Back to Journals » Nature and Science of Sleep » Volume 16
Sleep Duration and Kidney Function – Does Weekend Sleep Matter?
Authors Wu CC , Yang PL, Kao LT , Liu YC, Zheng CM, Chu P, Lu K , Chu CM, Chang YT
Received 15 August 2023
Accepted for publication 21 December 2023
Published 2 February 2024 Volume 2024:16 Pages 85—97
DOI https://doi.org/10.2147/NSS.S427687
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Valentina Alfonsi
Chia-Chao Wu,1,2 Pei-Lin Yang,3 Li-Ting Kao,4– 6 Yi-Chun Liu,7,8 Cai-Mei Zheng,9 Pauling Chu,1 Kuo‐Cheng Lu,10 Chi-Ming Chu,7,11– 13 Yu-Tien Chang7
1Division of Nephrology, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan; 2Department and Graduate Institute of Microbiology and Immunology, National Defense Medical Center, Taipei, Taiwan; 3School of Nursing, National Defense Medical Center, Taipei City, Taiwan; 4School of Pharmacy, National Defense Medical Center, Taipei, Taiwan; 5Graduate Institute of Life Sciences, National Defense Medical Center, Taipei, Taiwan; 6Department of Pharmacy Practice, Tri-Service General Hospital, Taipei, Taiwan; 7School of Public Health, National Defense Medical Center, Taipei City, Taiwan; 8Institute of Medical Sciences, National Defense Medical Center, Taipei City, Taiwan; 9Division of Nephrology, Department of Internal Medicine, Shuang Ho Hospital, Taipei Medical University, Taipei, Taiwan; 10Division of Nephrology, Fu-Jen Catholic Hospital, Fu-Jen Catholic University, Taipei, Taiwan; 11Department of Surgery, Songshan Branch of Tri-Service General Hospital, National Defense Medical Center, Taipei City, Taiwan; 12Division of Biostatistics and Informatics, Department of Epidemiology, School of Public Health, National Defense Medical Center, Taipei, Taiwan; 13Department of Public Health, China Medical University, Taichung City, Taiwan
Correspondence: Yu-Tien Chang, School of Public Health, National Defense Medical Center, No. 161, Sec. 6, Minquan E. Road, Neihu Dist, Taipei City, 11490, Taiwan, Tel +886-2-87923100#18454, Email [email protected]
Objective: Weekend sleep duration is linked to health issues, including mortality. However, how weekend sleep duration can impact chronic kidney disease (CKD) still needs to be understood. Therefore, we aimed to analyze how weekend sleep duration is associated with kidney function.
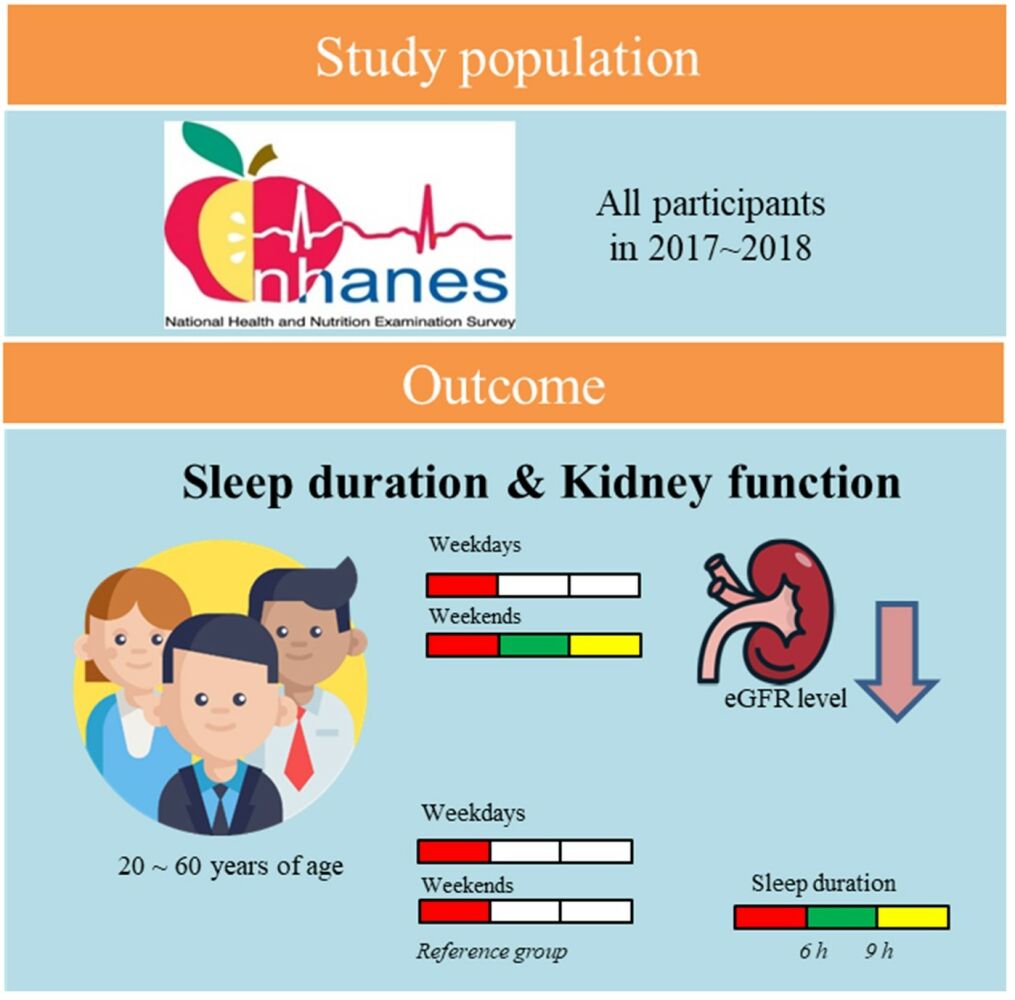
Methods: This is a cross-sectional study. Data were obtained from the 2017– 2018 National Health and Nutrition Examination Survey. We included 5362 study participants and categorized them into nine subgroups by sleep duration (short: ≤ 6 hours, normal: 6– 9 hours, and long: ≥ 9 hours) on weekdays and weekends and analyzed for the respective association with renal function using stratified multivariable linear regression.
Results: Weekend sleep duration for 9 hours or more was associated with decreasing estimated glomerular filtration rate (eGFR) levels by 2.8 to 6.4 mL/min/1.73 m2 among people with long to short weekday sleep duration compared with short weekday and weekend sleep durations (control group) after adjusting for demographic characteristics, body measurement, sleep quality, smoking, and comorbidities. The study population with short weekday sleep duration (sWK) and long weekend sleep duration (lWD) had the most significant decline in eGFR. For the study population with sWK, eGFR level significantly decreased by 1.1 mL/min/1.73 m2 as sleep duration on weekends increased by one hour.
Conclusion: The underlying mediators of lWD and CKD could be the dysregulation of human behaviors, metabolism, or biological functions. Longer weekend sleep duration was linked to a decrease in eGFR levels. It warrants further study to clarify the mediators.
Keywords: weekend sleep duration, kidney function, national health and nutrition examination survey, estimated glomerular filtration rate, eGFR, chronic kidney disease, CKD
Graphical Abstract:
Introduction
Chronic kidney disease (CKD) is characterized by progressive damage and loss of kidney function over time, affecting more than 10% of the global population.1 CKD, a well-known cardiovascular risk factor associated with all-cause mortality, has imposed a significant socioeconomic burden.2 Although the etiology of CKD remains to be elucidated, it has been linked with numerous biological (eg, age, medication, diabetes, hypertension, and obesity), environmental (eg, environmental pollutants, heavy metals, agricultural chemicals), and lifestyle factors (eg, sleep and dietary behaviors, physical activity, tobacco smoking).3,4 Among these factors, lifestyle factors are the most amenable to making behavioral modifications for preventing and/or slowing CKD progression.5
Many investigators have reported that sleep deficiency, including shorter or longer sleep duration (<6 hours or >8 hours) and poor sleep quality, is a significant risk for renal function deterioration.6 Moreover, untreated sleep deficiency may contribute to chronic activation of the sympathetic nervous system, endothelial dysfunction, and systemic inflammation and such physiological alterations can contribute to CKD development and progression.7 Therefore, there is a growing interest in studying sleep and its associations with renal health outcomes, particularly for an estimated glomerular filtration rate (eGFR) to prompt kidney health and prevent CKD-associated comorbidities.
Social factors such as work/school schedules can influence habitual sleep due to family and/or work commitments and social expectations surrounding daily routines.8 As a result, social factors shape sleep behaviors termed “social jetlag”, where weekday sleep debts and weekend catch-up sleep lead to irregular sleep-wake patterns between weekdays and weekends.9 Emerging evidence has suggested weekday-to-weekend sleep irregularity may contribute to enormous adverse impacts on health. For example, short weekend sleep was associated with increased mortality in subjects <65 years.10 Weekend catch-up sleep was associated with poor sleep quality and mental health issues.11 While prior studies have reported the association between the length of sleep duration and decreased eGFR, it remains unknown whether and how the weekday-to-weekend difference in sleep duration influences renal health outcomes. In light of what has been reported in the literature, we used data from the National Health and Nutrition Examination Survey (NHANES) to examine the relationships of kidney function with weekday and weekend sleep duration.
Methods
Ethics Statement
In accordance with ethical guidelines, this study was reviewed and determined to be exempt from full IRB review by Tri-Service General Hospital Institutional Review Board in Taiwan (IRB, TSGHIRB approval number: E202316032).
Data Source
We conducted a cross-sectional analysis using data from the NHANES survey. The NHANES is a broad research program that assesses the health and nutritional status of individuals, encompassing adults and children in the United States. Initiated in the early 1960s, it occurs regularly, tracking and assessing changes in national health and nutrition trends every two years. The survey annually examines a nationally representative sample of 5000 persons. The NHANES interview covers demographic, socioeconomic, dietary, and health-related questions. The examination component involves medical, dental, and physiological measurements, as well as laboratory tests administered by highly trained medical personnel. NHANES protocols were approved by the National Center for Health Statistics Ethics Review Board and written informed consent was obtained from all participants. The analysis workflow is shown in Figure 1. We included all participants (N = 9254) participated in the 2017–2018 cycle of the NHANES survey. After excluding missing data, there are 1859 participants included in multivariable analysis.
 |
Figure 1 Analysis workflow. |
Demographic and Clinical Characteristics
Demographic variables age, sex, race, education, and body mass index (BMI). The Body measures data were collected in the Mobile Examination Center (MEC) by trained health technicians. In addition, sleep difficulty (Have you ever told doctor had trouble sleeping?), smoking status, cotinine metabolite (serum hydroxycotinine), kidney function indicators (eGFR, blood urea nitrogen, serum creatinine, and albumin creatinine ratio), and comorbidities (kidney failure, kidney stones, congestive heart failure, stroke, emphysema, high blood pressure, and cholesterol) were included as clinical variables.
Sleep Measures
Sleep information were obtained through NHANES survey questions administered in participants’ homes by trained interviewers utilizing the Computer-Assisted Personal Interview system. The number of hours slept on weekdays and weekends is determined by calculating the stated normal sleep time and waking time throughout the major sleeping phase (night or day). Participants who experienced intermittent awakenings or brief sleep durations at night or throughout the day were instructed to provide details on the specific times they slept and woke up during their primary sleep phase. In the context of this study, weekdays, referred to as workdays, represent the days when study subjects are actively engaged in work, while weekends indicate the days when study subjects are not on duty.
Sleep duration variables included: (1) sleep duration on weekdays (WK), defined as the average sleep hours (h) on weekdays or workdays; (2) sleep duration on weekends (WKD), defined as the average sleep hours on weekend or non-workdays; and (3) weekend catch-up sleep is calculated by subtracting the duration of sleep on weekdays (h) from that on weekends (h). Further, sleep duration was categorized as three sleep duration categories: ≤6 h (short sleep duration), >6 to <9 h (normal sleep duration), and ≥9 h (long sleep duration).
To investigate weekday-to-weekend sleep duration differences, the participants were divided into nine sleep subgroups based on the combinations of sleep duration categories on weekdays and weekends. They are (1) short weekday sleep duration and short weekend sleep duration, termed as sWK-sWKD (2) short weekday sleep duration and normal weekend sleep duration, abbreviated as sWK-nWKD, (3) short weekday sleep duration and long weekend sleep duration, abbreviated as sWK-lWKD, (4) normal weekday sleep duration and short weekend sleep duration, abbreviated as nWK-sWKD, (5) normal weekday sleep duration and normal weekend sleep duration, abbreviated as nWK-nWKD, (6) normal weekday sleep duration and long weekend sleep duration, abbreviated as nWK-lWKD, (7) long weekday sleep duration and short weekend sleep duration, abbreviated as lWK-sWKD, (8) long weekday sleep duration and normal weekend sleep duration, abbreviated as lWK-nWKD, and (9) long weekday sleep duration and long weekend sleep duration, abbreviated as lWK-lWKD.
Renal Function
We used the R package of “CKDEpi.creat12” to calculate eGFR using the data of serum creatinine, sex, age, and ethnicity based on the following CKD-EPI equation:  , where SCr is the standardized serum creatinine in mg/dL, κ is 0.7 for females and 0.9 for males, a is −0.329 for females and −0.411 for males, min indicates the minimum of SCr /κ or 1, and max indicates the maximum of SCr /κ or 1. In this study, eGFR values were presented in mL/min/1.73 m2.
, where SCr is the standardized serum creatinine in mg/dL, κ is 0.7 for females and 0.9 for males, a is −0.329 for females and −0.411 for males, min indicates the minimum of SCr /κ or 1, and max indicates the maximum of SCr /κ or 1. In this study, eGFR values were presented in mL/min/1.73 m2.
As the distributions of renal functions were skewed, we used the R package “bestNormalize” to normalize data. Blood urea nitrogen (mmol/L) was normalized using center-scale transformation. The levels of serum creatinine (µmol/L), albumin–creatinine ratio (mg/g), and serum hydroxycotinine (ng/mL) were normalized using log transformation. The descriptive statistics of the testing index are presented as non-normalized figures for clinical use. Transformed figures were used in the linear regression models.
Statistical Analysis
We used R version 4.0.2 to perform all statistical analyses. We performed an analysis of variance (ANOVA) or χ2 to examine the differences among sWK, nWK, and lWK subgroups or among sWD, nWD, and lWD subgroups in demographic and clinical characteristics. Univariable and multivariable linear regression analyses were used to examine the relationship of eGFR (the renal function indicator) with weekday and weekend sleep durations. In addition to eGFR, significant demographic characteristics (age, gender, race, and education), body measurement, sleep quality, smoking, and comorbidities were included as covariates in sequential multivariable linear regression models. The association of sleep duration on weekdays/weekends, age, and eGFR levels were analyzed using Pearson correlation. The significant level, α is set to 0.05. An alluvial plot was drawn through the R package of “ggalluvial” to display the population distribution stratified by sleep duration and eGFR levels. Forest plots were used to present the difference in eGFR levels among the sleep duration subgroups and plotted using the R package of “forestplot”.
Results
Descriptive Statistics
Indicators of demographics, sleep difficulty, smoking status, and kidney function were compared among the three sleep duration categories on weekdays and weekends. The result was shown in Table 1. Both weekday and weekend sleep durations were significantly associated with age (p < 0.001), sex (p < 0.001), BMI (p < 0.05), race (p < 0.001), education levels (p < 0.001), sleep difficulty (p < 0.001), and smoking (p < 0.01). Of note, weekend sleep duration was associated with eGFR levels (p < 0.001), and blood urea nitrogen (p < 0.001), while weekday sleep duration was not.
 |
Table 1 Comparsions of Demographics, Sleep Difficulty, Smoking Status, and Kidney Function Indicators by Three Sleep Duration Categories on Weekdays and Weekends |
CKD-related comorbidities, including kidney failure, kidney stones, urinary incontinence, times of nocturia, congestive heart failure, stroke, emphysema, high blood pressure, and high cholesterol, were associated with weekday and/or weekend sleep durations (Table 2). We thus included these covariates in the multivariable linear regression models.
 |
Table 2 Comparsions of Comorbidities by Three Sleep Duration Categories on Weekdays and Weekends |
Figure 2 illustrates the raw eGFR levels within the nine subgroups delineated by combinations of sleep duration categories (short [s], normal [n], long sleep duration [l] on weekdays [WK], or weekends [KD], respectively). The estimated average eGFR levels were the lowest in the subgroup of sWK-sWKD (89.4 ± 25.3 mL/min/1.73 m2) and were the highest in the subgroup of sWK-lWKD (106 ± 22.3 mL/min/1.73 m2). The proportions of eGFR levels of <90 mL/min/1.73 m2 in the sleep subgroups of sWK-sWKD, nWK-nWKD, and lWK-lWKD were 48.9%, 45.1%, and 43.5%, respectively.
 |
Figure 2 Estimates of eGFR levels by nine sleep subgroups. |
Sensitivity Analysis
Figure 3 presents the results of multivariable linear regression of eGFR levels with nine sleep subgroups (n = 1859). The model was under the adjustment of BMI, age, gender, race, education, failing kidneys, serum hydroxycotinine (ng/mL), smoking status, congestive heart failure, stroke, emphysema, sleep difficulty, weekend catch-up sleep, high blood pressure, high cholesterol, times of nocturia, kidney stones, and urinary incontinence. The decreasing trends of eGFR levels were observed from sWKD to lWKD among all subgroups in the adjusted model. No matter how long the subjects slept on weekdays, longer weekend sleep durations were significantly associated with eGFR declines. Participants in sWK-lWKD subgroup were significantly associated with the lowest eGFR level (β: −6.4, 95% CI: −11.8~ −0.9, p = 0.02) compared to those in sWK-sWKD. The strength of eGFR declines in sWK (β = −6.4 for sWK-lWKD with p = 0.02; β = −5.0 for sWK-nWKD with p = 0.02) was more evident than those in nWK (β = −1.9 for sWK-nWKD with p = 0.11; β = −3.8 for sWK-lWKD with p = 0.03) and lWK (β = −3.6 for sWK-nWKD with p = 0.10; β = −2.8 for sWK-lWKD with p = 0.04).
 |
Figure 3 Coefficients of eGFRs (mL/min/1.73 m2) of the nine sleep subgroups using multivariable linear regression under the adjustment of possible covariates. |
Figure 4 presents the results of stepwise adjusted linear regression models used to examine the association of eGFR levels with sleep duration on weekends. The result under the unadjusted model (Model 1, n = 5362) demonstrated that long sleep duration on weekends was associated with higher eGFR levels. Model 2 was adjusted by BMI, age, gender, race, and education (n = 4779). Model 3 was adjusted for the covariates in Model 2, BMI, age, gender, race, education, kidney failure, serum hydroxycotinine (ng/mL), smoking status, congestive heart failure, stroke, emphysema, sleep difficulty, high blood pressure, high cholesterol, times of nocturia, kidney stones, and urinary incontinence (n = 1859). In the adjusted Model 3, short sleep duration on weekdays but longer sleep duration on weekends was associated with lower eGFR levels (β = −1.1, p < 0.05).
 |
Figure 4 Coefficients of eGFRs (mL/min/1.73 m2) levels of sleep duration subgroups in forest plots using univariable and multivariable regression models. |
Age was a strong factor negatively associated with eGFR levels (p < 2e-16, Pearson correlation coefficient r = −0.75) (Figure S1). The average eGFR levels of four age groups [<20, 20–39, 40–59, and above 60 years of age] were 136.2±18.8, 112.9±17.0, 95.3±17.4, and 74.7±19.8 mL/min/1.73 m2, respectively (Figure S2). We conducted the sensitivity analysis of the nine sleep subgroups and eGFR levels, stratified by age, and adjusted by BMI, age, gender, race, education, kidney failure, serum hydroxycotinine (ng/mL), smoking status, congestive heart failure, stroke, emphysema, catch-up sleep, sleep difficulty, high blood pressure, high cholesterol, times of nocturia, kidney stones, and urinary incontinence (Figure 5). No statistical difference was shown in eGFR levels among the nine sleep subgroups aged 40–59 years after adjusting for the covariates. In general, short weekend sleep duration for 6 hours or less was associated with increasing eGFR levels but had no statistical significance compared with sWK-sWKD except for those aged 20–39 years in the nWK group and the ones aged above 60 years in the lWK group. Beneficial association of eGFR levels and sleep duration on weekends in a dose–response manner were only observed in those aged 20–39 in the nWK group. Still, inverse effects were observed in those aged 40 or above in the nWK groups. The respective and combined impacts of weekday and weekend sleep duration on eGFR levels were variable with age.
 |
Figure 5 Coefficients of eGFRs (mL/min/1.73 m2) of sleep subgroups stratified by age in forest plots using multivariable linear regression model under the adjustment of possible covariates. |
Discussion
It has been studied that short or long sleep duration were linked to CKD.6,13 However, the combination effects of weekday and weekend sleep on CKD were not elucidated.
To the best of our knowledge, this is the first study to examine the relationships of sleep duration on weekdays and weekends with kidney function. This study demonstrated that long weekend sleep duration was associated with eGFR declines, regardless weekday sleep duration. The decrease in eGFR was especially severe in people with short weekday sleep duration.
Weekday and weekend sleep durations should be separately considered when analyzing the association between sleep duration and kidney functions. As discovered by Roenneberg et al, social time, particularly work schedules, significantly impacts sleep. When sleep duration is analyzed separately for work and free days, the strong social influence on sleep becomes clear.8 This finding is consistent with our study, where no significant association was found between weekday and weekend sleep duration difference and eGFR levels (data not shown). Yet a significant association was observed while we separately analyzed weekday and weekend sleep durations, which may explain some of the inconsistencies in the findings of the association between sleep duration and CKD,14–16 as well as the fact that social activity plays an important role in the relationship between sleep duration and kidney function.
In our findings, eGFR decreased more in sWK-lWKD and nWK-lWKD compared with sWK-sWKD. Weekend sleep duration, which was much higher than weekday sleep duration, was linked to a severe decrease in eGFR levels. Sleep duration on weekends is more likely to reflect the biological need for sleep due to less social influence on weekends (also termed as free days).17 sWK-lWK and nWK-lWK subgroups presented the sleep deprivation on weekdays. Numerous studies indicated the harmful effects of sleep deprivation on kidney functions through the dysregulation of human behavioral processes,18 ie, diet19 and physical activity,20 and biological functions, including immune,21 regular circadian rhythm, sympathetic nervous system activity,22 and physiological metabolism.23 The mechanism underlying the relationship between the weekday-to-weekend differences in sleep duration and renal function remains unclear but could be attributed to the abovementioned kidney health factors.
In addition, shorter weekday sleep and longer weekend catch-up sleep are associated with eating without hunger, which may cause weight gain19 and metabolic syndrome onset.24 Short sleepers are more likely to consume a greater percentage of carbohydrates, potentially leading to metabolic disorders24,25 and associated with a higher risk of inadequate hydration.26
It is known that weekend catch-up sleep was associated with the prevalence of proteinuria in a dose-dependent manner for the group with short weekday sleep durations for less than 6 hours,27 which echoed our findings on eGFR levels. On the contrary, weekend catch-up sleep behavior was significantly associated with better health-related quality of life than non-weekend catch-up sleep for participants who slept less or later on weekdays in the study of Yun Hwan Oh et al.28 However, regarding renal function, weekend catch-up sleep may not compromise kidney impairment, especially for those who sleep less on weekdays. A regular sleep schedule between weekdays and weekends was essential for protecting kidney functions.
However, as to lWK-lWKD, social jetlag may not be the mediate factor to eGFR declines. This finding aligned with previous studies that long sleep duration was associated with the prevalence and progression of CKD6,15 and was verified in a longitudinal study.29 Long sleep duration can serve as an initial symptom or the result of unmeasured diseases and conditions, such as sleep disorders and circadian rhythm abnormalities, and further linked to CKD.30
Sleep time is determined by numerous factors and chorotype, including health behaviors, lifestyle factors, cultural norms, demographic factors, psychological factors, medications, chronic health conditions, and poor sleep hygiene. We included the most available factors from NHANES and leveraged the missing data and variable numbers. Hence, we excluded nutrition factors.
There are certain limitations in this study. The indicators of sleep difficulty, sleep time, smoking, and self-disease reports are derived from surveys, which may be influenced by memory bias. The study did not take into account dietary factors such as the consumption of red meat, medicines such as Insulin, and exercise behaviors. Additionally, the observed association between sleep and kidney function in this study was not causal. It is crucial to note that determining a causal effect requires further elucidation. Specifically, prospective research is warranted to examine the impact of lifestyle factors on the relationship between weekday-to-weekend sleep durations and renal function.
Conclusion
Longer weekend sleep duration was associated with decreasing eGFR levels regardless of weekday sleep duration. This situation was the worst for those with a short weekday sleep duration. Hence, when exploring the relationship between sleep duration and CKD, weekday sleep duration (habitual sleep) and weekend sleep duration (unusual sleep) should be considered separately. Further research is required to validate the causal association and identify modifiable risk factors contributing to the prolonged weekend sleep linked to CKD. Subsequently, early intervention strategies could be implemented to prevent or mitigate kidney impairment.
Abbreviations
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; NHANES, National Health and Nutrition Examination Survey; WK, weekdays; WKD, weekends; ANOVA, analysis of variance.
Research Involving Human Participants and/or Animals
This study utilized secondary data from the NHANES (National Health and Nutrition Examination Survey), indicating that we did not directly involve or handle any human participants or animals in the actual research. NHANES is a large-scale survey conducted by government agencies, with prior informed consent obtained from the participants. Therefore, in conducting secondary analysis, we did not undertake new research involving human or animal participation and did not require additional ethical review approval. We respect the privacy and ethical principles of the original survey, ensuring that the use of research findings complies with relevant laws and standards.
Acknowledgments
Institution where work was performed: National Defense Medical Center.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Tri-Service General Hospital (TSGH-PH-E-112016, TSGH-C02-112030, TSGH-C03-113038 and TSGHPH-E-113009) and National Science and Technology Council (MOST111-2314-B-016-038-MY3) for Dr. Chia-Chao Wu.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl. 2022;12:7–11. doi:10.1016/j.kisu.2021.11.003
2. Airg-E EAFRRS, Set ONT. CKD: the burden of disease invisible to research funders. Nefrologia. 2022;42:65–84.
3. Kelly JT, Su G, Zhang L, et al. Modifiable lifestyle factors for primary prevention of CKD: a systematic review and meta-analysis. J Am Soc Nephrol. 2021;32(1):239–253. doi:10.1681/ASN.2020030384
4. Evangelidis N, Craig J, Bauman A, et al. Lifestyle behaviour change for preventing the progression of chronic kidney disease: a systematic review. BMJ Open. 2019;9(10):e031625. doi:10.1136/bmjopen-2019-031625
5. Hannan M, Ansari S, Meza N, et al. Risk factors for CKD progression: overview of findings from the CRIC study. Clin J Am Soc Nephrol. 2021;16(4):648–659. doi:10.2215/CJN.07830520
6. Park S, Lee S, Kim Y, et al. Short or long sleep duration and CKD: a Mendelian randomization study. J Am Soc Nephrol. 2020;31(12):2937–2947. doi:10.1681/ASN.2020050666
7. Hao Q, Xie M, Zhu L, et al. Association of sleep duration with chronic kidney disease and proteinuria in adults: a systematic review and dose–response meta-analysis. Int Urol Nephrol. 2020;52(7):1305–1320. doi:10.1007/s11255-020-02488-w
8. Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol. 2012;22(10):939–943. doi:10.1016/j.cub.2012.03.038
9. Caliandro R, Streng AA, van Kerkhof LWM, van der Horst GTJ, Chaves I. Social jetlag and related risks for human health: a timely review. Nutrients. 2021;13. doi:10.3390/nu13124543
10. Akerstedt T, Ghilotti F, Grotta A, et al. Sleep duration and mortality – does weekend sleep matter? J Sleep Res. 2019;28(1):e12712. doi:10.1111/jsr.12712
11. Lee H, Kim YJ, Jeon YH, Kim SH, Park EC. Association of weekend catch-up sleep ratio and subjective sleep quality with depressive symptoms and suicidal ideation among Korean adolescents. Sci Rep. 2022;12:10235. doi:10.1038/s41598-022-14352-1
12. Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi:10.1056/NEJMoa1114248
13. Bo Y, Yeoh E-K, Guo C, et al. Sleep and the risk of chronic kidney disease: a cohort study. J Clin Sleep Med. 2019;15(03):393–400. doi:10.5664/jcsm.7660
14. Cheungpasitporn W, Thongprayoon C, Gonzalez-Suarez ML, et al. The effects of short sleep duration on proteinuria and chronic kidney disease: a systematic review and meta-analysis. Nephrol Dial Transplant. 2017;32(6):991–996. doi:10.1093/ndt/gfw072
15. Choi H, Kim HC, Lee JY, et al. Sleep duration and chronic kidney disease: the Korean Genome and Epidemiology Study (KoGES)-Kangwha study. Korean J Intern Med. 2017;32(2):323–334. doi:10.3904/kjim.2015.400
16. Ye Y, Zhang L, Yan W, et al. Self-reported sleep duration and daytime napping are associated with renal hyperfiltration and microalbuminuria in an apparently healthy Chinese population. PLoS One. 2019;14(8):e0214776. doi:10.1371/journal.pone.0214776
17. Roenneberg T, Pilz LK, Zerbini G, Winnebeck EC. Chronotype and Social Jetlag: a (Self-) critical review. Biology. 2019;8(3):54. doi:10.3390/biology8030054
18. Liew SC, Aung T. Sleep deprivation and its association with diseases- a review. Sleep Med. 2021;77:192–204. doi:10.1016/j.sleep.2020.07.048
19. LeMay-Russell S, Tanofsky-Kraff M, Schvey NA, et al. Associations of weekday and weekend sleep with children’s reported eating in the absence of hunger. Nutrients. 2019;11(7):1658. doi:10.3390/nu11071658
20. Hargens TA, Kaleth AS, Edwards ES, Butner KL. Association between sleep disorders, obesity, and exercise: a review. Nat Sci Sleep. 2013;5:27–35. doi:10.2147/NSS.S34838
21. Garbarino S, Lanteri P, Bragazzi NL, Magnavita N, Scoditti E. Role of sleep deprivation in immune-related disease risk and outcomes. Commun Biol. 2021;4:1304. doi:10.1038/s42003-021-02825-4
22. Turek NF, Ricardo AC, Lash JP. Sleep disturbances as nontraditional risk factors for development and progression of CKD: review of the evidence. Am J Kidney Dis. 2012;60:823–833. doi:10.1053/j.ajkd.2012.04.027
23. Tasali E, Wroblewski K, Kahn E, Kilkus J, Schoeller DA. Effect of sleep extension on objectively assessed energy intake among adults with overweight in real-life settings: a randomized clinical trial. JAMA Intern Med. 2022;182(4):365–374. doi:10.1001/jamainternmed.2021.8098
24. Itani O, Kaneita Y, Tokiya M, et al. Short sleep duration, shift work, and actual days taken off work are predictive life-style risk factors for new-onset metabolic syndrome: a seven-year cohort study of 40,000 male workers. Sleep Med. 2017;39:87–94. doi:10.1016/j.sleep.2017.07.027
25. Mi SJ, Kelly NR, Brychta RJ, et al. Associations of sleep patterns with metabolic syndrome indices, body composition, and energy intake in children and adolescents. Pediatr Obes. 2019;14(6):e12507. doi:10.1111/ijpo.12507
26. Rosinger AY, Chang A-M, Buxton OM, et al. Short sleep duration is associated with inadequate hydration: cross-cultural evidence from US and Chinese adults. Sleep. 2019;42(2). doi:10.1093/sleep/zsy210
27. Aoki K, Yamamoto R, Shinzawa M, et al. Sleep debt and prevalence of proteinuria in subjects with short sleep duration on weekdays: a cross-sectional study. Clin Exp Nephrol. 2020;24(2):143–150. doi:10.1007/s10157-019-01808-4
28. Oh YH, Kim H, Kong M, Oh B, Moon JH. Association between weekend catch-up sleep and health-related quality of life of Korean adults. Medicine. 2019;98(13):e14966. doi:10.1097/MD.0000000000014966
29. Hirano K, Komatsu Y, Shimbo T, Nakata H, Kobayashi D. Longitudinal relationship between long sleep duration and future kidney function decline. Clin Kidney J. 2022;15(9):1763–1769. doi:10.1093/ckj/sfac107
30. Motohashi H, Tahara Y, Whittaker DS, et al. The circadian clock is disrupted in mice with adenine-induced tubulointerstitial nephropathy. Kidney Int. 2020;97(4):728–740. doi:10.1016/j.kint.2019.09.032
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
