studies report a wide range of unidentified or idiopathic etiology of pediatric uveitis: from 12.8% to 60%.1,4,7–13 Our rate of idiopathic etiology was higher than that reported in other studies, possibly due to some patients failing to receive sufficient follow-up (ie, some patients were unable to get bloodwork done or failed to follow-up at their rheumatology appointments). In our patient population, 9% of cases were attributable to infectious causes (with the most common infectious cause being toxoplasmosis, 5%). Infectious etiologies were identified as the cause of 5.2%–33.3% of pediatric uveitis cases in other studies.1,7,9–11,13 Interestingly, JIA (6.25%–33%) or juvenile rheumatoid arthritis (17.5%–36.3%) accounted for a substantial percentage of cases in several other studies, whereas JIA only accounted for 2% of our cases.1,4,7–13 Consistent with other studies indicating higher complications in children with uveitis, our complication rate was 74%. Khairallah et al reported on their experiences in Tunisia, North Africa, and described their complication rate to be 74.7%.13 Rosenberg et al similarly reported on their experiences in Miami, FL, USA, wherein they reported a complication rate of 34% at presentation and 86.3% by the third-year follow-up.11 Our most common complications, such as cataract, posterior synechiae, ocular hypertension/glaucoma, and band keratopathy were consistent with findings from other studies.1,4,9,11,12 However, macular scarring and other maculopathies were found in 0%–55.5% of patients in other studies and appeared to vary based on the type and location of the uveitis, whereas only 3% of our cases developed cystoid macular edema.1,4,9–13 Thirty percent of our patients underwent surgical management, compared to 7.8%–45.9% reported in other studies.4,11–13
For visual acuity, other studies found, at baseline, that 25%–71% of patients had visual acuity >20/40 (or >6/12), 20% had 20/40 to 20/200 (or 6/12 to 6/60), and 8%–53% had <20/200 (or <6/60).1,3,11–13 In our study at baseline, 27% of the H and 31% of NH patients had >20/40, 45% H and 54% NH had 20/40 to 20/200, and 28% H and 15% NH had <20/200. The other studies showed that visual outcome varied widely depending on the time at follow-up, which is especially exemplified by Rosenberg et al’s study that analyzed visual acuity separately at different follow-up times.11 In these studies, 17%–83% of patients had >20/40 (or >6/12), 13.7%–17% had 20/40 to 20/200 (or 6/12 to 6/60), and 0%–75% had <20/200 (or <6/60).1,3,9–13 In comparison, our study, where at final follow-up, visual acuity of >20/40 was noted in 50% NH and 42% H populations, while 29% NH and 35% H had 20/40 to 20/200, and 21% NH and 23% H had <20/200. These trends in our study indicate that either Hispanic or Latino children have uveitis detected at a more advanced stage with a greater impact on visual acuity at that point in the disease process resulting in more loss of vision or, alternatively uveitis syndromes in Hispanic/Latino populations are associated with more loss of vision.
The Hispanic population in Dallas County makes up a large proportion of patients treated at the Children’s Medical Center and made up 67% of our study population. The Hispanic/Latino population comprises 42.4% of the population in Dallas.14 This unique population deserves special care and attention and will likely have a large impact on the health care system due to the increasing size of this population, which is expected to grow by 113% in the USA from 2000 to 2025, and even more by 2050.15 The Latino population in the USA tends to have higher morbidity and mortality from illnesses secondary to multiple factors including, but not limited to, health care disparities and socioeconomic status.15 Lack of insurance combined with lower household incomes often leads to inferior health care and poorer outcomes for Hispanic children.16
Our findings of poorer outcomes in the Hispanic subgroup of our study population were consistent with a large retrospective, longitudinal study of pediatric uveitis by Smith et al, who evaluated demographics, uveitis disease characteristics, complications, treatments, and visual outcomes over a 10-year period from the National Eye Institute (Bethesda, MD, USA), University of Illinois at Chicago (Chicago, IL, USA), and Oregon Health Sciences University (Portland, OR, USA).4 They found that Hispanic ethnicity was associated with a higher incidence of infectious uveitis and a significantly greater risk of vision loss.4 Socioeconomic and health care disparities may have an influence on worsened outcomes in this subpopulation of patients, but the true etiology of the difference is unclear at this time.
Our study confirms that pediatric uveitis is a potentially devastating disease often requiring both medical and surgical therapy. Although the first choice of treatment in acute uveitis is topical or systemic corticosteroids, the treatment of chronic or recurrent uveitis often mandates the use of systemic immunosuppressives in children to avoid systemic complications, which are more worrisome in this age group. There are a few medications approved by the US Food and Drug Administration which are indicated for the treatment of pediatric uveitis. Methotrexate has been approved for use in children with JIA; however, its use is currently off-label in pediatric uveitis. Adalimumab is also approved for JIA in patients over 4 years of age, and its use has shown to be successful in gaining the control of inflammation in refractory or aggressive cases of uveitis.17
The limitations of our study include the retrospective design and lack of standardized follow-up visits. However, we did evaluate 10 years of data from a large tertiary referral center, revealing that multiple interventions were required for visual and clinical improvement and that complications (74%) and recurrences (24%) were common. In our study, we found that Hispanic patients may have poorer visual presentations and outcomes compared to their non-Hispanic counterparts. However, we cannot draw any definitive conclusions at this time with our smaller cohort size. Further studies are needed to determine whether others find a similar ethnic disparity in outcomes, and if so to elucidate some of the potential causes. Pediatric uveitis remains a continuing challenge for ophthalmologists due to the commonly indolent course with frequent complications in the pediatric population.
Acknowledgment
This study was supported by an unrestricted grant from Research to Prevent Blindness, Inc. (New York, NY, USA).
Disclosure
The authors alone are responsible for the content and writing of the paper. Doctor Jess T Whitson is a consultant for Alcon Laboratories, Inc. (Fort Worth, TX, USA) and Allergan, Inc. (Irvine, CA, USA). The authors report no other conflicts of interest in this work.
References
Kump LI, Cervantes-Castañeda RA, Androudi SN, Foster CS. Analysis of pediatric uveitis cases at a tertiary referral center. Ophthalmology. 2005;112(7):1287–1292. | ||
Wentworth BA, Freitas-Neto CA, Foster CS. Management of pediatric uveitis. F1000Prime Rep. 2014;6:41. | ||
Cunningham E. Uveitis in children. Ocul Immunol Inflamm. 2000;8(4):251–261. | ||
Smith JA, Mackensen F, Sen HN, et al. Epidemiology and course of disease in childhood uveitis. Ophthalmology. 2009;116(8): 1544–1551. | ||
Lowder C, Belfort R Jr, Lightman S, et al; Ozurdex HURON Study Group. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011;129(5):545–553. | ||
Jabs DA, Nussenblatt RB, Rosenbaum JT; Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509–516. | ||
BenEzra D, Cohen E, Maftzir G. Uveitis in children and adolescents. Br J Ophthalmol. 2005;89(4):444–448. | ||
Päivönsalo-Hietanen T, Tuominen J, Saari KM. Uveitis in children: population-based study in Finland. Acta Ophthalmol Scand. 2000;78(1):84–88. | ||
Hamade IH, Al Shamsi HN, Al Dhibi H, Chacra CB, Abu El-Asrar AM, Tabbara KF. Uveitis survey in children. Br J Ophthalmol. 2009;93(5):569–572. | ||
Paroli MP, Spinucci G, Liverani M, Monte R, Pezzi PP. Uveitis in childhood: an Italian clinical and epidemiological study. Ocul Immunol Inflamm. 2009;17(4):238–242. | ||
Rosenberg KD, Feuer WJ, Davis JL. Ocular complications of pediatric uveitis. Ophthalmology. 2004;111(12):2299–2306. | ||
Azar D, Martin F. Paediatric uveitis: a Sydney clinic experience. Clin Experiment Ophthalmol. 2004;32(5):468–471. | ||
Khairallah M, Attia S, Zaouali S, et al. Pattern of childhood-onset uveitis in a referral center in Tunisia, North Africa. Ocul Immunol Inflamm. 2006;14(4):225–231. | ||
QuickFacts United States [webpage on the Internet]. Washington, DC: United States Census Bureau; 2014. Available from: https://www.census.gov/quickfacts/table/PST045215/00. Accessed June 15, 2016. | ||
Vega WA, Rodriguez MA, Gruskin E. Health disparities in the Latino population. Epidemiol Rev. 2009;31:99–112. | ||
Pickens SS, Swanson T, Tietz MK. Dallas County Health Checkup. Online publication of Dallas County Health & Human Services; 2000:1–46. | ||
Díaz-Llopis M, Salom D, Garcia-de-Vicuña C, et al. Treatment of refractory uveitis with adalimumab: a prospective multicenter study of 131 patients. Ophthalmology. 2012;119(8):1575–1581. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
