Back to Journals » Medical Devices: Evidence and Research » Volume 16
3D Printing in the Fight Against Covid-19
Authors Płatek P , Daniel N, Cieplak K, Sarzyński M , Siemiński P , Sadownik B, Andruszkiewicz P, Wróblewski Ł
Received 11 February 2023
Accepted for publication 16 May 2023
Published 6 July 2023 Volume 2023:16 Pages 167—182
DOI https://doi.org/10.2147/MDER.S406757
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Paweł Płatek,1 Natalia Daniel,1 Kamil Cieplak,1 Marcin Sarzyński,1 Przemysław Siemiński,2 Bartosz Sadownik,3,4 Paweł Andruszkiewicz,3 Łukasz Wróblewski3
1Faculty of Mechatronics, Armament and Aviation, Military University of Technology, Warsaw, Poland; 2Faculty of Automotive and Construction Machinery Engineering, Warsaw University of Technology, Warsaw, Poland; 3 2nd Department of Anaesthesiology and Intensive Care, Medical University of Warsaw, Central Teaching Hospital, Central Teaching Hospital, Warsaw, Poland; 4Department of Descriptive and Clinical Anatomy, Medical University of Warsaw, Warsaw, Poland
Correspondence: Paweł Płatek, Military University of Technology, gen. Sylwestra Kaliskiego 2, Warsaw, 00-908, Poland, Tel/Fax +48 261839657, Email [email protected]
Purpose: The paper describes the design concept and findings from technological and initial clinical trials conducted to develop a helmet for non-invasive oxygen therapy using positive pressure, known as hCPAP (Helmet Continuous Positive Airway Pressure).
Methods: The study utilized PET-G filament, a recommended material for medical applications, along with the FFF 3D printing technique. Additional technological investigations were performed for the production of fitting components. The authors proposed a parameter identification method for 3D printing, which reduced the time and cost of the study while ensuring high mechanical strength and quality of the manufactured elements.
Results: The proposed 3D printing technique facilitated the rapid development of an ad hoc hCPAP device, which was utilized in preclinical testing and treatment of Covid-19 patients, and yielded positive results. Based on the promising outcomes of the preliminary tests, further development of the hCPAP device’s current version was pursued.
Conclusion: The proposed approach offered a crucial benefit by significantly reducing the time and costs involved in developing customized solutions to aid in the fight against the Covid-19 pandemic.
Keywords: 3D printing, fused filament fabrication, Covid-19, SARS-CoV-2, respiratory failure, hCPAP
Graphical Abstract:
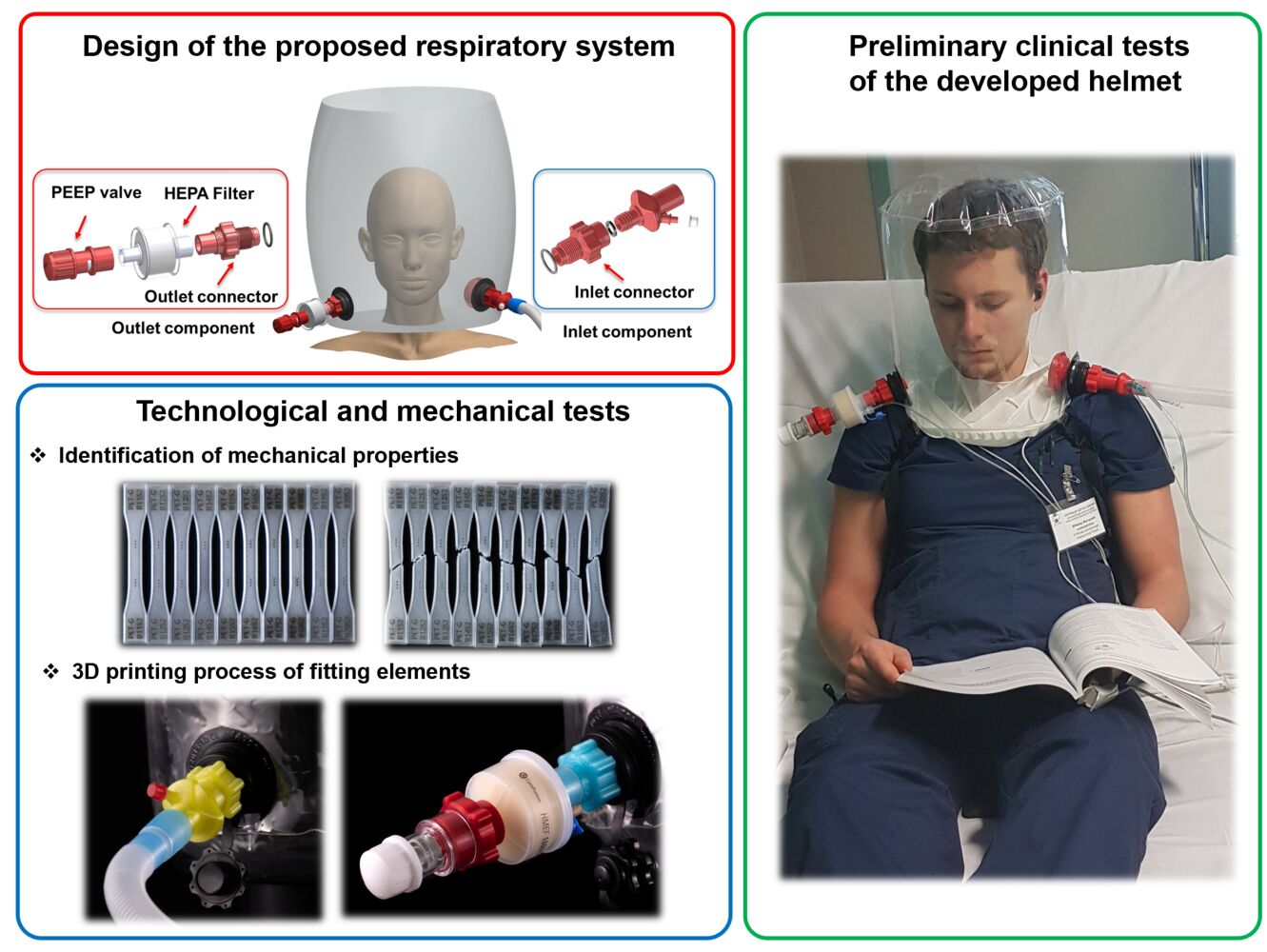
Introduction
Additive Manufacturing (AM), also called 3D printing, is one of the most disruptive technologies that have been invented in the ‘90s.1 A characteristic feature that describes this manufacturing process is the method that describes the way the object is built. Contrary to the standard subtractive technique, the object is created “layer by layer” according to the data defined based on the 3D model.1,2 Over the last three decades, the number of available 3D printing techniques as well as the range of commonly used materials have significantly increased.3,4 The introduction of both new materials5,6 with high mechanical strength and a user-friendly 3D printing manufacturing process resulted in a growing number of research articles related to AM.7,8 In the last two decades, the 3D printing technique has been generally used in the most advanced and demanding branches of industry (such as aviation, aerospace, automotive, mechatronics, and robotics).9 The design freedom it offers allowed for the manufacture of objects with complex geometry that was impossible to obtain with the use of standard subtractive technological approaches. Time and cost-effectiveness are another advantage of the AM technique that allows for increasing workflow during the development and introduction of new cutting-edge products on market. In the last ten years, 3D printing techniques have also started to be used in bioengineering and medical applications.10–12 They have enabled the fabrication of various prostheses, and implants13,14 dedicated to anthropometric characteristics of patients with the use of biocompatible materials.15 Additionally, AM techniques are also used in the fabrication process of medical devices that enhance clinical operations16 as well as in dental applications. A wide range of special materials17,18 dedicated to bio-engineering and medical applications caused this group of manufacturing techniques to also attract the attention of researchers and engineering focused on the bioengineering and medical field. It appears to be a relatively inexpensive and very effective way that allows filling demands arriving in the design process of medical equipment.19
The global coronavirus (SARS-CoV-2) pandemic resulted in respiratory failure that spread throughout the world in 2019 and caused a deep crisis in the healthcare system.20 Explosive progression of coronavirus infection has led to massive hospitalizations and resulted in radically limited access to personal protective equipment (PPE), as well as medical devices used in respiratory failure treatment.21 Social and economic restrictions introduced by many countries quickly affected global capacity shortages and posed a serious risk of total destabilization of healthcare systems. This motivated many engineers and researchers all over the world to find alternative solutions that could be used effectively to solve the problems. Within a few weeks after the pandemic outbreak, social media related to the 3D printing branch published many information demonstrating the potential application of the 3D printing process in the ad hoc manufacturing process of PPEs.22,23 News about other cutting-edge inventions followed soon after: printing face shields,24–26 nasopharyngeal swabs,27 and HEPA filter adapters dedicated to diving masks,28,29 parts and connectors to ventilators as well as low-cost portable pulse oximeter devices.30
The outbreak of the Covid-19 pandemic also affected the Polish healthcare system in March 2020. The number of patients required hospitalization increased dramatically within next few months.31 Furthermore, very quickly the problems with purchasing PPE, as well as ventilators, also affected the Polish healthcare system and caused a serious risk that hospitals will not be able to treat patients with acute hypoxemia.32 This situation was an impulse that led an academic society to undertake projects that allowed one to find alternative solutions to minimize the risk of the collapse of the healthcare system. Taking inspiration from other European academic societies and other social groups integrating enthusiasts of 3D printing techniques, many actions were undertaken in Poland to produce ad hoc PPE (mainly face shields) and other devices (HEPA filter adapters dedicated to diving masks) supporting medical staff in fighting against the Covid-19 pandemic.
The main aim of this paper is to present the results of studies carried out by interdisciplinary research groups that have participated in the development of helmets for oxygen therapy and non-invasive positive pressure ventilation dedicated to the treatment of patients with acute hypoxemia caused by Covid-19. At the beginning, the functional assumptions adopted necessary to carry out the design process are presented. The other section contains the description of 3D CAD models of the proposed fitting elements required to connect the helmet with oxygen lines, CPAP or ventilators. The experimental method of identifying the most effective 3D printing parameters is also presented. The conclusions formulated based on the results of carried out tests are presented in the final part of the paper.
Main Assumption of the Proposed Respiratory System
The main concept of the helmet project undertaken was proposed based on the results of the technical and functional analysis carried out in the field of helmet continuous positive airway pressure (hCPAP) devices. hCPAP is highlighted as an effective treatment method used in the treatment of acute respiratory failure since the 1990s.33 It delivers a constant positive pressure (higher than the atmospheric pressure) with a defined oxygen fraction into the airways during inspiration and expiration. This method is well known based on the results of the treatment of obstructive sleep apnea syndrome.34 Positive pressure during mechanical ventilation is a recognised method of treatment of acute respiratory distress syndrome (ARDS) in intensive care. The utility of CPAP in the treatment of respiratory failure is highly reported during the SARS-CoV-1 pandemic.35 The main benefit of this model in the treatment of acute hypoxemic respiratory failure (AHRF) is that there is no need for tracheal intubation, sedation, and mechanical ventilation.36 Furthermore, the advantage of a helmet interface that is put on the patient’s head, in comparison to other used interfaces, is that it allows for effective isolation of infected patients and enables a significant reduction of the risk of contamination and transmission of infection. One of the most commonly used CPAP devices was described in detail by Amirfarzan et al in33 where the MaxVenturi device was used. The proposed solution allows natural humidification and easy transition to HFNO (High Flow Nasal Oxygenation) during breaks and is a simple and safe configuration that allows the patient to transport. In that solution, helmets were made with armpit braces which keep the helmet in an unambiguous and stable position. The other variant of the CPAP device was characterized in detail by Bibiano-Guillen et al.28 The authors recommended in addition to the application of stationary oxygen source application of a high-pressure hospital circuit or portable oxygen container, where air reaches the mask through a high-flow tube connected to a flowmeter at the outlet of oxygen sources. The mask represents the first generation of Easybreath (Decathlon) assembled for diving (called adapted diving mask, ADM). The connecting parts, such as the adapters used in this variant, were produced by the 3D printing method. The next CPAP system presented by37 poses a transparent nontoxic autoclavable PVC hood, where a silicone rubber collar neck seal is attached to a polypropylene ring. An inhalation port (inlet) and an exhalation port (outlet) are mounted on the hood. To adjust the hood to different patient neck circumferences, an additional silicone rubber seal was used. The ELMO 1.0 is a non-invasive ventilation device that prevents air leakage and droplet dispersion and also provides CPAP. Regarding advantages reported in the literature,35,38 CPAP therapy includes high availability, low cost, no need for high medical competence, no patient-device asynchrony, and low risk of treatment.
The advantages of non-invasive positive pressure oxygen therapy presented above inspired a research group from the Department of Anaesthesiology and Intensive Care of Medical University of Warsaw, Military University of Technology, and Warsaw University of Technology to carry out studies on the development, manufacturing, and testing of helmets with devices enabling high flow and maintenance of positive pressure. Based on the state-of-the-art, it was decided that helmets will be designed in two defined variants presented in schemes below (Figure 1).
 |
Figure 1 Main view of non-invasive oxygen-positive pressure helmet conceptions. |
In the first helmet required condition of treatment, such as positive pressure, the flow of oxygen and air will be defined and controlled by the dedicated ventilator, the second one will be based on existing oxygen hospital lines. According to data published in many research papers, the effective and safe value of positive pressure inside the helmet should range from 5 to 20 cmH20 and gas inflow should be at least 20 lpm to reduce the risk of carbon dioxide cumulation.39 However, considering the limited access to ventilators caused by the pandemic situation, it was assumed that the main application will be obtained using a hospital oxygen installation.
According to the assumed design concept, the helmet will be made from flexible, thermoplastic polyurethane material (one size with approximate diameter 250 mm and height 300 mm) connected with two additional sleeves made from a PTFE material. The first sleeve, placed in the bottom part of the helmet minimizes uncontrolled air leakage by closely fitting to the patient’s neck after being put on. The upper sleeve of the helmet, after rolling and closing by buckle, poses an easy and quick locking solution that guarantees non-air leakage. Furthermore, the upper sleeve allows for quick access to the patient’s head in case of emergency or provides an easy way for patients to feed and provide hygienic care. The proposed PTFE material does not cause an allergic reaction under long contact with the skin.
Characterization of the Developed CAD Model of the Helmet and the Dedicated Fitting System
Based on the formulated functional assumptions, the design process of the fitting elements necessary to connect the helmet to medical devices was carried out. The 3D models of particular parts were developed in the SolidWorks 2020 CAD (Computer-Aided Design) system. Additionally, the shape, dimensions, and geometrical features of the designed elements were adopted to satisfy the requirements of a fused filament fabrication 3D printing technique which was selected to conduct the manufacturing process. The main view of the CAD model of the helmet with designed inlet and outlet subassemblies is presented in Figure 2
 |
Figure 2 Main view of the designed helmet: (a) left-hand view, (b) frontal view, (c) right-hand view. |
Two standard elastic sockets used in the production of inflatable products (eg, mattresses) were proposed to join the inlet and outlet ports with the helmet. The sockets are placed symmetrically in the frontal plane. The inlet subassembly illustrated in Figure 3 consists of an inlet connector that is screwed in a socket with a sealing O-ring and an additional interchangeable element responsible for supplying oxygen and air mixture to the helmet through the oxygen line port and the spiro tube port. The shape and dimension of the spiro tube port were defined according to the geometric requirements defined in the ISO 5356–1:2004 standard.
 |
Figure 3 The view of the inlet port with particular parts. |
Inside the helmet, an additional airflow diffuser part is connected to the inlet port to improve patient tolerance to high gas flow by dispersing the air stream in the helmet. Furthermore, a piston with a cylindrical spring block is placed inside the air diffuser to prevent airflow back to the connected CPAP devices (Figure 4).
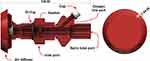 |
Figure 4 Cross-sectional view of the inlet subassembly elements. |
The outlet subassembly is placed on the other side of the helmet (Figure 5). Its main function is to release the breathed air from the helmet. The outlet connector with the O-ring is screwed into the outlet socket port (Figure 6). The HEPA filter that minimizes viral transmission to the environment is tightly connected with the external tip of the connector. The PEEP valve designed by the authors is connected to the HEPA filter. It allows adjusting the pressure value inside the volume of the helmet if there is no automatic system to control the pressure. When the pressure and gas flow are generated and controlled through the standard ventilators, this element is unnecessary.
 |
Figure 5 The view of the outlet port with particular parts. |
 |
Figure 6 Cross-sectional view of the outlet subassembly elements. |
Technological Studies of the Proposed 3D Printing FFF Technique
One of the key issues that had to be considered during the designing process of the connecting fittings was the choice of the most effective manufacturing technique. Taking into account the production of a large batch of designed elements, it is reasonable to use plastic injection moulding technology. Unfortunately, due to the restrictions of the Covid-19 pandemic, the application of the standard approach to the production process was severely limited. The reduced number of technical staff, feedstock shortages, extended supply times and limitations in the production of necessary tools such as injection molds led 3D printing to become an effective ad-hoc method of the manufacturing process. Considering available solutions, a fused filament fabrication (FFF), 3D printing technique seemed to be one of the most effective that can be applied in this situation. FFF is a polymer extrusion-based 3D printing technology. It is most widely used by engineers, researchers, and hobbyists. It has a great number of applications in many industrial branches such as aerospace, automotive, electrical, and electronics, biomedical, food, jewellery, textile and various others.40 Furthermore, it is also used in the medical field in the fabrication of customized products such as implants and surgical tools.11 The FFF technique uses a filament material as a feedstock that is spooled to the extruder mechanism placed in the printing head. The material is heated to the melting temperature of the polymer in the extruder and can be easily deposited through the nozzle on the working table according to the G-code program prepared in dedicated software. The real object is built by depositing layer by layer on the table in a raster pattern according to the 3D printing parameters. Based on the data provided in many papers,41–43 the most essential parameters are nozzle and working table temperature values, the height of the deposited layer of material, the defined number of solid layers, infill parameters like fill density, fill pattern, fill angle, and generation of the support structures. Mentioned parameters affect the geometric quality of manufactured objects and their mechanical properties. They determine the duration of the 3D printing process. Depending on the applied FFF 3D printer, there are a few solutions of the applied kinematic system responsible for the movement of a working head and table. Furthermore, many available 3D printers are equipped with a double nozzle extruder or additional adapter that allows using a few materials at the same time. The popularity of the FFF 3D printers along with user-friendly and cost-effective usage resulted in a growing range of available filaments. Besides typical polymeric materials like ABS (Acrylonitrile Butadiene Styrene) and PLA (Polylactic Acid), there are also other filaments with high mechanical strength like PC (polycarbonate), PEEK (polyether ether ketones), or with high flexibility, like TPU (thermoplastic polyurethane). Furthermore, there are also other groups of filaments, which are characterized by specific chemical, physical, and mechanical properties. They are dedicated to particular applications like, eg, bioengineering and medicine. On the basis of the market survey, it was found that two filaments declared by producers as recommended for medical applications (Polyethylene Terephthalate Glycol-modified PET-G from Devil Design Corp.) and (ABS Medical from Spectrum Filaments Corp.) were available on the Polish market. Taking into consideration the significance of shrinkage sensitivity of the ABS materials during the 3D printing process, the authors decided that the designed connecting fitting elements will be manufactured from PET-G filament. Furthermore, the 3D printing task was carried out with the use of Prusa i3 MK3S FFF 3D printers (form Prusa Corp.).
Identification of the 3D Printing Parameters of PET-G Suitable for the FFF Technique
The PET-G material can be applied in the production of medical elements that are foreseen to be in contact with human skin and food. Furthermore, it demonstrates a high mechanical strength (ultimate strength approx. 40 MPa), and it does not require special 3D printing conditions, as well as additional post-processing. Its chemical composition does not favor the accumulation and multiplication of bacteria and viruses. The fitting element fabrication process has proceeded with additional technological tests carried out to identify the most effective 3D printing parameters of PET-G that would guarantee the high geometric quality, homogeneity of the material structure, and high mechanical strength. Table 1 lists the main 3D printing parameters recommended by the manufacturer of the used filament.
 |
Table 1 PET-G 3D Printing Parameters Recommended by the Devil Design Filament Producer |
Nevertheless, it is difficult to state if these values are optimized and enable us to meet assumed requirements. For this reason, the authors proposed their technological studies. Based on suggestions presented in many papers, there is a close relationship between the geometrical quality of a 3D printed object, such as surface roughness and its mechanical strength.44 The singular layer of the specimen manufactured by the FFF technique consists of many parallel strands that are defined according to the specified pattern. The internal structure of the object is similar to the composite structure where the subsequent layers of the material are defined in different angular orientations, eg, 45 and −45 degrees. The main goal of the 3D printing parameter identification process refers to the proper deposition of strands and layers to avoid the presence of small gaps and voids that result in the structure porosity and finally on the surface layer roughness. The method proposed by the authors assumed that the value of the extruder temperature and the so-called default extrusion width would be changed where the other parameters were constant to minimize the number of variables. The rectangular shape specimen with dimension presented in Figure 7 was proposed to carry out tests aimed at defining the relation between top surface roughness and 3D printing process parameters.
 |
Figure 7 Geometry of samples used in roughness measurements. |
Based on the result of roughness measurements, the proper values of parameters subjected to evaluation were identified. For all the tested variants considered, the heat bed temperature was defined as 80 °C, the printing speed was 50 mm/s and the following retraction parameters (1 mm and 45 mm/s) were adopted. The layer height significantly affects the roughness of the side surfaces, as well as the printing time. Therefore, it was assumed that the layer height should be defined as 0.2 mm (equal to the radius value of the nozzle). This value is commonly used and recommended in many manuals related to the 3D printing process because it guarantees a good ratio between high surface quality and the 3D printing time process. Proposed technological studies were carried out based on the measurement results of 36 specimens manufactured with different nozzle temperatures and different default extrusion width values. Roughness measurements Ra and Rz were performed with the use of a Waveline W20 profilometer (Jenoptik, Germany). The results obtained are presented in Table 2 and Table 3. Furthermore, to better analyze the relationship between the parameters studied and their effect on the top surface quality of the specimen, the results were presented graphically on charts (Figure 8).
 |
Table 2 Results of Ra Roughness Measurements of 3D Printed PET-G Material Samples with Different Nozzle Temperature and Default Extrusion Width Values |
 |
Table 3 Results of Rz Roughness Measurements of 3D Printed PET-G Material Samples with Different Nozzle Temperature and Default Extrusion Width Values |
 |
Figure 8 Results of the toughness tests of PET-G specimens manufactured with the use of different 3D printing parameters. |
The main goal of the analysis performed was to identify the parameters that resulted in the minimum value of the roughness of the top surface (Ra and Rz). On the basis of the obtained results, it was found that the optimal values of the parameters studied for PET-G material were as follows: nozzle temperature 240 ℃ and default extrusion width 0.47 mm. They were used in further 3D printing works related to the manufacturing of tensile test specimens and manufacturing elements of the designed fitting system. When the identified value of the nozzle temperature was compared with the one recommended by the producer of the PET-G filament, it could be stated that it is within the proposed temperature range. However, this value can differ depending on the applied 3D printer (distributed by the other producers) and for a case where different nozzle sizes will be used.
Characterization of the Mechanical Properties of the 3D Printed PET-G Material
The subsequent stage of the conducted studies was related to the characterization of the mechanical properties of 3D printed PET-G material. Tensile tests were performed with the use of a standard dog bone material sample (ASTM E466: 96). The shape and dimensions are generally recommended to conduct creep fatigue tests; nevertheless, in many research papers, this standard is proposed to carry out studies related to the mechanical characterization of 3D printed thermoplastic materials. Detailed information on the shape and dimensions of the specimen is presented in Figure 9. They were manufactured only in one orientation according to the 0Z direction. Before tensile tests, all samples (10 pieces) were marked with the FIBER ATMS 2020 PRO laser marker (ATM Solution, Poland). This operation allowed for a precise definition of measurement base points on the top surface of specimens and describing the identification notation. Furthermore, a Baty Venture XT multisensory optical measurement machine (Bowers, UK) was used to verify the geometric quality control (Figure 10) of marked reference points. Tensile tests were carried out with the use of a universal strength machine MTS Criterion C 45.105 (MTS, USA). Additionally, a Canon EOS R (Canon, Japan) digital camera with a 200 mm macro lens was utilized to register the sample deformation process. The tests were repeated 10 times with the same deformation velocity equal to 1 mm/s. Based on the obtained movies, a precise estimation of specimen elongation was possible in the TEMA 2022 Classic software (Image system, Sweden). The main view of the applied laboratory stand used in the tensile tests is presented in Figure 11.
 |
Figure 9 The main view of the ASTM E466:96 tensile test specimen (a) Shape and dimensions, (b) Orientation of manufacturing (c) Real specimens used in tests. |
 |
Figure 11 The main view of the laboratory stand used to perform the tensile tests of PET-G material. |
Based on the tensile tests performed, the engineering stress–strain curves were defined (Figure 12) and the main mechanical parameters were estimated (Table 4). Comparing obtained results of determined mechanical properties with the available data published online, it can be stated that the adopted parameters of the 3D printing process ensure a high strength that is equal or even higher than the data provided by producers of different types of FFF 3D printers. Furthermore, the proposed parameters enable the surface roughness to be minimized, which is an important issue in terms of medical device production.
 |
Table 4 The Mechanical Properties of PET-G are Estimated on the Basis of the Results of the Tensile Tests |
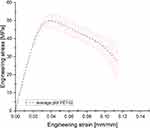 |
Figure 12 The average stress–strain curve with standard deviation determined on the basis of the results of the tensile tests. |
Manufacturing Process of Fitted Elements with the Use of the 3D Printing FFF Technique
The subsequent stage of the studies was related to the manufacturing process of the fitting elements which enables the connection of the helmet to the different configurations of respiratory devices. This task was carried out with the use of Prusa i3 MK3S 3D printers and the PET-G filament tested. The information about the geometry of particular elements was exported to the *.stl file in the SolidWorks 2020 CAD system. Subsequently, these data were imported into Prusa Slicer software, where the parameters of the 3D printing process established based on the previous identification process were defined. The Prusa Slicer software enabled preparing the G-code file that is required to perform the 3D printing process. The main idea of preparing the G-code file is presented in Figure 13. Depending on the user’s skills and experience, it is possible to select one of the three different modes: simple, advanced, and expert. The first one allows for defining the model orientation on the working table, selection of the type of material which is used to fabricate the object, definition of the temperature values of the printing nozzle and working table, and choice of the model infill (type and density ratio). The advanced and expert modes give additional functionality related to the definition of layer option (eg, seam contour position, material flow rate) and other extra options referring to support structure definition, and more advanced functions of print motion, velocity, and acceleration control.
The proposed threaded joints between the fitting elements and the helmet required the application of additional support structures during the 3D printing process. They were defined automatically where the angle of the overhanging surfaces of the geometry was higher than 45 degrees. These supports affect the time of the 3D printing process and require additional post-processing to remove them. Nevertheless, the available options for support structure definition in Prusa Slicer software, caused by the post-processing, were not time-consuming and difficult. Furthermore, to improve the screwing in parts together, the threaded elements of the inlet and outlet connectors were scaled down (99% in the 0XY direction). Figure 14 illustrates the 3D printing process during the execution of the task and the view of fabricated parts at the end of the process. The low-density thin-walled support structures and the relatively high stiffness of the PET-G material allowed easy separation of the support material structure from the manufactured parts with the use of a filament cutter (Figure 15). Based on the assumed technological assumptions, the authors did not use any other additional mechanical operation to prepare fitting elements for further tests. Particular elements, including inlet and outlet adjustment subassemblies, were mounted together to assess their geometrical correctness and dimensional tolerances (Figure 16). The inlet sub-assembly allowed the helmet to be connected to the silicone breathing tube and oxygen line. The outlet subassembly was connected with a standard HEPA filter and an optional PEEP valve that was planned to be used in the case of respiratory device shortage.
 |
Figure 14 The main view of the manufacturing process of the fitting elements (a) during the 3D printing process, (b) the final result. |
 |
Figure 15 The main view of 3D printed fitting elements (a) before, (b) after removing support structures. |
 |
Figure 16 The main view of the inlet and outlet fitting elements mounted in the helmet ports. |
Preliminary Clinical Tests of the Developed Helmet
Helmets made of polyurethane and equipped with 3D printed fitting elements were subjected to preliminary tests. They were conducted with the approval of the Bioethics Committee of the Medical University of Warsaw, Approval reference number: 177/2020, Date of approval: 09/11/2020. Both subassemblies presented in the figure (Figure 16) were connected to the helmet and placed on the medical phantom to validate the adopted functional and exploitation assumptions (Figure 17a). First attempts were made to verify the airtightness between the connected fitting elements. The most critical issue was to provide a proper screw connection between the helmet port and 3D printed connectors. The application of additional sealing enabled the solution of this problem. Then, different values of pressure (5–20 cm H2O) and airflow (15–60L/min) were applied according to recommendations related to treatment of acute respiratory failure. The positive results of the tests allowed us to perform preliminary clinical tests with 10 volunteers (medical students) who signed agreement that they declare participation in tests (Figure 17b) and provided content for publishing their images (Figure 17b and c). During the tests, the concentration of O2, CO2, as well as temperature, humidity, and noise with the use of different types of air diffusers, flows and pressure were evaluated. Based on the results obtained, it was decided that the tests with the first group of patients will be carried out with the use of an oxygen flow of 30 L/m and a pressure ranging from 5 to 20 cm H2O depending on the stage of respiratory failure. The first group of participants included 30 volunteers-patients. All of them were infected with Covid-19, developed respiratory failure, and needed hospitalization in the ICU (Figure 17c). According to recommendations, they were qualified to oxygenation with positive pressure because passive oxygenation was not adequate. The application of the developed helmet with CPAP and oxygen allowed achieving the goal of therapy defined as increasing oxygen saturation (SpO2) up to at least 92% during the first hour. The mean time of treatment was 5 days, and the longest time of observation was 19 days. No complications related to helmet use were recorded.
Conclusions
The outbreak of the global Covid-19 pandemic will be remembered in history as one of the significant challenges that affected humanity in the 21st century. The necessity of staying in isolation and quarantine caused that in a short time we witnessed everydays’ product shortages due to logistic chain breakdowns and drastically reduced production. Furthermore, the growing number of Covid-19 patients put enormous pressure on the global healthcare system. In such a scenario many ad hoc initiatives have been carried out to support health care and minimize the negative results of the pandemic. In this paper, we present our practical solution of application of the 3D printing technique in manufacturing elements of medical equipment which can be used in respiratory failure. Based on the carried out project, the following conclusions can be drawn:
- the developed helmet solution is versatile and can be used in treating patients with respiratory failure. The solution can incorporate a wide range of medical devices, such as ventilators, CPAP machines, and oxygen lines. Additionally, the helmet reduces the risk of virus transmission.
- the geometry of the fitting elements was parametrized, allowing for easy adaptation to other healthcare port standards.
- 3D printing was used to manufacture the fitting elements, significantly reducing the production time. Concept design to initial testing took only a few months, and different design concepts were evaluated quickly thanks to 3D printing.
- Fused Filament Fabrication (FFF) 3D printing was used and proved to be one of the most versatile and user-friendly techniques. The designed parts can be produced using other FFF 3D printers and PET-G filaments.
- the authors’ method of identifying 3D printing parameters results in high strength and geometrical quality. However, depending on the 3D printer and PET-G filament used, some discrepancies may arise.
- the PET-G filament selected by the authors is commercially available and is specifically designed for contact with food and human skin.
- clinical tests with student volunteers and Covid-19 infected patients showed that hCPAP with the developed helmet was a safe and effective respiratory failure treatment. Oxygen saturation of at least 92% was achieved during the first hour of therapy.
Further Reading
Disclosure
Łukasz Wroblewski, Paweł Andruszkiewicz, and Bartosz Sadownik report financial support was provided by Polish Ministry of Science and Higher Education, National Centre for Research and Development (grant NCBR 52/2020). The authors declare that they have no other competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Prakash KS, Nancharaih T, Rao VVS. Additive manufacturing techniques in manufacturing -an overview. Mater Today Proc. 2018;5(2):3873–3882. doi:10.1016/j.matpr.2017.11.642
2. Singh S, Ramakrishna S, Singh R. Material issues in additive manufacturing: a review. J Manuf Process. 2017;25:185–200. doi:10.1016/j.jmapro.2016.11.006
3. Li N, Huang S, Zhang G, et al. Progress in additive manufacturing on new materials: a review. J Mater Sci Technol. 2019;35(2):242–269. doi:10.1016/j.jmst.2018.09.002
4. Valino AD, Ryan J, Dizon C, et al. Progress in polymer science advances in 3D printing of thermoplastic polymer composites and nanocomposites. Prog Polym Sci. 2019;98:101162. doi:10.1016/j.progpolymsci.2019.101162
5. Ngo TD, Kashani A, Imbalzano G, Nguyen KTQ, Hui D. Additive manufacturing (3D printing): a review of materials, methods, applications and challenges. Compos Part B Eng. 2018;143:172–196. doi:10.1016/j.compositesb.2018.02.012
6. Herzog D, Seyda V, Wycisk E, Emmelmann C. Additive manufacturing of metals. Acta Mater. 2016;117:371–392. doi:10.1016/j.actamat.2016.07.019
7. Yuan S, Shen F, Chua CK, Zhou K. Polymeric composites for powder-based additive manufacturing: materials and applications. Prog Polym Sci. 2019;91:141–168. doi:10.1016/j.progpolymsci.2018.11.001
8. Buchanan C, Gardner L. Metal 3D printing in construction: a review of methods, research, applications, opportunities and challenges. Eng Struct. 2019;180:332–348. doi:10.1016/j.engstruct.2018.11.045
9. Singh R, Gupta A, Tripathi O, et al. Powder bed fusion process in additive manufacturing: an overview. Mater Today Proc. 2019;26:3058–3070. doi:10.1016/j.matpr.2020.02.635
10. Palmara G, Frascella F, Roppolo I, Chiappone A, Chiadò A. Functional 3D printing: approaches and bioapplications. Biosens Bioelectron. 2021;175:112849. doi:10.1016/j.bios.2020.112849
11. Mohd Pu’ad NAS, Abdul Haq RH, Mohd Noh H, Abdullah HZ, Idris MI, Lee TC. Review on the fabrication of fused deposition modelling (FDM) composite filament for biomedical applications. Mater Today Proc. 2020;29:228–232. doi:10.1016/j.matpr.2020.05.535
12. Haryńska A, Carayon I, Kosmela P, et al. A comprehensive evaluation of flexible FDM/FFF 3D printing filament as a potential material in medical application. Eur Polym J. 2020;138:109958. doi:10.1016/j.eurpolymj.2020.109958
13. Javaid M, Haleem A. Additive manufacturing applications in orthopaedics: a review. J Clin Orthop Trauma. 2018;9(3):202–206. doi:10.1016/j.jcot.2018.04.008
14. Dall’Ava H, Di L, Henckel H. 3D Printed acetabular cups for total hip arthroplasty: a review article. Metals. 2019;9(7):729. doi:10.3390/met9070729
15. Petersmann S, Spoerk M, Van De Steene W, et al. Mechanical properties of polymeric implant materials produced by extrusion-based additive manufacturing. J Mech Behav Biomed Mater. 2020;104:103611. doi:10.1016/j.jmbbm.2019.103611
16. Zabaleta J, Aguinagalde B, López I, et al. Creation of a multidisciplinary and multicenter study group for the use of 3D printing in general thoracic surgery: lessons learned in our first year experience. Med Devices Evid Res. 2019;12:143–149. doi:10.2147/MDER.S203610
17. Tang Y, Zhang Y, Meng Z, et al. Accuracy of additive manufacturing in stomatology. Front Bioeng Biotechnol. 2022;10:1–12. doi:10.3389/fbioe.2022.964651
18. Chia HN, Wu BM. Recent advances in 3D printing of biomaterials. J Biol Eng. 2015;9(1):1–14. doi:10.1186/s13036-015-0001-4
19. Berard D, Navarro JD, Bascos G, et al. Novel expandable architected breathing tube for improving airway securement in emergency care. J Mech Behav Biomed Mater. 2021;114:104211. doi:10.1016/j.jmbbm.2020.104211
20. Sharma A, Tiwari S, Deb MK, Marty JL. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): a global pandemic and treatment strategies. Int J Antimicrob Agents. 2020;56(2):106054. doi:10.1016/j.ijantimicag.2020.106054
21. Vordos N, Gkika DA, Maliaris G, et al. How 3D printing and social media tackles the PPE shortage during Covid – 19 pandemic. Saf Sci. 2020;130:104870. doi:10.1016/j.ssci.2020.104870
22. François PM, Bonnet X, Kosior J, Adam J, Khonsari RH. 3D-printed contact-free devices designed and dispatched against the COVID-19 pandemic: the 3D COVID initiative. J Stomatol Oral Maxillofac Surg. 2020;122:381–385. doi:10.1016/j.jormas.2020.06.010
23. Jafferson J, Pattanashetti S. Use of 3D printing in production of personal protective equipment (PPE) - a review. Mater Today Proc. 2021;46:1247–1260. doi:10.1016/j.matpr.2021.02.072
24. Xu R, Yang L, Qin Z. Design, manufacture, and testing of customized sterilizable respirator. J Mech Behav Biomed Mater. 2022;131:105248. doi:10.1016/j.jmbbm.2022.105248
25. Manero A, Smith P, Koontz A, et al. Leveraging 3D printing capacity in times of crisis: recommendations for COVID-19 distributed manufacturing for medical equipment rapid response. Int J Environ Res Public Health. 2020;17(13):1–17. doi:10.3390/ijerph17134634
26. Tareq MS, Rahman T, Hossain M, Dorrington P. Additive manufacturing and the COVID-19 challenges: an in-depth study. J Manuf Syst. 2021;60:787–798. doi:10.1016/j.jmsy.2020.12.021
27. Manoj A, Bhuyan M, Raj Banik S, Ravi Sankar M. 3D printing of nasopharyngeal swabs for COVID-19 diagnose: past and current trends. Mater Today Proc. 2020;44:1361–1368. doi:10.1016/j.matpr.2020.11.505
28. Bibiano-Guillen C, Arias-Arcos B, Collado-Escudero C, et al. Adapted Diving Mask (ADM) device as respiratory support with oxygen output during COVID-19 pandemic. Am J Emerg Med. 2021;39:42–47. doi:10.1016/j.ajem.2020.10.043
29. Pedraja J, Maestre JM, Rabanal JM, Morales C, Aparicio J, Del Moral I. Role of 3D printing in the protection of surgical and critical care professionals in the COVID-19 pandemic. Rev Española Anestesiol y Reanim. 2020;67(8):417–424. doi:10.1016/j.redare.2020.10.001
30. Nemomssa HD, Raj H. Development of low-cost and portable pulse oximeter device with improved accuracy and accessibility. Med Devices Evid Res. 2022;15:121–129. doi:10.2147/MDER.S366053
31. Dar M, Swamy L, Gavin D, Theodore A. Mechanical-ventilation supply and options for the COVID-19 pandemic leveraging all available resources for a limited resource in a crisis. Ann Am Thorac Soc. 2021;18(3):408–416. doi:10.1513/AnnalsATS.202004-317CME
32. Mularczyk-Tomczewska P, Zarnowski A, Gujski M, et al. Barriers to accessing health services during the COVID-19 pandemic in Poland: a nationwide cross-sectional survey among 109,928 adults in Poland. Front Public Heal. 2022;10:986996. doi:10.3389/fpubh.2022.986996
33. Amirfarzan H, Cereda M, Gaulton TG, et al. Use of Helmet CPAP in COVID-19 – a practical review. Pulmonology. 2021;27:413–422. doi:10.1016/j.pulmoe.2021.01.008
34. Johnson KG, Johnson DC. Treatment of sleep-disordered breathing with positive airway pressure devices: technology update. Med Devices Evid Res. 2015;8:425–437. doi:10.2147/MDER.S70062
35. Czajkowska-Malinowska M, Kania A, Kuca P, et al. Treatment of acute respiratory failure in the course of Covid-19. Practical hints from the expert panel of the assembly of intensive care and rehabilitation of the Polish respiratory society. Adv Respir Med. 2020;88(3):245–266. doi:10.5603/ARM.2020.0109
36. Borg U, Aviano J, Ginani M, Li K. Evaluation of common nasal cannulas in neonatal noninvasive ventilation (NIV) using a novel neonatal nasal model. Med Devices Evid Res. 2022;15:307–315. doi:10.2147/MDER.S374418
37. Holanda MA, Tomaz BS, Menezes DGA, Lino JA, Gomes GC. ELMO 1.0: a helmet interface for CPAP and high-flow oxygen delivery. J Bras Pneumol. 2021;47(3):e20200590. doi:10.36416/1806-3756/e20200590
38. Davidson AC. Continuous positive airway pressure. Non-Invasive Vent Weaning Princ Pract. 2010;2010:30–35.
39. Carron M, Freo U, Bahammam AS, et al. Complications of non-invasive ventilation techniques: a comprehensive qualitative review of randomized trials. Br J Anaesth. 2013;110(6):896–914. doi:10.1093/bja/aet070
40. Singh S, Singh G, Prakash C, Ramakrishna S. Current status and future directions of fused filament fabrication. J Manuf Process. 2020;55:288–306. doi:10.1016/j.jmapro.2020.04.049
41. Masood SH. Advances in fused deposition modeling. Compr Mater Process. 2014;10:69–91. doi:10.1016/B978-0-08-096532-1.01002-5
42. Tajik AR, Khan TI, Parezanovic V. Raster angle impact on fdm-based additive manufactured fluidic oscillator. SSRN Electron J. 2022. doi:10.2139/ssrn.4178239
43. Qamar Tanveer M, Mishra G, Mishra S, Sharma R. Effect of infill pattern and infill density on mechanical behaviour of FDM 3D printed Parts- a current review. Mater Today Proc. 2022;62:100–108. doi:10.1016/j.matpr.2022.02.310
44. Fischer D, Eßbach C, Schönherr R, Dietrich D, Nickel D. Improving inner structure and properties of additive manufactured amorphous plastic parts: the effects of extrusion nozzle diameter and layer height. Addit Manuf. 2022;51. doi:10.1016/J.ADDMA.2022.102596
45. Polymers P. Prusament PETG Technical Data Steet. Available from: https://prusament.com/media/2020/01/PETG_TechSheet_ENG.pdf.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



