Back to Journals » Journal of Pain Research » Volume 11
10 kHz spinal cord stimulation: a retrospective analysis of real-world data from a community-based, interdisciplinary pain facility
Authors DiBenedetto DJ , Wawrzyniak KM , Schatman ME , Kulich RJ , Finkelman M
Received 26 September 2018
Accepted for publication 2 November 2018
Published 20 November 2018 Volume 2018:11 Pages 2929—2941
DOI https://doi.org/10.2147/JPR.S188795
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor E Alfonso Romero-Sandoval
David J DiBenedetto,1,2 Kelly M Wawrzyniak,1,2 Michael E Schatman,1,3 Ronald J Kulich,2,4 Matthew Finkelman5
1Boston PainCare, Waltham, MA, USA; 2Department of Diagnostic Sciences, Tufts School of Dental Medicine, Boston, MA, USA; 3Department of Public Health and Community Medicine, Tufts School of Medicine, Boston, MA, USA; 4Department of Anesthesia Critical Care and Pain Medicine, Harvard Medical School/Massachusetts General Hospital, Boston, MA, USA; 5Division of Biostatistics and Experimental Design, Tufts School of Dental Medicine, Boston, MA, USA
Objective: To evaluate clinical outcomes and health care utilization at 12 months post spinal cord stimulator (SCS) implantation compared with baseline and a matched sample of patients receiving conventional medical management (CMM) for the treatment of low back and lower extremity pain.
Patients: A retrospective study of patients with at least 24 months of active treatment at an interdisciplinary community pain center between December 1, 2014 and December 31, 2017. Thirty-two patients receiving implantation of a high-frequency (10 kHz) SCS and 64 patients receiving CMM were identified through propensity matching at a ratio of 2:1.
Methods: Data were extracted from medical records, including pain severity, prescribed opioid dose in morphine milligram equivalents, patient perception of disability, and volume of interventional pain procedures and total office visits to the pain center.
Results: Reductions in opioid dose were significantly greater for the SCS group than the CMM group. The 26.2 mg morphine equivalent dose reduction represents a 28% reduction from baseline, with 71.4% of those prescribed opioids in the SCS group reducing their dose at 12 months post-implant. Among those with SCS, there were significant within-group reductions in numerical pain score for low back and lower extremity pain, reducing by 46.2% and 50.9% from baseline, respectively. Change in functional pain score was not significant for either SCS group or CMM. Both groups had significant within-group reduction in disability. Reduction of interventional procedure volume was significant for both groups with a greater reduction observed in the SCS group. Office visit volume reduction was significant for the CMM group, but this was not a significant difference from the SCS group.
Conclusions: Results support the efficacy of 10 kHz SCS for analgesia, reduction of opioid utilization, reduction of interventional pain procedures, and patient perception of disability.
Keywords: high-frequency (10 kHz) spinal cord stimulation, analgesia, opioids, disability, economic variables
Corrigendum for this paper has been published
Introduction
Recent advancements in the field of neuromodulation have yielded significant improvements in treatment outcomes and have expanded the application of spinal cord stimulation (SCS) treatment to a wider range of chronic pain patients. Primary among these advancements has been the development of high-frequency SCS. Clinical investigation of 10 kHz SCS was first reported in an abstract in 2010,1 in which the authors reported not only significant reductions of radicular lower extremity pain, but of axial back pain and self-reported disability scores as well. In 2013, van Buyten et al2 published similar findings, and noted fewer self-reported awakenings from sleep and a reduction in those using opioid analgesics.
Al-Kaisy et al3 were the first to report on the sustained effectiveness of 10 kHz SCS in 2014, examining outcomes from a cohort of patients with chronic low back pain 24 months after undergoing implantation at two large treatment centers in Europe. In addition to findings of decreased low back pain at 6 and 24 months post-implantation, the authors reported sustained decreases in perceived disability and opioid dose. In 2015, the results of the US non-inferiority study required for US Food and Drug Administration (FDA) approval of 10 kHz SCS, and the first to compare two SCS technologies in a pivotal study, were published.4 This multicenter, randomized, controlled trial demonstrated superiority in terms of opioid dose reduction and relief of low back and lower extremity pain at 3 and 12 months post-implantation when compared with a commonly used low-frequency stimulator. The 10 kHz SCS treatment was also associated with greater improvements in quality of life measures examining function, pain interference, patient satisfaction and sleep.5 A follow-up study6 published in 2016 by these authors examined 24 months of data and reported results similar to those from the original study in terms of improved pain ratings, self-reported disability scores, and patient satisfaction from 10 kHz SCS compared with traditional low-frequency SCS.
Although the results of these investigations are promising, substantiation in a real-world setting, outside the confines of a clinical study protocol, is needed. As with any large-scale health care investigation, generalizability remains a barrier in the absence of investigations that are conducted within the standards of practice in which patients are routinely managed. Moreover, despite the well-documented positive impact of interdisciplinary care on chronic pain treatment outcomes,7,8 the role of integrated care is rarely addressed in SCS investigations.
Finally, given the US health care system’s shift in focus from fee for service or capitated payment models to value-based care, the assessment of both efficacy and cost with regard to emerging health care treatments and technologies is critical.9,10 Encouragingly, the initial investigation of the cost-effectiveness of 10 kHz SCS published in 201411 demonstrated a favorable incremental cost-effectiveness ratio when compared with conventional medical management (CMM). However, this study was conducted in Europe, and therefore, these results may not generalize to settings without socialized medical systems.
This investigation presents retrospective treatment outcome data from a population of chronic low back pain patients evaluated and treated in a community-based interdisciplinary care setting in which treatment outcome expectations, functional goal setting, and targeted opioid reduction are addressed throughout treatment. The primary aim of this study was to evaluate the impact of 10 kHz SCS in addition to CMM on analgesia and perceived disability at 12 months post-implant compared with a matched sample of patients receiving CMM only. Secondary study aims were to examine changes in health care utilization, including the use of opioid medications, in patients receiving 10 kHz SCS in addition to CMM compared with those receiving CMM only.
Methods
A retrospective records review was performed using data from patients with chronic low back pain with or without radicular lower extremity pain, treated at one community-based, interdisciplinary pain management center between December 1, 2014 and December 31, 2017. The Tufts University Health Sciences Institutional Review Board reviewed the protocol and determined that this study was not research involving human subjects as defined by Department of Health and Human Services and FDA regulations. Thirty-two subjects underwent implantation of a 10 kHz SCS unit (Senza™ system, Nevro Corp., Redwood City, CA, USA) during this study period, had been active patients of the pain center for at least 12 months prior to and after the date of implant, and had not had an explant of the 10 kHz SCS during the study period. These subjects were referred to as the SCS + CMM group. A matched cohort of 274 patients met the criteria of having a diagnosis of chronic low back pain with or without radicular lower extremity pain, engaged in treatments for at least 24 months during this same study period, and did not have an implanted SCS during this time period. These subjects were referred to as the CMM group. To ensure an adequately matched sample, a propensity-matching technique was used with a ratio of 2:1 to identify a CMM group of 64 subjects to be included in data analysis, further described in the Statistical Analyses section.
Prior to 10 kHz SCS implant, patients were evaluated by an interventional anesthesiologist to determine medical appropriateness, and then evaluated by a pain psychologist. Self-report screening measures were completed as part of the evaluation process. Since this was a retrospective investigation, selection of measures was developed over time and not all measures were administered to all subjects at their 10 kHz SCS evaluations. The collateral treating clinicians (eg, outpatient psychiatrist and referring surgeon) were consulted when this was indicated to aid in the evaluation. Patients who were determined to likely benefit from 10 kHz SCS were recommended to proceed to trial for 7–10 days. Collaborative meetings were scheduled with the patient, treating pain physician, nurse practitioner, and psychologist to establish specific functional goals, opioid reduction targets, and determine any barriers to adherence or success. At the end of the trial, the pain physician assessed pain relief and functional improvement to determine if the patients met criteria of at least 50% reduction in pain and marked improvement in function. All patients who proceeded to permanent implantation were asked to follow-up at least every 6 months post-implant with the pain physician to monitor progress with the 10 kHz SCS device, administer the screening measures to monitor psychosocial and functional domains, ensure compliant use of 10 kHz SCS, assess self-reported pain, and review functional goals.
Data collection
Sociodemographic data included marital status, employment status, tobacco use, gender, and age at the earliest time point in the study period for each subject.
Opioid dose
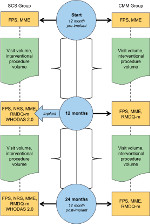
Daily opioid dose in morphine milligram equivalent (MME) was extracted at three time points for all subjects (Figure 1). For those with SCS + CMM, 12 months pre-implant, date of implant, and 12 months post-implant were used. For those receiving CMM, the start, 12- and 24-month time points during the study period were used. MME was calculated using the conversions developed and published by the Washington State Agency Medical Directors’ Group.12 MME at 12 months pre-implant/start of study period was used for propensity matching and a dose of 0 MME represents someone who was not prescribed a daily opioid dose in the pain center’s opioid prescribing program, a structured program to closely monitor safety and effectiveness of chronic opioid therapy. For those subjects who did participate in the center’s opioid prescribing program, change in opioid dose was calculated by subtracting MME at 12 months post-implant from time of implants for the SCS + CMM group and subtracting MME at month 24 from month 12 for the CMM group.
Visit volume
Office visit volume and interventional procedure volume were calculated by counting the total number of visits and procedures, respectively, over the time period of 12 months pre-implant to the time of implant and again from the time of implant to 12 months post-implant for the SCS + CMM group, and for those with CMM using totals of start date to month 12, and month 12 to month 24 (Figure 1). Office visits included all evaluations and follow-ups across providers at the pain center (eg, medical follow-up visits, appointments for minor office procedures, such as trigger point or bursa injections, behavioral medicine visits, and physical therapy) excluding routine medication management follow-up visits and visits related to the 10 kHz SCS device (eg, evaluation, follow-up, and programming follow-ups). Interventional procedures included epidural steroid injections, facet joint injections, radiofrequency ablations, and major joint injections and excluded 10 kHz SCS trial visits.
Self-report measures
Pain severity was measured using two different scales. A Functional Pain Scale (FPS) is administered at each office visit for all patients at the pain center. This scale is anchored at 0 representing “no pain” and 10 representing “worst imaginable pain.” Each number on this 11-point scale has a functional description; for example, a 3 on the scale is “Pain that is starting to affect your ability to perform the current activity,” and a 7 on the scale is “You cannot use or move the painful area. You have difficulty talking and concentrating on anything but the pain.” While initially validated only among the elderly,13 a recent study14 reported adequate reliability and validity of a modified version in a sample of patients with chronic pain. FPS data were extracted at three time points for all subjects and the initial point value was used for propensity matching (Figure 1). For those with SCS + CMM, 12 months pre-implant, date of implant, and 12 months post-implant were used. For subjects in the CMM group, the start, 12-month, and 24-month time points during the study period were used. Change in FPS severity was calculated by subtracting FPS rating at 12 months post-implant from time of implants for the SCS + CMM group and subtracting FPS rating at month 24 from month 12 for the CMM group.
The second pain severity measure used with only 10 kHz SCS implant patients was a numeric rating scale (NRS) to assess low back pain and lower extremity pain at the time of 10 kHz SCS implant and at 12 months post-implant. The NRS requires patients to report their current level of pain on an 11-point Likert scale, with 0 representing “no pain” and 10 representing “worst pain possible”. Although widely used for many years in pain medicine, a 2007 review noted that it was not developed or validated as a measure of pain.15 Test–retest reliability has been found only to be fair, although responsiveness in treated patients with chronic pain was determined to be “adequate”.16 A more recent study of low back and radiating lower extremity patients17 failed to demonstrate its construct validity. However, it remains the standard measure in pain outcomes studies, including those evaluating SCS. Change in NRS rating was calculated by subtracting NRS rating at 12 months post-implant from those at the time of implants for the SCS + CMM group.
The pain catastrophizing scale18 was administered at the time of 10 kHz SCS evaluation. Each of the 13 items is a statement exemplary of a catastrophizing thought, and the patients are asked to rate the degree to which the item was true for them using a 5-point Likert scale anchored at 0 representing “not at all” and 4 representing “all the time”. Total scores range from 0 indicating no pain catastrophization to 52 indicating the most severe pain catastrophization possible, with a score of ≥30 indicating a clinically relevant level of catastrophizing.19 The factor structure, reliability, and validity of the measure have been empirically established in the pain patient population,20 and the assessment tool has already been used in a study of high-frequency SCS.21
The Patient Health Questionnaire (PHQ)-922 is a 9-item measure of depression severity that was administered at the time of 10 kHz SCS evaluation, the validity and reliability of which were established and confirmed through numerous studies. It has been widely used in investigations of chronic pain patients,23 including those examining chronic pain patients receiving opioid therapy24 and SCS.25 Each item is rated for its frequency over the past 2 weeks, using a Likert scale anchored at 0 representing “not at all” and 3 representing “nearly every day”, with total scores ranging from 0 indicating no depression to 27 indicating severe depression with suicidal ideation.
The PHQ-15 is a 15-item measure developed to measure somatization26 and was administered at the time of 10 kHz SCS evaluation. Its validity and reliability have been established,27 although it has not previously been used in a study of SCS. Each item is a physical symptom (eg, dizziness and chest pain) and is rated on the extent to which the symptom has bothered the individual over the past 4 weeks: not bothered, bothered a little, and bothered a lot. Total scores range from 0 to 30, with a total score of ≥15 indicating high somatic symptom severity.
The Generalized Anxiety Disorder-728 is a measure of anxiety severity that was administered at the time of 10 kHz SCS evaluation. The validity and reliability have been established in the general population29 as well as in the population of patients with chronic pain30 and has been recommended as a prescreening measure for selection of patients for SCS.31 Each item is rated for its frequency over the past 2 weeks, using a Likert scale anchored at 0 representing “not at all” and 3 representing “nearly every day”, with total scores ranging from 0 indicating no anxiety to 21, and scores of ≥10 indicating the likely presence of an anxiety disorder.
The WHO Disability Assessment Schedule 2.0 (WHODAS 2.0) is a 36-item measure used to assess disability and was administered at the time of 10 kHz SCS evaluation and at follow-up post 10 kHz SCS implant. Each item uses a 5-point Likert scale to rate the degree of difficulty over the past 30 days in various tasks ranging from “none” to “extreme or cannot do”. It has demonstrated high concurrent validity with comparable instruments designed to measure disability in day-to-day functioning across activity domains.32 Internal consistency and reliability of the measure have been established as good.33 The simple scoring method was used wherein the summary score is an average weighted score of each of the six domains, reported as one number ranging from 1.00 to 5.00.
The Roland–Morris Disability Questionnaire (RMDQ)34 is a 24-item self-report measure of disability due to back pain and was modified (RMDQ-m) to describe pain in general (eg, “I walk more slowly than usual because of my back” was modified to “I walk more slowly than usual because of my pain”). Modifications to the items have been described in the literature and supported by the authors of the RMDQ.35 Items were either endorsed if true on the current day or left blank if not true on the current day. Scores range from 0 to 24, with 0 representing no disability from pain and 24 representing maximum disability from pain. Subjects in the SCS + CMM group may have completed this measure at SCS evaluation and post-implant follow-up visits and/or as part of routine care if enrolled in the opioid prescribing program. Subjects in the CMM group who were also enrolled in the opioid prescribing program were administered the RMDQ-m as part of routine care, and scores were obtained from month 12 and month 24 for both groups.
Statistical analyses
A propensity score-matching approach was undertaken using nearest-neighbor matching without replacement. Age, gender, initial pain severity, combined office visit and interventional procedure volume over the initial 12 months, and initial opioid dose category (initial dose =0 MME, initial dose between 0 and 90 MME, or initial dose ≥90 MME) were included in the logistic regression model. Visit volume was clinically important to use in the matching model for two main reasons: 1) patients who are in the clinic monthly vs once per year receive a different level of engagement in education and exposure to the clinic’s philosophy to prioritize functional outcomes and 2) the patients who are candidates for SCS are clinically different from those coming infrequently for visits, such as those doing well receiving one spine procedure each year. The ratio of subjects in the CMM group to subjects in the SCS + CMM group was 2:1. The R package “MatchIt” was used to select matched samples. Following the propensity score-matching procedure, the CMM and SCS + CMM groups were compared with respect to each of the above potential confounders. The groups were compared in terms of age using the independent-samples t-test. Due to non-normality of data, they were compared in terms of initial pain severity and combined office visit and interventional procedure volume over the initial 12 months using the Mann–Whitney U test. Comparisons between the groups in terms of gender and initial opioid dose category were conducted via the chi-squared test.
Descriptive statistics (counts and percentages for categorical variables; means, medians, SDs, and IQRs for discrete/continuous variables) were computed. Comparison of the CMM and SCS + CMM groups in terms of tobacco status was performed using the chi-squared test. The two groups were compared in terms of marital status and employment status via Fisher’s exact test (which was used in lieu of the chi-squared test due to sparse expected cell counts for these particular analyses). Due to non-normality, between-group comparisons of change in pain score, RMDQ-m score, MME, visit volume, and procedure volume were performed using the Mann–Whitney U test. Within-group comparisons were performed using the Wilcoxon signed-rank test. The significance level was set at 0.05. SPSS Version 24 was used in the analysis.
Results
Propensity-matching technique identified 64 subjects in the CMM group who were best matched to the 32 subjects in the SCS + CMM group on five variables: gender, age, pain severity at month 0, MME at month 0, and overall visit and procedure volume over the initial 12 months. Additionally, the CMM group did not significantly differ from the SCS + CMM group in terms of tobacco use, employment status, or marital status (Table 1). Of those in the SCS + CMM group who had completed self-report measures as part of their psychological evaluations for SCS, their median and mean scores were below thresholds used to indicate major depression, generalized anxiety disorder, somatic symptom severity, and pain catastrophizing (Table 2).
  | Table 2 Self-report measures at the time of spinal cord stimulator evaluation Note: aIQR. |
Pain severity
The SCS + CMM group reported a reduction in FPS of approximately one point (M=−0.9, SD=1.9) from baseline to 12 months follow-up; however, this did not quite reach statistical significance (P=0.06). The CMM group reported no change in FPS (M=0.0, SD=2.3, P=0.97), and these changes in FPS were not significantly different between groups (P=0.07). Subjects in the SCS + CMM group also reported a significant reduction in low back pain severity of 42.6% as measured by the NRS (M=−3.1, SD=2.6, n=30, P<0.001) and a significant reduction in lower extremity pain of 50.9% on the NRS (M=−2.9, SD=2.2, n=16, P=0.01). Mean and median pain severity data are presented in Table 3.
Disability
Of those completing the RMDQ-m on at least 2 time points during the study period, both the SCS+CMM group (n=21) and CMM group (n=38) reported a significant reduction in RMDQ-m scores (M=−3.1, SD=5.5, P=0.02; M=−1.5, SD=4.0, P=0.01, respectively). There was not a statistically significant difference between groups in the change of RMDQ-m scores (P=0.13). Some subjects in the SCS + CMM group additionally completed the WHODAS 2.0 measure of disability at the time of evaluation for SCS and at 12 months post-implant (n=19). The total score was low at evaluation (M=1.97, SD=0.42), indicating mild disability across domains and did not significantly change (M=−0.05, SD=0.57, P=0.57). Mean and median disability data are presented in Table 4.
Opioid dose
Among those prescribed daily opioid medications during the study period, those in the SCS + CMM group (n=21) had a dose of 92.2 MME at the time of implant (SD=51.3) and a significant reduction in dose at 12 months post-implant (M=−26.2, SD=32.8, P=0.001). The CMM group (n=38) had a starting dose of 89.1 MME (SD=52.7) and no significant change in dose (M=−5.8, SD=34.2, P=0.11). There was a statistically significant difference between groups in the change of MME (P=0.01), indicating more reduction in opioid doses among the SCS + CMM group. Mean and median opioid data are presented in Table 5. Five subjects in the SCS + CMM group had dose reductions of at least 60%, including one who tapered completely off, while only one subject in the CMM group had a dose reduction of at least 60%. Information on number of subjects with and without dose changes is presented in Table 6.
  | Table 6 Opioid dose change data by group Abbreviations: CMM, conventional medical management; MME, morphine milligram equivalent; SCS, spinal cord stimulator. |
Health care utilization: office visits and procedures
The SCS + CMM group had a reduction in office visit volume, but this change was not statistically significant (M=−0.9, SD=5.6, P=0.46). The CMM group had a statistically significant reduction in office visit volume (M=−1.3, SD=4.3, P=0.02). There was no statistically significant difference between groups in the change of visit volume (P=0.62) and the significant finding for the CMM group but not the SCS + CMM group may be related to the difference in sample size of each group.
Both the SCS + CMM and CMM groups had similar interventional procedure volume over the initial 12 months of the study period (M=2.5, SD=2.1 and M=2.6, SD=2.5, respectively) and experienced significant reductions in interventional procedure volume (M=−1.8, SD=1.9, P<0.001; M=−0.8, SD=2.5, P=0.01, respectively) during the following 12 months. The SCS + CMM group reduced the number of interventional procedures by 72.0% while the CMM group reduced by 34.6%, and this difference between groups was significant (P=0.03) with those in the SCS + CMM group undergoing less than half the number of interventional procedures than the CMM group in the latter 12 months of the study period. Mean and median office visit and interventional procedure data are presented in Table 7.
Discussion
This retrospective, matched sample, cohort study demonstrates that 10 kHz SCS treatment is associated with improvements in low back and lower extremity pain and perceived disability. As hypothesized, the use of 10 kHz SCS resulted in significant reductions in opioid dosage. Analysis of opioid dose change also revealed statistically significant dose reductions among those receiving 10 kHz SCS in addition to CMM compared with those undergoing CMM only. Importantly, the study results suggest the potential for decreased health care utilization and costs given the marked reductions in both medication dosage and interventional procedure volume with 10 kHz SCS treatment.
Our finding of significant opioid dose reduction 1-year post 10 kHz SCS implantation is consistent with the extant literature.2–4 Most of the patients in this group (71.4%) underwent a dose reduction, including one who tapered off completely from 120 MME. Only one patient in this group increased 4 MME after SCS implant, compared with 12 (31.6%) of the CMM group who were titrated up over the study period, one with an increase of 135 MME. The 28% mean reduction in MME observed among subjects receiving 10 kHz SCS treatment in the current investigation was substantially greater than that reported in the previous US study of high-frequency SCS.4 One explanation for the differing results may relate to the screening process used during the evaluation for SCS treatment. All candidates must undergo a comprehensive behavioral health assessment focused on identifying meaningful treatment outcome goals that include medication reduction. As such, it is possible that our assessment process may serve to select patients more willing to engage in opioid reduction after permanent implantation. Establishing the goal of opioid reduction during the assessment process may also serve to set expectations for post-implant dose reduction among the treatment team, thereby increasing the efforts devoted to medication reduction. Additionally, the use of the interdisciplinary care model to help establish treatment outcome expectations and provide education and support to patients undergoing targeted opioid reductions may serve to increase the likelihood of achieving more significant reductions.36,37
Conversely, the reduction in opioid dose observed in our study was lower than those reported in European studies2,3 and may reflect differing cultural and prescribing attitudes toward the use of opioid medications. Data indicate that the use of prescription opioids has historically been substantially higher in the US than in Europe.38 Hauser et al39,40 have suggested that the lack of a prescription opioid crisis in Germany, for example, may be largely due to more stringent guidelines regarding the continuation and discontinuation of opioid trials in chronic pain patients. Hauser et al39 also cited increased access to evidence-based non-pharmacological treatments due to the emphasis placed on integrated pain treatment as a factor in limiting opioid prescribing. Fischer et al41 have also postulated that dynamics, including direct-to-consumer drug marketing, a predominantly fee for service reimbursement model, and a lack of sufficient specialty pain services relative to the number afflicted with chronic pain may have contributed to viewing the use of prescription drugs in the US as “the most desirable, feasible or incentivized intervention for care providers, yet also one expected mainly from many patients as a satisfactory form of medical care” (p. 179).
Our results also demonstrated a statistically significant difference in opioid reduction in the SCS+ CMM group compared with those undergoing CMM only, a finding of particular importance given the increased emphasis on reducing opioid prescribing in the US as reflected in current Centers for Disease Control and Prevention guidelines.42 An interdisciplinary approach to pain management can facilitate efforts at opioid reduction as reported in a recent Veterans Health Administration study43 and may help to explain the reduced opioid dosing observed in the SCS+ CMM group. However, the absence of similar dose reductions in the CMM group suggests a beneficial effect of 10 kHz SCS treatment beyond that derived from the model of care.
Similar to previous study findings,1–4 subjects using 10 kHz SCS in addition to CMM reported a significant decrease in disability measured by the RMDQ-m. Interestingly, a significant decrease in RMDQ-m scores was also observed in the CMM group. The use of an interdisciplinary approach to chronic pain treatment has been associated with improved treatment outcomes in published literature37,38 and may account for the reduction in self-reported physical disability in both study groups. Despite significant reductions in RMDQ-m scores, the SCS + CMM group that completed the WHODAS 2.0 did not report significant change. This may have been due to a difference in the theoretical frameworks upon which each measure was constructed. The RMDQ-m is predicated on a biomedical approach in which disability is viewed as a result of pathophysiologic changes and physical impairments. In contrast, the WHODAS 2.0 was developed by the WHO and encompasses six domains reflecting the biopsychosocial conceptualization of disability embodied in the International Classification of Functioning, Disability, and Health: Cognition (understanding and communicating), Mobility (moving and getting around), Self-care (hygiene, dressing, eating, and staying alone), Getting along (interacting with other people), Life activities (domestic responsibilities, leisure, work, and school), and Participation (joining in community activities).34 Thus, effective but localized treatment for low back and lower extremity pain may result in more significant decreases in self-reported disability when assessed by measures focused on physical functioning, such as with the RMDQ-m rather than the multidimensional WHODAS 2.0.
Pain relief has been considered by many to be the primary objective of pain management, and concerns have been raised that this approach has directly contributed to the opioid crisis facing the US.44,45 Furthermore, despite escalating costs associated with chronic pain treatment from 1996 to 2013,46 there is no evidence that treatment outcomes have improved. In response to these concerns, the need to prioritize functional improvement over reduction in pain has been advocated15,16 and has prompted the development of newer assessment tools, such as the FPS. The FPS is a global (as opposed to site-specific) instrument that incorporates both objective and subjective components of pain experience,13 thus making it a more complex tool than a traditional pain visual analog scale or NRS. The objective functional anchors include descriptions across seven domains of functioning (sitting, standing, walking, lifting, carrying, pushing, and pulling, as well as generally using both upper and lower extremities). These scores are not based on pain severity per se, but rather on the perceived level of disability that results from patients’ pain. Despite the significant reductions observed in opioid dosing and self-reported disability, the reduction in reported pain severity as measured by the FPS among 10 kHz SCS patients did not reach statistical significance (P=0.06). Examination of prospective data from a larger study sample and the recording of more frequent real-time assessments, such as use of a daily pain diary, may be needed to demonstrate improvement in pain severity linked to functional impairment. Additionally, the global nature of this instrument may also account for the lack of significant change on the FPS. For example, a patient who is suffering from headache on the day of an assessment would likely report a higher pain level than he or she would report if asked specifically about low back or lower extremity pain. In contrast, pain NRS data collected from subjects receiving 10 kHz SCS implant demonstrated statistically significant reductions in low back and lower extremity pain with 10 kHz SCS treatment at 12 months post-implant. Low back pain severity was reduced 42.6% from baseline and lower extremity pain reduced 50.9% from baseline ratings. Our findings on change in pain NRS data are comparable with those identified in previous studies of 10 kHz SCS..2–4,6
It is well documented that chronic low back pain is costly to treat,47 with recent data suggesting that expenditures may be even greater for those patients treated with opioids.48 Accordingly, chronic low back pain has been noted as a source of frustration for third-party payers.49 Despite a recent review’s conclusions that SCS results in “cost savings and efficient use of health care resources relative to current standards of care”,50 insurers may remain hesitant to cover SCS implantation given the considerable initial costs associated with SCS treatment. As such, additional community-based data may serve to reassure third-party payers with regard to the value of this pain management modality. The current study’s health care utilization finding of a significant reduction in post-implantation interventional procedures supports the potential for long-term cost reduction with the use of 10 kHz SCS treatment. Furthermore, the reduction in opioid dosing observed with 10 kHz SCS also represents the potential for further cost savings, the financial burden of which has not gone unnoticed by health care agencies.51 While a full cost analysis was beyond the scope of this study, future studies will help to further clarify the costs and benefits associated with 10 kHz SCS treatment compared with CMM by examining additional variables, including SCS surgical revisions, explantation of the SCS device, and side effects associated with medications or interventional procedures.
Limitations are inherent in any retrospective cohort study. First, such designs are associated with the risk that patients who receive a specific treatment may differ from untreated patients both in terms of measured and unmeasured baseline characteristics.52 Propensity matching was used to address this concern; however, the possibility of between-group differences remains. Second, subjective patient-reported measures of disability used in this study are susceptible to response biases caused by issues, such as social desirability, secondary gain, and inaccurate memory, and may result in either the over- or underestimation of an individual’s true level of physical functioning. Future prospective studies of SCS outcomes in clinical settings should consider the use of objective measures of disability and function, such as functional capacity evaluations and digital monitoring devices. Third, sample size in this study was relatively small due to the convenience sample of patients with 10 kHz SCS implant at a single pain management center, and therefore, our study may not have been sufficiently powered to detect within- and between- group differences. While smaller than prior multi-site investigations of 10 kHz SCS, our sample size was within the range of other studies (n=20–153) with Level I and II evidence evaluating SCS in patients with failed back surgery syndrome.50 Fourth, follow-up data were collected for only 12 months post-SCS implant; however, longer follow-up data are needed to more thoroughly evaluate the clinical and economic impacts of this treatment. Finally, the integrated, biopsychosocial model of care used in this investigation focused on structured goal setting that underscored the importance of improved function and medication reductions. While we attempted to control this effect by utilizing a matched sample of subjects receiving CMM only within the same pain management center, it is possible that the model of care had an impact on our results.
Strengths of this study include the use of real-world retrospective data that reflect the clinical practice of a community-based interdisciplinary treatment team. The retrospective nature of the study reflects clinical decisions made in usual daily medical care, in the absence of specific research objectives at the time of decision-making. A prospective study of SCS + CMM compared with CMM could not have been blinded and therefore, the clinicians might have been biased in their treatment decisions in order to produce more favorable outcomes for the SCS + CMM group. As already discussed, the SCS + CMM group of patients was pre-screened for certain clinical comorbidities as well as clinical strengths (eg, acceptance of pain), but this was not as rigorous as is typical in a clinical trial, thereby providing data on a more typical chronic pain sample with medical, psychological, and socioeconomic complexities.
Conclusions and future directions
These findings underscore the potential role for integrating 10 kHz SCS treatment in an interdisciplinary, community-based pain management center. Importantly, our patients with 10 kHz SCS implant in addition to CMM demonstrated a significant reduction in opioid use, a finding consistent with the current direction of controlled substance risk mitigation. As with earlier prospective clinical trials, our results demonstrate that 10 kHz SCS is associated with an improvement in low back and lower extremity pain and self-reported disability.
This investigation also offers a proof-of-concept for a model in which the delivery of cost-effective pain care is possible when 10 kHz SCS treatment is integrated into the patient’s overall interdisciplinary treatment plan. Neuromodulation should be considered as component to the overall interdisciplinary plan of care rather than as a stand-alone treatment. Future research focused on examining the possible differences in 10 kHz SCS outcomes when used within and without the framework of interdisciplinary, goal-focused care could address the generalizability of our results and provide important guidance on optimizing the value of 10 kHz SCS treatment.
Acknowledgements
This study was supported by an unrestricted grant from Nevro Corp. Sponsors were not involved at any stages of this study.
Disclosure
Dr Schatman serves as a consultant and speaker for Kaleo Pharma. The other authors report no conflicts of interest in this work.
References
Smet I, van Buyten JP, Al-Kaisy A. Successful treatment of low back pain with a novel neuromodulation device. In: Proceedings of the 14th North American Neuromodulation Society Annual Meeting; 2010; Las Vegas, NV, USA. | ||
van Buyten JP, Al-Kaisy A, Smet I, Palmisani S, Smith T. High-frequency spinal cord stimulation for the treatment of chronic back pain patients: results of a prospective multicenter European clinical study. Neuromodulation. 2013;16(1):59–66. | ||
Al-Kaisy A, van Buyten JP, Smet I, Palmisani S, Pang D, Smith T. Sustained effectiveness of 10 kHz high-frequency spinal cord stimulation for patients with chronic, low back pain: 24-month results of a prospective multicenter study. Pain Med. 2014;15(3):347–354. | ||
Kapural L, Yu C, Doust MW, et al. Novel 10-kHz high-frequency therapy (HF10 therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: The SENZA-RCT randomized controlled trial. Anesthesiology. 2015;123(4):851–860. | ||
Amirdelfan K, Yu C, Doust MW, et al. Long-term quality of life improvement for chronic intractable back and leg pain patients using spinal cord stimulation: 12-month results from the SENZA-RCT. Qual Life Res. 2018;27(8):2035–2044. | ||
Kapural L, Yu C, Doust MW, et al. Comparison of 10-kHz high-frequency and traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: 24-month results from a multicenter, randomized, controlled pivotal trial. Neurosurgery. 2016;79(5):67–677. | ||
Cheatle MD. Biopsychosocial approach to assessing and managing patients with chronic pain. Med Clin North Am. 2016;100(1):43–53. | ||
Hylands-White N, Duarte RV, Raphael JH. An overview of treatment approaches for chronic pain management. Rheumatol Int. 2017;37(1):29–42. | ||
Porter ME. A strategy for health care reform--toward a value-based system. N Engl J Med. 2009;361(2):109–112. | ||
Burwell SM. Setting value-based payment goals--HHS efforts to improve U.S. health care. N Engl J Med. 2015;372(10):897–899. | ||
Annemans L, van Buyten JP, Smith T, Al-Kaisy A. Cost effectiveness of a novel 10 kHz high-frequency spinal cord stimulation system in patients with failed back surgery syndrome (FBSS). J Long Term Eff Med Implants. 2014;24(2–3):173–183. | ||
Washington State Agency Medical Directors’ Group. Interagency Guideline On Opioid Dosing for Chronic Noncancer Pain: An Educational Aid to Improve Care and Safety with Opioid Treatment. Olympia, WA: Washington State Department of Labor and Industries; 2010. Available from: http://www.agencymeddirectors.wa.gov/Files/OpioidGdline.pdf. Accessed November 15, 2018. | ||
Gloth FM, Scheve AA, Stober CV, Chow S, Prosser J. The functional pain scale: reliability, validity, and responsiveness in an elderly population. J Am Med Dir Assoc. 2001;2(3):110–114. | ||
Arnstein PM, Gentile D. 3D Reliability and validity of the functional pain scale for hospitalized chronic pain patients. Pain Management Nursing. 2018;19(2):97–98. | ||
Krebs EE, Carey TS, Weinberger M. Accuracy of the pain numeric rating scale as a screening test in primary care. J Gen Intern Med. 2007;22(10):1453–1458. | ||
Young IA, Cleland JA, Michener LA, Brown C. Reliability, construct validity, and responsiveness of the neck disability index, patient-specific functional scale, and numeric pain rating scale in patients with cervical radiculopathy. Am J Phys Med Rehabil. 2010;89(10):831–839. | ||
Cleland JA, Whitman JM, Houser JL, Wainner RS, Childs JD. Psychometric properties of selected tests in patients with lumbar spinal stenosis. Spine J. 2012;12(10):921–931. | ||
Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524–532. | ||
Sullivan MJL. The Pain Catastrophizing Scale: User Manual; 2009. Available from: http://sullivan-painresearch.mcgill.ca/pdf/pcs/PCSManual_English.pdf. Accessed September 19, 2018. | ||
Osman A, Barrios FX, Kopper BA, Hauptmann W, Jones J, O’Neill E. Factor structure, reliability, and validity of the pain catastrophizing scale. J Behav Med. 1997;20(6):589–605. | ||
Al-Kaisy A, Palmisani S, Smith T, Harris S, Pang D. The use of 10-kilohertz spinal cord stimulation in a cohort of patients with chronic neuropathic limb pain refractory to medical management. Neuromodulation. 2015;18(1):18–23. | ||
Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. | ||
Dobscha SK, Corson K, Perrin NA, et al. Collaborative care for chronic pain in primary care: a cluster randomized trial. JAMA. 2009;301(12):1242–1252. | ||
Tsui JI, Lira MC, Cheng DM, et al. Chronic pain, craving, and illicit opioid use among patients receiving opioid agonist therapy. Drug Alcohol Depend. 2016;166:26–31. | ||
Lancer K. A brief demographic and psychological portrait of VA patients evaluated as candidates for spinal cord stimulators. J Pain. 2018;19(3):S77. | ||
Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64(2):258–266. | ||
Gierk B, Kohlmann S, Toussaint A, et al. Assessing somatic symptom burden: a psychometric comparison of the patient health questionnaire-15 (PHQ-15) and the somatic symptom scale-8 (SSS-8). J Psychosom Res. 2015;78(4):352–355. | ||
Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. | ||
Löwe B, Decker O, Müller S, et al. Validation and standardization of the generalized anxiety disorder screener (GAD-7) in the general population. Med Care. 2008;46(3):266–274. | ||
Kroenke K, Outcalt S, Krebs E, et al. Association between anxiety, health-related quality of life and functional impairment in primary care patients with chronic pain. Gen Hosp Psychiatry. 2013;35(4):359–365. | ||
Stephens KA, Ward A. Patient selection for spinal cord stimulators: mental health perspective. Curr Pain Headache Rep. 2014;18(3):398. | ||
Ustün TB, Chatterji S, Kostanjsek N, et al. Developing the World Health Organization Disability Assessment Schedule 2.0. Bull World Health Organ. 2010;88(11):815–823. | ||
Federici S, Bracalenti M, Meloni F, Luciano JV. World Health Organization disability assessment schedule 2.0: an international systematic review. Disabil Rehabil. 2017;39(23):2347–2380. | ||
Roland M, Morris R. A study of the natural history of back pain. Part I: development of a reliable and sensitive measure of disability in low-back pain. Spine. 1983;8(2):141–144. | ||
Roland M, Fairbank J. The Roland-Morris disability questionnaire and the Qswestry disability questionnaire. Spine. 2000;25(24):3115–3124. | ||
Turk DC, Swanson K. Efficacy and cost-effectiveness of treatment for chronic pain: an analysis and evidence-based synthesis. In: Schatman ME. Campbell A. editors. Chronic Pain Management: Guidelines for Multidisciplinary Program Development. New York: Informa Health care; 2007:15–38. | ||
Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. 2014;69(2):119–130. | ||
International Narcotics Control Board [webpage on the Internet]. Availability of narcotic drugs for medical use. Available from: https://www.incb.org/incb/en/narcotic-drugs/Availability/availability.html. Accessed August 9, 2018. | ||
Häuser W, Petzke F, Radbruch L, Tölle TR. The opioid epidemic and the long-term opioid therapy for chronic noncancer pain revisited: a transatlantic perspective. Pain Manag. 2016;6(3):249–263. | ||
Häuser W, Schug S, Furlan AD. The opioid epidemic and national guidelines for opioid therapy for chronic noncancer pain: a perspective from different continents. Pain Rep. 2017;2(3):E599. | ||
Fischer B, Keates A, Bühringer G, Reimer J, Rehm J. Non-medical use of prescription opioids and prescription opioid-related harms: why so markedly higher in North America compared to the rest of the world? Addiction. 2014;109(2):177–181. | ||
Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65(1):1–49. | ||
Seal K, Becker W, Tighe J, Li Y, Rife T. Managing chronic pain in primary care: It really does take a village. J Gen Intern Med. 2017;32(8):931–934. | ||
Kolodny A, Courtwright DT, Hwang CS, et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. 2015;36:559–574. | ||
Baker DW. History of The joint commission’s pain standards: lessons for today’s prescription opioid epidemic. JAMA. 2017;317(11):1117–1118. | ||
Dieleman JL, Baral R, Birger M, et al. US spending on personal health care and public health, 1996-2013. JAMA. 2016;316(24):2627–2646. | ||
US Department of Health and Human Services [webpage on the Internet]. Healthy People 2020, 2020 Topics and Objectives: Arthritis, Osteoporosis, and Chronic Back Conditions. Updated 2016. Available from: http://www.healthypeople.gov/2020/topicsobjectives2020/overview.aspx?topicid=3. Accessed August 2, 2018. | ||
Zgierska AE, Ircink J, Burzinski CA, Mundt MP. Cost of opioid-treated chronic low back pain: Findings from a pilot randomized controlled trial of mindfulness meditation-based intervention. J Opioid Manag. 2017;13(3):169–181. | ||
Itz C, Huygen F, Kleef MV. A proposal for the organization of the referral of patients with chronicnon-specific low back pain. Curr Med Res Opin. 2016;32(11):1903–1909. | ||
Hoelscher C, Riley J, Wu C, Sharan A. Cost-effectiveness data regarding spinal cord stimulation for low back pain. Spine. 2017;42(Suppl 14):S72–S79. | ||
Gellad WF, Cunningham FE, Good CB, et al. Pharmacy use in the first year of the veterans choice program: a mixed-methods evaluation. Med Care. 2017;55(Suppl 7 Suppl 1):S26–S32. | ||
Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150–161. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.