Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 11
Xanthelasma palpebrarum – a brief review
Received 30 June 2017
Accepted for publication 13 October 2017
Published 18 December 2017 Volume 2018:11 Pages 1—5
DOI https://doi.org/10.2147/CCID.S130116
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Video abstract presented by Pragya A Nair.
Views: 69685
Pragya A Nair, Rochit Singhal
Department of Dermatology, Venereology and Leprosy, Pramukhswami Medical College, Gujarat, India
Abstract: Xanthelasma palpebrarum is the most common cutaneous xanthoma, characterized by yellowish plaques over eyelids – most commonly, over the inner canthus of the upper lid. It is triggered by hyperlipidemia, thyroid dysfunction, and diabetes mellitus. Xanthelasma results from perivascular infiltration of mono- and multinucleated foam cells within lipid-laden cytoplasmic vacuoles in the superficial reticular dermis. Different modalities of treatment, such as simple surgical excision, cryotherapy, chemical peeling with trichloroacetic acid, radiofrequency, and laser, are used in the treatment of xanthelasma palpebrarum. A brief review of current treatment strategies is presented here.
Keywords: xanthelasma palpebrarum, xanthoma, hyperlipidemia, laser, cryotherapy
Introduction
The term “xanthelasma” is derived from the Greek word xanthos (yellow) and elasma (beaten metal plate).1 Xanthelasma palpebrarum (XP) is the most common cutaneous xanthoma, with a prevalence of roughly 1.1% in women and 0.3% in men.2 It is characterized by yellowish plaques occurring most commonly near the inner canthus of the eyelid, more often on the upper, rather than the lower, lid. Lesions are symmetrically distributed, and may be singular or multiple, nodular or flat and soft, and semisolid or calcareous. Hyperlipidemia, thyroid dysfunction, and diabetes mellitus are possible pathogenic triggers.3 Moreover, XP has been reported following erythroderma, inflammatory skin disorders, and allergic contact dermatitis despite normal lipid profiles.4
A benign condition which never limits function, XP is cosmetically disturbing; therefore, patients consult dermatologists, ophthalmologists, or plastic surgeons for lesion removal.2 Several methods are used to treat XP and these include simple surgical excision, cryotherapy, chemical peeling with trichloroacetic acid (TCA), radiofrequency (RF), and laser treatment. Each modality has its own advantages and disadvantages. This article reviews all currently accepted modes of treatment and describes how to apply an algorithmic management approach according to the size and location of the lesion. Furthermore, it is mandatory to treat the underlying medical cause, if any.
Pathophysiology
Xanthomas are cholesterol-rich depositions that can appear anywhere in the body during various disease states. XP is a type of xanthoma that occurs over the eyelids, with the absence of xanthomas elsewhere. Xanthomas can be associated with primary hyperlipidemias, such as types II and IV, having low high-density lipoprotein (HDL) levels, or secondary hyperlipidemias, such as hypothyroidism, diabetes mellitus, drugs5 (glucocorticoids, cyclosporine, cimetidine, estrogens, some antihypertensive medications, retinoids, certain antiepileptic drugs, anabolic steroids, tamoxifen, etc.), and food (diets rich in saturated fats, cholesterol, and alcohol). XP can occur in normolipidemic persons with low HDL levels.
Histopathology
XP is composed of xanthoma cells or foam cells, which are histiocytes laden with intracellular fat deposits, primarily located within the upper reticular dermis or in perivascular and periadnexal areas. Intrahistiocytic vacuoles contain esterified cholesterol.6
Clinical features
XP is rare in the general population, with a variable incidence of 0.56%–1.5% in Western countries.5 It is more common in women – 32%, versus 17.4% in men. The age of onset ranges from 15 to 73 years, with a peak incidence between 30 and 50 years.7 Clinically, it presents as yellowish papules, plaques, or nodules, and is soft in consistency, but can be semisolid or hard. Lesions are usually symmetrically distributed on the medial side of the upper eyelids, but can also involve the lower eyelids. XP can be easily diagnosed on the basis of clinical background.8 In cases which are ambiguous, surgical excision and histopathology should be undertaken. Lesions of XP have no premalignant potential.
Clinically, necrobiotic xanthogranuloma, syringomas, adult-onset asthma and periocular xanthogranuloma (AAPOX), palpebral sarcoidosis, and sebaceous hyperplasia are the main conditions to consider as differential diagnoses. Atypical lesions of XP may have to be differentiated from Erdheim–Chester disease – a systemic xanthogranulomatous disorder (lesions are indurated) – and lipoid proteinosis (lesions appear as a string of nodules along the lid margin, plus other mucocutaneous involvement is present).6 Retinal surgery with silicone oil in tissue was reported to mimic xanthelasma – an entity termed a pseudo-xanthelasma.9
XP can be considered a risk factor for ischemic heart disease, independent of other well-known cardiovascular risk factors (eg, plasma cholesterol, triglyceride concentrations). Arcus senilis of the cornea is seen in patients of XP, but is not considered to be an independent predictor of risk.10
Management
Patients with XP have been seen to have lipid disorders; therefore, plasma lipid levels including triglycerides, cholesterol, low density lipoprotein and HDL, and apolipoprotein B100 levels should be assessed.
Various treatment options are available for XP, but none of them produce satisfactory results. Medical management involves lifestyle modifications such as regular physical exercise and low-fat diet in addition to lipid-lowering drugs. Although important in the overall care of a patient with abnormal lipids, medical management has a limited role in the treatment of XP. Various surgical modalities available for XP treatment are simple surgical excision, laser therapy, chemical cauterization with TCA, RF, and cryotherapy.
Surgical excision
Surgical excision has been the treatment of choice of XP for decades. It is undertaken in:
- cases with defined familial hyperlipoproteinemia;
- involvement of all four eyelids;
- more than one recurrence.
According to Lee et al,11 patients can be classified into four grades depending on the location and extent of the lesion. Grade I are patients with lesions on the upper eyelids only. Grade II are patients in whom lesions extended to the medial canthal area. Grade III are patients with lesions on the medial side of both upper and lower eyelids. Grade IV are patients with diffuse involvement on medial and lateral sides of the upper and lower eyelids. Moreover, the height of the lesions should be noted.
Simple excision with or without blepharoplasty and medial epicanthoplasty can be conducted in grades I and II lesions, whereas, in advanced cases, uncapping surgery, local flaps, and skin grafts can be carried out. The most common method of surgery is full-thickness skin excision. In XP that infiltrates the muscle layer, muscle resection is required.12
There are many disadvantages associated with surgery. There is always need of systemic or local anesthesia for the procedure. Surgical excision is often followed by slight scarring, regardless of whether wound closure is achieved through primary closure, full-thickness skin grafting,13,14 or granulation.15 It can cause ectropion and dyspigmentation as postoperative complications.
Laser therapy
The first report of light for the treatment of XP was given by Meyer-Schwickerath.16 He used xenon light in a procedure which was not simple but required several sittings.
Laser is an ideal therapy for XP because of its superficial location. Laser therapy works on the principle of destruction of perivascular foam cells through the caloric energy that originates from coagulation of vessels present in the stratum corneum. Furthermore, the coagulation of vessels prevents recurrence by blocking lipid leakage into tissues.17 Precise photoablation and coagulation of the skin allow bloodless removal of lesions, with minimal scarring, pain, and perilesional inflammation; moreover, it reduces the risk of secondary infection.18
Various types of lasers have been tried, including carbon dioxide laser,2 Argon laser,17 Er: YAG laser,19 Q-switched Nd: YAG laser,20 and pulsed dye laser.21
A slit-lamp-mounted argon laser (blue–green) with average wavelength of 514 nm, spot size 700 μm on continuous mode duration, and energy output from 500 to 750 mW, based on the tissue response, is used. Energy is absorbed by skin chromophores and is then converted into heat, thus altering the foam cells, leading to resorption of lipoid material with adjacent thermal damage to the overlying epidermis.
Carbon dioxide and argon lasers have been used with good results, but with risk of scarring and pigmentary changes. A high recurrence rate within the first 12–16 months was also seen with argon lasers. The carbon dioxide laser provides better hemostasis and, thus, is better suited for deeper lesions.
Er: YAG and Q-switched Nd: YAG lasers are reported to induce greater swelling, bleeding, and crusting and are also less efficacious.22
Pulsed dye laser can be carried out without anesthesia, with excellent cosmetic results; however, it is effective in early vascular lesions.
Complications of laser therapy include persistent erythema, superficial depigmentation, scars, severe burns, transitory or permanent lower lid ectropion, and corneal injuries or ocular perforation if the procedure is undertaken in the periocular region.
Advantages of lasers include better acceptance, avoidance of surgery, minimal tissue loss, good functional and cosmetic results, and therapy repeatability. Moreover, the procedure is easy to perform and gives fast results. Disadvantages include high cost and unpredictable results. In addition, it is not possible to obtain a histopathological specimen.
Radiofrequency
For XP, RF is considered to be a easy, safe, quick, inexpensive, and effective treatment. In RF procedures, thermal energy induces ionic agitation with vaporization at the cellular level in tissues. It uses a controlled RF current to reduce the tissue volume in a precise and controlled mode. RF leads to fibrotic changes and volume reduction in tissues during the healing period.23 The necrotic tissue in the lesions is gradually reabsorbed as part of the body’s natural process, thus reducing the tissue volume.
Treatment sites should be first cleaned with 10% povidone iodine, normal standardized solution, followed by topical anesthetic cream (lidocaine with prilocaine) applied to the lesions 30 min before treatment. Cosmetic results are satisfactory. This technique treats lesions, with minimal impact on the surrounding tissues, making it appropriate for delicate areas. Temporary side effects include pain, pruritus, burning, swelling, and erythema. Complications, such as hypopigmentation, hyperpigmentation, and ectropion are noted, but rarely.
Trichloroacetic acid
TCA is an affordable and versatile treatment modality, particularly in the Indian setup. It is a short, simple, and inexpensive procedure. It has been observed that 100% TCA gives the best results in papulonodular lesions, 100% or 70% TCA give similar results in flat plaque xanthelasma, and, in macular lesions, 50% TCA is sufficient.20 The technique requires the applicator to be rotated in a circular fashion with the greatest amount of TCA at the margin of the lesion, followed by neutralization with sodium bicarbonate. Hypopigmentation is the commonest side effect, followed by hyperpigmentation, irritation, and pain. Scarring and atrophy are other rare side effects. A Koebner-like phenomenon was also reported with TCA application.24 Moreover, the depth of tissue penetration by the chemicals is hardly controllable; therefore, quite often, the therapeutic effect of chemical measures is unsatisfactory.25
Cryosurgery
Cryosurgery is one of the modern methods of treating XP. It is an outpatient procedure that is safe, relatively painless, effective, cosmetically acceptable, and free of any major complications. However, it requires multiple sittings, and post-inflammatory pigmentation can occur after the procedure. Dewan et al studied 100 cases of XP wherein they used a closed probe cryojet with nitrous oxide gas as the cryogen. After a single session of a freeze–thaw cycle lasting 15 s, all cases were followed up for 6 months. Lesions resolved in all patients except in a few who developed post-procedural post-inflammatory hypopigmentation. Twenty-six cases had XP recurrence. No scarring or milia were observed in any of the treated cases.26
Choice of treatment
If the patient has an underlying medical condition with abnormal lipid profile, the patient should be referred to an internal medicine specialist. If the patient is normolipidemic with no underlying medical condition, the lesion should be removed. The modality to be used depends upon the size and location of the lesion. For lesions limited to the superficial dermis, of height ≤5 mm, soft in consistency, and onset ≤1 year, surgery is generally not required. In these cases, other modalities such as laser therapy, RF, TCA peel, and cryotherapy can be individualized depending upon the patient’s need. For lesions involving the deep dermis and/or muscle, of height ≥5 mm, hard in consistency, and onset ≥1 year in addition to skin laxity, blepharochalasia, and need of aesthetic refinement, surgical excision is the most appropriate therapeutic option.9
An algorithm to treat a case of XP is given in Figure 1.
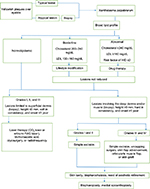  | Figure 1 Algorithm for management of a patient with xanthelasma palpebrarum. Abbreviations: IHD, ischemic heart disease; LDL, low density lipoprotein. |
Prognosis
Recurrence is common regardless of mode of treatment. Mendelson and Masson reported that 40% of patients with XP had recurrence after primary surgical excision, 60% after secondary excision, and 80% when all four eyelids were involved. He did not mention lesion extension in regard to depth, which may be the cause of a relatively high recurrence rate, if surgical excision is not deep.27 Of these failures, 26% occur within the first year and are more likely to occur in patients with hyperlipidemia syndromes and in those with all four eyelids affected.
Deep lesions should not be treated by lasers. Only small, shallow lesions (<5 mm) treated with laser therapy heal by secondary intention.28 Deep lesions may result in a partial eyelid defect. Therefore, surgical management is preferred over other modalities for deep lesions as there are lesser chances of eyelid deformity, with a better tolerated scar, and the recurrence rate is minimal.
Conclusion
XP is a common cutaneous xanthoma, which is a cosmetic concern for most patients. Different modalities of treatment such as simple surgical excision, cryotherapy, chemical peeling with TCA, RF, and laser are used for treating XP, and these need to be individualized according to the need of the patient.
Disclosure
The authors report no conflicts of interest in this work.
References
Segal P, Insull W Jr, Chambless LE, et al. The association of dyslipoproteinemia with corneal arcus and xanthelasma. The lipid research clinics program prevalence study. Circulation. 1986;73(1 Pt 2):I108–I118. | ||
Jonsson A, Sigfiisson N. Significance of xanthelasma palpebrarum in the normal population. Lancet. 1976;76:372. | ||
Gangopadhyay DN, Dey SK, Chandra M, Pal D, Chaudhary S. Serum lipid profile in xanthelasma. Indian J Dermatol. 1998;43:53–56. | ||
Raulin C, Schoenermark MP, Werner S, Greve B. Xanthelasma palpebrarum: treatment with the ultrapulsed CO2 laser. Lasers Surg Med. 1999;24(2):122–127. | ||
Rohrich RJ, Janis JE, Pownell PH. Xanthelasma palpebrarum: a review and current management principles. Plast Reconstr Surg. 2002;110(5):1310–1313. | ||
Martinez-Rovira GR. Xanthelasma in association with hyperthyroidism. JAMA. 1968;206(5):1081–1083. | ||
Pathania V, Chatterjee M. Ultrapulse carbon dioxide laser ablation of xanthelasma palpebrarum: a case series. J Cutan Aesthet Surg. 2015;8(1):46–49. | ||
Nair PA, Patel CR, Ganjiwale JD, Diwan NG, Jivani NB. Xanthelasma palpebrarum with arcus cornea: a clinical and biochemical study. Indian J Dermatol. 2016;61(3):295–300. | ||
Santaella RM, Ng JD, Wilson DJ. Carbon dioxide laser-induced combustion of extravasated intraocular silicone oil in the eyelid mimicking xanthelasma. Ophthal Plast Reconstr Surg. 2011;27(6):e163–e165. | ||
Christoffersen M, Frikke-Schmidt R, Schnohr P, Jensen GB, Nordestgaard BG, Tybjærg-Hansen A. Xanthelasmata, arcus corneae, and ischaemic vascular disease and death in general population: prospective cohort study. BMJ. 2011;343:d5497. | ||
Lee HY, Jin US, Minn KW, Park YO. Outcomes of surgical management of xanthelasma palpebrarum. Arch Plast Surg. 2013;40(4):380–386. | ||
Mittelviefhaus H, Kreusser C, Bohringer D, Auw-Hädrich C. The underestimated depth of tissue invasion of xanthelasma: a histological study. Klin Monbl Augenheilkd. 2011;228(1):14–18. | ||
Parkes ML, Waller TS. Xanthelasma palpebrarum ectropion. Laryngoscope. 1994;1984:1238–1240. | ||
Rose EH, Vistnes LM. Unilateral invasive xanthelasma palpebrarum. Ophthal Plast Reconstr Surg. 1987;3(2):91–94. | ||
Eedy DJ. Treatment of xanthelasma by excision with secondary intention healing. Clin Exp Dermatol. 1996;21(4):273–275. | ||
McBurney E. Clinical usefulness of the argon laser for the 1990s. J Dermatol Surg Oncol. 1993;19(4):358–362. | ||
Abdelkader M, Alashry SE. Argon laser versus erbium: YAG laser in the treatment of xanthelasma palpebrarum. Saudi J Ophthalmol. 2015;29(2):116–120. | ||
Hintschich C. Argonlaserkoagulation von Xanthelasmen [Argon laser coagulation of xanthelasmas]. Ophthalmologe. 1995;92:885–891. German | ||
Borelli C, Kaudewitz P. Xanthelasma palpebrarum: treatment with the erbium: YAG laser. Lasers Surg Med. 2001;29(3):260–264. | ||
Fusade T. Treatment of xanthelasma palpebrarum by 1064-nmQ-switched Nd: YAG laser: a study of 11 cases. Br J Dermatol. 2008;158(1):84–87. | ||
Karsai S, Czarnecka A, Raulin C. Treatment of xanthelasma palpebrarum using a pulsed dye laser: a prospective clinical trial in 38 cases. Dermatol Surg. 2010;36(5):610–617. | ||
Karsai S, Schmitt L, Raulin C. Is Q-switched neodymium-doped yttrium aluminium garnet laser an effective approach to treat xanthelasma palpebrarum? Results from a clinical study of 76 cases. Dermatol Surg. 2009;35(12):1962–1969. | ||
Mehmet Akdag. The effects of radiofrequency in xanthelasma of eyelid: case report. J Int Dent Med Res. 2013;6:128–131. | ||
Haque MU, Ramesh V. Evaluation of three different strengths of trichloroacetic acid in xanthelasma palpebrarum. J Dermatolog Treat. 2006;17(1):48–50. | ||
Akhyani M, Daneshpazhooh M, Jafari AK, Naraghi ZS, Farahami F, Toosi S. Koebner phenomenon in xanthelasma after treatment with trichloroacetic acid. Dermatol Online J. 2006;12(2):12. | ||
Dewan SP, Kaur A, Gupta RK. Effectiveness of cryosurgery in xanthelasma palpebrarum. Indian J Dermatol Venereol Leprol. 1995;61(1):4–7. | ||
Mendelson BC, Masson JK. Xanthelasma: follow-up on results after surgical excision. Plast Reconstr Surg. 1976;58(5):535–538. | ||
Ozdöl S, Sahin S, Tokgözoğlu L. Xanthelasma palpebrarum and its relation to atherosclerotic risk factors and lipoprotein (a). Int J Dermatol. 2008;47(8):785–789. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
