Back to Journals » OncoTargets and Therapy » Volume 11
99mTc-labeled sodium phytate and stannous chloride injection accurately detects sentinel lymph node in axillary of early stage breast cancer: a randomized, controlled study
Authors Yang S, Bao W, Bai X, Gao C, Zhang B, Jiang Z
Received 26 October 2017
Accepted for publication 4 January 2018
Published 4 April 2018 Volume 2018:11 Pages 1891—1898
DOI https://doi.org/10.2147/OTT.S155265
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Suisheng Yang,* Weiyu Bao,* Xiaorong Bai, Chen Gao, Binming Zhang, Zhuanji Jiang
Department of Breast Surgery, Gansu Provincial Cancer Hospital, Lanzhou, China
*These authors contributed equally to this work
Aim: The aim of this study was to assess the sentinel lymph node (SLN) detection rate and accuracy of 99mTc-labeled sodium phytate and stannous chloride (99mTc-PHY) injection versus 99mTc-labeled sulfur colloid (99mTc-SC) injection in sentinel lymph node biopsy (SLNB) in patients with early stage breast cancer.
Methods: A total of 146 consecutive female patients with early stage breast cancer were recruited in this open-labeled, randomized, controlled study. SLNB was conducted on all patients, and 99mTc-PHY or 99mTc-SC was used as the radioactive agent (RA). Axillary lymph node dissections were performed in all patients post SLN dissections.
Results: The detection rate of 99mTc-PHY group was higher compared with that of 99mTc-SC group (p=0.023), but no difference in the detection rate by dye alone (p=0.190) or by RAs alone (p=0.615) was found between the two groups, and the number of identified SLNs (p=0.100), number of identified SLNs by dye alone (p=0.161), and number of identified SLNs by RA alone (p=0.242) were similar between the two groups. In addition, the sensitivity, specificity, false-negative rate, false-positive rate, and accuracy rate of SLNB showed no difference between 99mTc-PHY and 99mTc-SC groups (sensitivity: p=0.645; specificity: p=0.511; false-negative rate: p=0.645; false-positive rate: p=0.511; accuracy rate: p=0.464).
Conclusion: Our study revealed that 99mTc-PHY was qualified to be a convincing radiopharmaceutical in SLNB.
Keywords: breast cancer, sentinel lymph node biopsy, 99mTc, sodium phytate, stannous chloride, detection rate
Introduction
Breast cancer, the most frequent cancer among females, shows escalations of both incidence and mortality in Asia, South America, and Africa while a decline in Europe due to advanced screening programs in general population.1,2 Compared with other cancers, breast cancer is characterized by hormone-related and human epidermal growth factor receptor-2-related features in its pathogenesis.3,4 Treatment procedures for breast cancer include systemic therapy and resections, among which breast preservation becomes a preferred approach in order to improve the quality of life.5
Axillary lymph node dissection (ALND) has become a routine and a standard procedure in the management of breast cancer patients and a method for lymph metastasis staging over decades.6 Until now, ALND combined with histopathological examination is still a gold standard for assessing the axillary lymph node involvement.7 Nevertheless, patients who received ALND have high morbidity rate, and the acute complication rate could reach 20%–30%.8 The axillary lymph node metastasis is the integral risk factor of poor prognosis in breast cancer patients; however, the high morbidity and ALND caused complications, including edema, pain, and physical inactivity of the infected side, which largely limit the practicability of ALND in clinical practice, leading to the existence of sentinel lymph node biopsy (SLNB), which has become the standard process for assessing axillary lymphatic metastasis in early stage breast cancer.1,9,10
SLNB is a minimally invasive surgery that detects the axillary lymphatic metastasis by sequentially locating and performing histopathological biopsy on sentinel lymph node (SLN).11 In previous studies, SLNB presented a relatively low false-negative rate of 5%–10% as well as a high sensitivity of 90%–95%, indicating that SLNB is a procedure of high accuracy and might be an optimal option for reducing unnecessary ALNDs.12,13 Location of SLN is normally assessed by injecting blue dye or radioisotope technetium-labeled nano colloid, which could also be other isotopic carriers such as rituximab and dextran.14–16 Despite that sulfur colloid (SC) or albumin colloid is the most common radiotracer, the detection rate, true-positive rate, and false-negative rate varied among studies and were not satisfactory, thus, no standard or optimal carriers were identified in SLN location.17,18 Sodium phytate and stannous chloride (PHY) has been used in lymphoscintigraphies for liver, spleen, and bone marrow, and it has been reported that PHY could be used as radiotracer in SLNB for patients with papillary thyroid carcinoma.19,20 However, PHY has not been studied and used as an isotopic carrier for localization of SLN in breast cancer.
Therefore, the aim of this study was to assess the SLN detection rate and accuracy of 99mTc-labeled sodium phytate and stannous chloride (99mTc-PHY) injection versus 99mTc-labeled sulfur colloid (99mTc-SC) injection in patients with early stage breast cancer.
Methods
Participants
A total of 146 consecutive female patients with breast cancer from February 2012 to December 2015 at the Department of Breast Surgery in Gansu Provincial Cancer Hospital were recruited in this open-labeled, randomized, controlled study. The inclusion criteria were as follows: 1) age >18 years; 2) diagnosed of breast cancer based on clinical manifestations and radiological and histological findings; 3) clinical stage at Tis–T3; 4) clinically negative lymph node metastasis; 5) diagnosed as eligible for resections; and 6) no preoperative clinical or radiologic evidence for metastases. Patients with the following features were excluded: 1) previous major surgery on breast or axillary with lymph backflow destruction; 2) multicenter or multifocal breast cancer; 3) confirmed axillary lymph node metastasis; 4) previous treatments of radiotherapy, chemotherapy, or targeted therapy; 5) pregnant or lactating patients; and 6) cognitive impairment or poor adherence and could not understand the study protocol. This study was approved by the ethics committee of Gansu Provincial Cancer Hospital and conducted in accordance with the Declaration of Helsinki. All the participants provided signed informed consents.
Study design and randomization
This research was an open-labeled, noninferior, randomized, controlled study. The calculated minimum size sample was 63 for each group (126 totally) according to noninferiority trial balanced design (in this study, 146 participants were enrolled; the sample size was >126). All patients were randomized in 1:1 ratio to 99mTc-PHY and 99mTc-SC groups, using a block randomization method by SAS software. The randomization was conducted by a separate statistical analyzer, and the documents were sent and kept in Shanghai Qeejen bio-tech Company (a medical and statistic service company). When a patient was eligible for the study, a call was made to Qeejen Company and a unique subject identification number was provided from the randomized module.
Equipment and materials
In this study, the Neoprobe 2000 γ probe detector (Johnson & Johnson, New Brunswick, NJ, USA) was used for identifying SLN. Single-photon emission computed tomography (SPECT) was achieved by dual-head Discovery VH SPECT scanner (GE Healthcare, Pittsburgh, PA, USA). Esaote MyLab Twice Color Doppler Ultrasonic Diagnosis Apparatus (Esaote, Florence, Italy) was used for locating the SLN and performing aspiration cytology. 99mTc was provided by Beijing Atom Hi-Tech Co., Ltd. (Beijing, China), and Liquid-based Thin-layer Cell Test Machine was provided by Hologic (Marlborough, MA, USA). Leica cm-1950 cryostat microtome was purchased from Leica Microsystems (Wetzlar, Germany). PHY for injection was obtained from Jiangsu Atom Medicine Research Institute Jiangyuan Pharmaceutical Factory (Jiangsu, China). The methylene blue was provided by Jumpcan Pharmaceutical Group Co., Ltd. (Jiangsu, China), and ultrasound contrast agent Sonovue was provided by Bracco Co., Ltd. (Milan, Italy).
Procedures
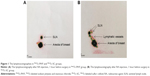
Before surgeries, the ultrasound contrast and aspiration biopsy were performed prior to lymphoscintigraphy on patients to rule out some of the patients with SLN metastasis but was diagnosed as SLN metastasis negative in clinical practice before enrollment of our study for the purpose of lowering the false-negative rate. Three to eight hours prior to surgery, 99mTc-PHY (0.5 mL) or 99mTc-SC (0.5 mL) was injected into single site under the mammary areola subcutaneously, and the radiation intensity was 0.4–3.2 mCi. Lymphoscintigraphy was performed on the affected side and same-side axilla of each patient at 30–120 minutes after the injection. The examples of lymphoscintigraphies in 99mTc-PHY and 99mTc-SC groups were presented in Figure 1A and B, which were obtained 1 hour after RA injection. Meanwhile, methylene blue was subcutaneously injected 10–15 minutes before the surgeries; the injection sites included four peritumoral sites and one subareolar site. In addition, the blue dye was injected at different time points because blue dye has distinct flow velocity in lymphatic vessel compared to radiotracers and was injected at different sites because after the radiotracer injection there might be inflammatory responses that could cause edema that blocks the lymphatic vessels. All the blue-stained and intensively radioactive SLNs were detected by the handheld γ probe detector, which were identified as the lymph node with the highest radioactivity count rate and lymph nodes with radioactivity count rate more than 10% of the highest radioactivity count rate (Figure 2A), and the radiation count rate of the SLN in Figure 2A was 805 (Figure 2B).21,22 The imprint cytology, intraoperative frozen section, and postoperative histopathological examination were carried out on dissected SLNs immediately. After SLN dissections, ALNDs were performed in all patients. The SLNB procedure was performed according to standard protocols described elsewhere.23,24
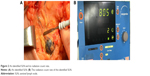  | Figure 2 An identified SLN and its radiation count rate. |
Primary and secondary endpoints
The primary endpoint was the detection rate of SLN in each group. The secondary endpoints were as follows: detection rate of SLN by dye alone; detection rate of SLN by radioactive agent (RA) alone; number of identified SLNs; sensitivity, specificity, false-negative rate, false-positive rate, and accuracy rate of SLNB.
Evaluation criteria
Sensitivity, specificity, false-negative rate, false-positive rate, and accuracy rate of SLNB were calculated according to criteria of SLNB by Louisville University as follows:25 Sensitivity (%) = SLN true-positive cases/(SLN true-positive cases + SLN false-negative cases) * 100%; specificity (%) = SLN true-negative cases/(SLN true-negative cases + SLN false-positive cases) * 100%; false-negative rate (%) = SLN false-negative cases (SLN true-positive cases + SLN false-negative cases) * 100%; false-positive rate = SLN false-positive cases/(SLN true-negative cases + SLN false-positive cases) * 100%; accuracy rate = (SLN true-positive cases + SLN true-negative cases)/(SLN true-positive cases + SLN false-positive cases + SLN false-positive cases + SLN true-negative cases) * 100%; detection rate = cases detected with SLN/all cases.
Statistical analysis
Statistical analysis was performed using the SSPS 21.0 software. Data were mainly presented as mean ± standard deviation (SD) and count (percentage). Difference between two groups was compared by t-test or chi-squared test. A p-value <0.05 was considered significant.
Results
Characteristics
As presented in Figure 3, 208 participants were screened for eligibility, among which 62 cases were excluded (29 for exclusion criterion, 26 for disagreement with informed consent, and 7 for other reasons). The remaining 146 participants were randomly allocated to 99mTc-PHY group (n=73) and 99mTc-SC group (n=73) in 1:1 ratio, and there was no withdrawal during the study for any reasons, thus finally 73 patients in 99mTc-PHY group and 73 patients in 99mTc-SC group were included in this analysis.
The 73 patients in 99mTc-PHY group had a mean age of 46.5±10.2 years, among which 35 cases (48%) had left breast cancer and 38 cases (52%) had right breast cancer, whereas patients in 99mTc-SC group had a mean age of 48.3±10.8 years, with 39 left breast cancer cases (53%) and 34 right breast cancer cases (47%). No difference in age, body mass index, side of breast cancer, menstrual status, primary tumor location, histopathologic type, and clinical T stage was found between 99mTc-PHY and 99mTc-SC groups as shown in Table 1.
Primary endpoint
SLNs were detected in all 73 patients in 99mTc-PHY group with the detection rate of 100%, which was higher than 99mTc-SC group with 68 out of 73 patients being detected with a detection rate of 93.2%, p=0.023 (Table 2).
Secondary endpoints
As listed in Table 2, SLNs were identified by dye in 70 patients (95.9%) in 99mTc-PHY group compared with 66 patients (90.4%) in 99mTc-SC group, no difference between the two groups was observed (p=0.190). Consistently, no difference in detection rate by RA was found between 99mTc-PHY and 99mTc-SC groups either (89.0% vs 86.3%, p=0.615).
In 99mTc-PHY group, one SLN was detected in 16 patients (22%), two SLNs in 30 patients (41%), three SLNs in 20 patients (27%), four SLNs in four patients (5%), five SLNs in two patients (3%), and six SLNs in one patient (1%). While in 99mTc-SC group, 0 SLN was detected in five patients (7%), one SLN in 17 patients (23%), two SLNs in 30 patients (41%), three SLNs in 14 patients (19%), and four SLNs in seven patients (10%). The number of identified SLNs was similar between the two groups (2.30±1.05 vs 2.01±1.05, p=0.100) (Table 2), and as to number of identified SLNs by dye or RA, the results were the same (2.23±1.14 vs 1.97±1.09, p=0.161; 2.15±1.24 vs 1.92±1.15, p=0.242). In addition, the detection rates by RA and by dye were of no difference both in 99mTc-PHY group (p=0.117) and 99mTc-SC group (p=0.439).
In 99mTc-PHY group, SLNB illustrated that 18 cases were lymph node positive, among which 17 cases were true-positive cases and one was a false-positive case according to ALND results. The false-positive case was a patient without lymph node metastasis confirmed by the pathological examination from ALND but was diagnosed as lymph node metastasis positive according to the biopsy from SLNB. Meanwhile, SLNB showed that 55 cases were lymph node negative, among which 51 cases were true-negative cases and four were false-negative cases (p<0.001) (Table 3).
In 99mTc-SC group, SLNB disclosed that 17 cases were lymph node positive, among which 15 cases were true-positive cases and two were false-positive cases according to ALND results. Meanwhile, SLNB presented that 51 cases were lymph node negative, among which 46 cases were true-negative cases and five were false-negative cases (p<0.001) (Table 4).
The sensitivity, specificity, false-negative rate, false-positive rate, and accuracy rate of SLNB showed no difference between 99mTc-PHY and 99mTc-SC groups as presented in Table 5 (sensitivity: 81.0% vs 75.0%, p=0.645; specificity: 98.1% vs 95.8%, p=0.511; false-negative rate: 19.0% vs 25.0%, p=0.645; false-positive rate: 1.9% vs 4.2%, p=0.511; accuracy rate: 93.2% vs 89.7%, p=0.464, respectively).
Safety
During and within 24 hours postoperation, no side effect was observed in either 99mTc-PHY group or 99mTc-SC group of this study.
Discussion
In the present study, the results elucidated that 1) the detection rate in 99mTc-PHY group was elevated compared with 99mTc-SC group; 2) no difference of identified SLN numbers was discovered between the two groups; 3) similar sensitivity, specificity, false-negative rate, false-positive rate, and accuracy rate of SLNB between the two groups were observed.
Females diagnosed with breast cancer, in all likelihood, will receive radical resections.1 Fortunately, the advance of imaging techniques has led to an increasing possibility of small primary tumors to be discovered, which makes less invasive procedures and organ saving possible among patients.26–28 Thus, the ALND, which is one of the standard surgeries and resulted in several complications like lymphedema, should be avoided once verified unnecessary. SLNB, strikingly reduced the unnecessary ALNDs, has been developed regarding its efficacy and safety over years. In addition, for primary breast cancer patients, the adjuvant systemic therapy is determined by the lymph node metastasis condition, which could be detected by the SLNB.29
SLN location is essential in SLNB, often achieved by lymphoscintigraphy or blue dye or their combination, which is confirmed to be of better accuracy compared with lymphoscintigraphy or blue dye alone.10,15,30 During decades of development of lymphoscintigraphy technology, various radiopharmaceuticals have been used for SLN localization, and whether the radiopharmaceutical is qualified for SLNB is determined by the anatomical and physiologic features of SLN,26 which means the features, especially the particle size, of radiopharmaceutical should allow for its proper flow velocity in lymphatic vessel and accumulation in SLN for the purpose of better identification of the SLN. A successful SLN localization requires for the right method and site of injection and the right dose as well as particle size, among which particle size is demonstrated to be most crucial.31 Particle size is negatively associated with migrate speed and remaining time in SLN, which means a large-sized particle tends to migrate slowly in lymph vessels and remain longer in SLNs.32 In the United States, 99mTc-SC and 99mTc-labeled tilmanocept are the most common radiotracers, and both of them are approved by the Food and Drug Administration of the United States,33 and 99mTc nano colloid, to be exactly, the colloidal albumin, is preferred by the European surgeons.34
PHY, white powder soluble in water, is used for the preparation of 99mTc-PHY in clinical practice for years.35 Post injection, phytate reacts with plasma calcium immediately to synthesize soluble colloid with the particle size of 100–1,000 nm, which is similar to that of SC; PHY is approved by the China Food and Drug Administration (CFDA) however the SC is not.35 In the study of Takei et al, 99mTc-PHY has been proved to be superior to 99mTc-labeled human serum albumin for SLN detection in patients with breast cancer.36 Similarly, 99mTc-PHY also demonstrated a better efficiency than 99mTc-tin colloid in SLNB for breast cancer patients.25 In this study, 99mTc-PHY showed a more favorable detection rate than 99mTc-SC; however, no difference of the detected numbers of SLN, sensitivity, specificity, false-negative rate, as well as accuracy rate was found compared with 99mTc-SC. The probable explanations of our results are: 1) in a previous study, 99mTc-PHY had a more intense radioactivity in SLNs compared with 99mTc human serum albumin, indicating PHY had a greater accumulation in the SLN, which might be a specific feature of PHY that is superior to SC;36 2) the particle size of radiotracer should not be too large to move in the lymphatic vessels, or too small to accumulate in the SLNs, thus it is reasonable that PHY outscored SC in detection rate with similar or even larger particle size.26
On the other hand, learning curve is another factor that has influence on the accuracy of SLN detection in SLNB. The training and maturity of the surgeons’ skill is essential in SLNB, and using blue dye curtails the learning curve of surgeons in SLNB using 99mTc.37 A retrospective study elucidated that dye-only injection into the SA plexus had a favorable SLN detection rate in patients with breast cancer.38 In addition, the detection rates by RA alone were numerically lower than that by dye alone in both the two groups, which is partially different from the previous reports, which might be caused by the surgeons who are more skilled in using blue dye than using radiotracers.
Some limitations existed in this study: 1) 146 consecutive female breast cancer patients were recruited in this open-labeled randomized study, the sample size was relatively small which might lead to that differences in some parameters were not observed; 2) surgeon skill was not considered in this study, and it was determined by the stages of learning curve, which was proved to be important in a successful SLNB.39
Conclusion
Our study demonstrated that 99mTc-PHY was qualified to be a convincing radiopharmaceutical in SLNB.
Acknowledgment
This study was supported by the Special Project for Technical Research and Development of Gansu Province, China (No 3013GS08735).
Disclosure
The authors report no conflicts of interest in this work.
References
Harbeck N, Gnant M. Breast cancer. Lancet. 2017;389(10074):1134–1150. | ||
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. | ||
Jordan NV, Bardia A, Wittner BS, et al. HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature. 2016;537(7618):102–106. | ||
Leo AD, Jerusalem G, Petruzelka L, et al. Final overall survival: fulvestrant 500 mg vs 250 mg in the Randomized CONFIRM Trial. J Natl Cancer Inst. 2014;106(1):349–351. | ||
McLaughlin SA. Surgical management of the breast: breast conservation therapy and mastectomy. Surg Clin North Am. 2013;93(2):411–428. | ||
Parmigiani G, Berry DA, Winer EP, Tebaldi C, Iglehart JD, Prosnitz LR. Is axillary lymph node dissection indicated for early-stage breast cancer? A decision analysis. J Clin Oncol. 1999;17(5):1465–1473. | ||
NIH consensus conference. Treatment of early-stage breast cancer. JAMA. 1991;265(3):391–395. | ||
Hsueh EC, Hansen N, Giuliano AE. Intraoperative lymphatic mapping and sentinel lymph node dissection in breast cancer. CA Cancer J Clin. 2000;50(5):279–291. | ||
Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23(30):7703–7720. | ||
McMasters KM, Tuttle TM, Carlson DJ, et al. Sentinel lymph node biopsy for breast cancer: a suitable alternative to routine axillary dissection in multi-institutional practice when optimal technique is used. J Clin Oncol. 2000;18(13):2560–2566. | ||
Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220(3):391–398; discussion 398–401. | ||
Veronesi U, Viale G, Paganelli G, et al. Sentinel lymph node biopsy in breast cancer: ten-year results of a randomized controlled study. Ann Surg. 2010;251(4):595–600. | ||
Borgstein PJ, Pijpers R, Comans EF, van Diest PJ, Boom RP, Meijer S. Sentinel lymph node biopsy in breast cancer: guidelines and pitfalls of lymphoscintigraphy and gamma probe detection. J Am Coll Surg. 1998;186(3):275–283. | ||
Li N, Wang X, Lin B, et al. Clinical evaluation of 99mTc-rituximab for sentinel lymph node mapping in breast cancer patients. J Nucl Med. 2016;57(8):1214–1220. | ||
Wei L, Chen F, Zhang X, et al. (99m)Tc-dextran lymphoscintigraphy can detect sentinel lymph node in breast cancer patients. Exp Ther Med. 2015;9(1):112–116. | ||
Mayes E, Douek M, Pankhurst Q. Magnetic Nanoparticles: From Fabrication to Clinical Applications. Nguyyen TTT, editor. New York: CRC Press; 2012. | ||
Mirzaei S, Rodrigues M, Hoffmann B, et al. Sentinel lymph node detection with large human serum albumin colloid particles in breast cancer. Eur J Nucl Med Mol Imaging. 2003;30(6):874–878. | ||
Chen SL, Iddings DM, Scheri RP, Bilchik AJ. Lymphatic mapping and sentinel node analysis: current concepts and applications. CA Cancer J Clin. 2006;56(5):292–309; quiz 316–297. | ||
Qiao G, Cong Y, Zou H, et al. False-negative frozen section of sentinel lymph node biopsy in a Chinese population with breast cancer. Anticancer Res. 2016;36(3):1331–1337. | ||
Lee J, Na KY, Lee J, et al. The usefulness and accuracy of sentinel lymph node biopsy using single photon emission computed tomography/computed tomography with 99mTc phytate to detect locoregional lymph node metastases in patients with papillary thyroid carcinoma. J Korean Surg Soc. 2013;84(4):195–201. | ||
Giammarile F, Bozkurt MF, Cibula D, et al. The EANM clinical and technical guidelines for lymphoscintigraphy and sentinel node localization in gynaecological cancers. Eur J Nucl Med Mol Imaging. 2014;41(7):1463–1477. | ||
Gradishar WJ, Anderson BO, Balassanian R, et al. Invasive Breast Cancer Version 1. 2016. J Natl Compr Canc Netw. 2016;14(3):324–354. | ||
Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14(7):609–618. | ||
Schaafsma BE, Verbeek FP, Rietbergen DD, et al. Clinical trial of combined radio- and fluorescence-guided sentinel lymph node biopsy in breast cancer. Br J Surg. 2013;100(8):1037–1044. | ||
Seok JW, Choi YS, Chong S, et al. Sentinel lymph node identification with radiopharmaceuticals in patients with breast cancer: a comparison of 99mTc-tin colloid and 99mTc-phytate efficiency. Breast Cancer Res Treat. 2010;122(2):453–457. | ||
Moncayo VM, Aarsvold JN, Grant SF, Bartley SC, Alazraki NP. Status of sentinel lymph node for breast cancer. Semin Nucl Med. 2013;43(4):281–293. | ||
Cady B, Stone MD, Schuler JG, Thakur R, Wanner MA, Lavin PT. The new era in breast cancer. Invasion, size, and nodal involvement dramatically decreasing as a result of mammographic screening. Arch Surg. 1996;131(3):301–308. | ||
Kiderlen M, Ponti A, Tomatis M, et al. Variations in compliance to quality indicators by age for 41,871 breast cancer patients across Europe: a European Society of Breast Cancer Specialists database analysis. Eur J Cancer. 2015;51(10):1221–1230. | ||
Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1992;339(8785):71–85. | ||
Newman EA, Newman LA. Lymphatic mapping techniques and sentinel lymph node biopsy in breast cancer. Surg Clin North Am. 2007;87(2):353–364, viii. | ||
Linehan DC, Eberlein TJ. Mechanisms of radiocolloid localization in sentinel node biopsy. Ann Surg Oncol. 2000;7(2):77. | ||
Paganelli G, De Cicco C, Cremonesi M, et al. Optimized sentinel node scintigraphy in breast cancer. Q J Nucl Med. 1998;42(1):49–53. | ||
Goldfarb LR, Alazraki NP, Eshima D, Eshima LA, Herda SC, Halkar RK. Lymphoscintigraphic identification of sentinel lymph nodes: clinical evaluation of 0.22-micron filtration of Tc-99m sulfur colloid. Radiology. 1998;208(2):505–509. | ||
Pijpers R, Meijer S, Hoekstra OS, et al. Impact of lymphoscintigraphy on sentinel node identification with technetium-99m-colloidal albumin in breast cancer. J Nucl Med. 1997;38(3):366–368. | ||
Fernandes RS, Mota LG, Kalbasi A, et al. 99mTc-phytate as a diagnostic probe for assessing inflammatory reaction in malignant tumors. Nucl Med Commun. 2015;36(10):1042–1048. | ||
Takei H, Suemasu K, Kurosumi M, et al. 99mTc-phytate is better than 99mTc-human serum albumin as a radioactive tracer for sentinel lymph node biopsy in breast cancer. Surg Today. 2006;36(3):219–224. | ||
Bass SS, Cox CE, Ku NN, Berman C, Reintgen DS. The role of sentinel lymph node biopsy in breast cancer. J Am Coll Surg. 1999;189(2):183–194. | ||
D’Eredita G, Ferrarese F, Cecere V, Massa ST, de Carne F, Fabiano G. Subareolar injection may be more accurate than other techniques for sentinel lymph node biopsy in breast cancer. Ann Surg Oncol. 2003;10(8):942–947. | ||
Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8(10):881–888. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.