Back to Journals » Research and Reports in Urology » Volume 13
What are the Optimal Renal Ultrasound Parameters for Detecting Small Kidney in Young Children?
Authors Kon M, Nakamura M, Moriya K , Nishimura Y, Hirata Y, Nishida M , Higuchi M, Kitta T , Shinohara N
Received 7 May 2021
Accepted for publication 8 September 2021
Published 27 October 2021 Volume 2021:13 Pages 767—772
DOI https://doi.org/10.2147/RRU.S318793
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jan Colli
Masafumi Kon,1 Michiko Nakamura,1 Kimihiko Moriya,1,2 Yoko Nishimura,1 Yurie Hirata,1 Mutsumi Nishida,3 Madoka Higuchi,1 Takeya Kitta,1 Nobuo Shinohara1
1Department of Renal and Genitourinary Surgery, Hokkaido University Graduate School of Medicine, Sapporo, Japan; 2Department of Urology, Sapporo City General Hospital, Sapporo, Japan; 3Diagnostic Center for Sonography and Division of Laboratory and Transfusion Medicine, Hokkaido University Hospital, Sapporo, Japan
Correspondence: Kimihiko Moriya
Department of Urology, Sapporo City General Hospital, 1-1 North 11, West 13, Chuo-ku, Sapporo, 060-8604, Japan
Tel +81-11-726-2211
Fax +81-11-726-7912
Email [email protected]
Introduction: Recent guidelines do not recommend routine screening of vesicoureteral reflux after a first febrile urinary tract infection in children without abnormal findings on ultrasound or atypical/recurrent urinary tract infection. Currently, there are no clear ultrasonographic parameters for detecting abnormalities in renal size, especially in young children. The aim of the present study was to determine an optimal cutoff value for detecting small kidney in children without apparent congenital anomalies except vesicoureteral reflux by retrospective chart review.
Patients and Methods: Children aged ≤ 3 years who had undergone nuclear renal scans and ultrasound were enrolled. Small kidney was defined as split renal function of < 40%. Optimal cutoff values of various ultrasonographic parameters for detecting small kidney were calculated.
Results: Of the 69 children included in the present study, small kidney was identified in 20. There was a significant difference in renal size between each kidney in patients with small kidney, whereas there was no significant difference in those without small kidney. With a ratio of estimated renal area of 74.26%, maximum area under the curve with the highest sensitivity, specificity, positive predictive value, negative predictive value, and accuracy rate were obtained. In addition, simple measurement of renal length with a cutoff of 4.97 cm showed high specificity comparable with estimated renal area.
Conclusion: Small kidney may be screened by two-dimensional measurement on ultrasonographic examination, even in young children. With the cutoff described, risk stratification or an individualized approach may be possible.
Keywords: small kidney, ultrasound, screening, cutoff value
Introduction
Primary vesicoureteral reflux (VUR) is the most common congenital anomaly of the urinary tract (UT). In infancy, VUR is commonly diagnosed following demonstration of prenatally detected dilatation of the UT or during investigation of UT infection (UTI). While routine voiding cystourethrography (VCUG) to detect VUR after the first febrile UTI was not recommended in recent guidelines, it is indicated when abnormal findings on ultrasound (US) are detected or when atypical or recurrent UTI is observed.1–3
Small kidney is a common finding in infants or young children with high-grade VUR, especially in boys. Small kidney is recognized as a hypo/dysplastic kidney resulting from disordered renal development4,5 because of an abnormal origin of the ureteral bud, which interacts suboptimally with the metanephric mesenchyme,6,7 or high-pressure voiding during gestation because of incomplete sphincter relaxation.5,8 Renal abnormalities on US or nuclear renal scan have been reported in 12%–50% of patients with VUR detected in infancy.9–11
To date, no simple US parameters for detecting renal abnormalities, especially small kidney, have been reported, although VCUG is recommended when abnormal findings are observed on US. It has been demonstrated that volumetry on computed tomography or magnetic resonance imaging predicts the affected kidney’s function, which is traditionally evaluated on nuclear renal scan.12,13 We speculated that small kidney may be screened by measuring bilateral renal size, including length, area, and volume, on US.
We routinely perform 99mTc- DMSA renal scans and US of kidney and bladder for evaluation after a febrile UTI or US abnormality that is suspected to be a congenital anomaly of the kidney and UT. The aim of the present study was to determine an optimal cut-off value for detecting small kidney on US in young children without apparent congenital anomalies except VUR by retrospective chart review.
Patients and Methods
Patients
Medical charts of children aged ≤3 years who had undergone 99mTc-DMSA renal scans and US for the evaluation of UTI, VUR, or US abnormalities between September 2011 and May 2019 were retrospectively reviewed. Patients with grade 3 or grade 4 hydronephrosis were excluded from this study, because dilation of the renal pelvis may affect US evaluation of kidney size. Patients with congenital anomalies, such as ureterocele, posterior urethral valve, anorectal malformation, cloacal anomaly, spina bifida, hypospadias, or fused kidney were also excluded. Informed consent was waived due to the retrospective nature of the study.
Imaging Studies
In patients who presented with febrile UTI, 99mTc-DMSA renal scans and US evaluation were performed at 3 months or later after UTI. US evaluations were performed without sedation. Renal length and width of each kidney were measured on US longitudinal scans (Figure 1). 99mTc-DMSA renal scans were performed under sedation. Small kidney was defined when split renal function (SRF) was <40% on 99mTc-DMSA uptake.
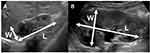 |
Figure 1 Renal size measurement. Longitudinal scan of a small kidney (A) and contralateral kidney (B). Length (L) and width (W) of each kidney were measured. |
Length, estimated renal area (eArea; length × width × pi/4), and estimated renal volume (eVol; length × width × width × Pi/6) in the smaller kidney were used to evaluate the optimal parameters for detection of small kidney in the present study. In addition, ratio of length, eArea, and eVol between both kidneys (smaller divided by larger kidney) were also calculated.
Statistical Analyses
Mann–Whitney U and Chi-square tests were used to compare patients with or without small kidney. Wilcoxon signed-rank tests were for comparison of US parameters between each of the kidneys. A receiver-operating characteristic (ROC) curve was used to determine cutoff values of each parameter for detecting small kidney. Sensitivity, specificity, positive predictive value, and negative predictive value were calculated for each parameter.
The protocol for the present study was approved by the Ethics Committee of Hokkaido University Hospital (018-0346).
Results
Patient Characteristics
The present study enrolled 69 patients (41 boys and 28 girls), and their characteristics are summarized in Table 1. Median age at US and 99mTc-DMSA renal scan was 10.9 months. Small kidney was identified in 20 children (29%), and incidence in boys was significantly higher (p=0.001). VCUG was performed in all, except one case without small kidney. VUR was identified more frequently in patients with small kidney had who received VCUG; however, the difference was not significant (p=0.0748). However, the incidence of high-grade VUR (grade 4–5) was significantly higher in patients with small kidney (p=0.0342). Age at US or 99mTc-DMSA renal scan or incidence of mild hydronephrosis (SFU1–2) were not significantly different between patients with or without small kidney.
 |
Table 1 Characteristics of patients |
Comparison of Kidney Size Between Each Side
Median SRF of the lower uptake side on 99mTc-DMSA renal scan was 30.4% (13.0%–39.1%) in patients with small kidney compared to 47.7% (40.0%–49.9%) in those without small kidney. In 20 patients with small kidney, the kidney with lower SRF had a shorter length in all, whereas lower eArea or eVol was identified in 19 patients. Conversely, in 49 patients without small kidney, the kidney with lower SRF showed shorter length, lower eArea, or eVol in 25, 28, and 28, respectively. While length, eArea, and eVol were not significantly different between each side in patients without small kidney, these parameters were significantly different among patients with small kidney (Table 2).
 |
Table 2 Difference in parameters between kidneys in patients with or without small kidney |
Impact of Each Parameter for Detecting Small Kidney
Cutoff values for detection of small kidney estimated by the ROC curve was 4.97 cm length, 11.02 cm2 eArea, 17.42 mL eVol, 90.85% ratio of length, 74.26% ratio of eArea, and 63.84% ratio of eVOL. Among these parameters, maximum area under the curve (AUC) was obtained using a ratio of eArea of 74.26%, with the highest sensitivity (80.0%), specificity (89.8%), positive predictive value (76.2%), negative predictive value (91.67%), and accuracy rate (86.96%) when compared with other parameters. Simple measurement of length showed high specificity (87.76%) comparable with the ratio of eArea (Table 3).
 |
Table 3 Impact of each parameter on detecting small kidney |
Discussion
In this retrospective study, we report that small kidney may be screened by two-dimensional measurement of renal US in patients aged ≤3 years. By using a ratio of eArea of 74.26%, we obtained maximum AUC with highest sensitivity, specificity, positive predictive value, negative predictive value, and accuracy rate. In addition, simple measurement of renal length with a cutoff of 4.97 cm showed high specificity, followed by ratio of eArea.
UTI is one of the most common bacterial infections in children. Its incidence in infants and young children aged between 2 months and 2 years is reported to be about 15%.14 While recurrent febrile UTI is bothersome and there is a risk of renal impairment, recent guidelines do not recommend routine VCUG to detect VUR after the first febrile UTI in patients without abnormal findings on US or atypical or recurrent UTI.1–3
US is the preferred tool to evaluate the kidneys and UT in pediatric patients because it is safe, readily available, nonionizing, and cost-effective. While it is credited with low sensitivity and positive and negative predictive value in detecting renal scars,15 it is the accepted method for assessment of renal size in children.16 Nomograms of renal size using US in healthy children with normal renal function have been reported;17,18 however, accepted cutoff values for evaluating abnormal renal size have not been reported. While SRF in children was estimated by measuring kidney size on US in a limited number of studies, those that focused on infants or very young children were scarce.19,20
Kidney length is the simplest and most commonly used approach to evaluate renal size. In the present study, a cutoff of 4.97 cm showed high specificity, indicating that a kidney longer than this is less likely to be small kidney. In a previous study investigating kidney length in normal healthy children in an Australian population, Coombs et al found that mean kidney lengths among infants were 5.6 (95% CI 5.5–5.8) cm for the left and 5.5 (95% CI 5.3–5.6) cm for the right.18 Furthermore, Luk et al reported that mean kidney lengths among infants were 5.67 cm for the left and 5.55 cm for the right kidney.17 According to these values, a cutoff of 4.97 cm is suitable for screening for small kidney by US. Although racial variation in renal size has been reported,17,21 our data were based on a Japanese population, whereas the studies by Coombs et al18 and Luk et al17 were based on an Australian population with diverse ethnicity and an Asian population, respectively. On the other hand, Farhat et al demonstrated that sonographic findings of decreased renal length (<50th percentile for age) were strongly correlated with renal hypoplasia on renal scans.19 Comparison with age-related renal size would be a reasonable approach to define the cutoff, because kidney size in children changes with their growth. However, development of a referenced normal size across various races at each age would be relatively complex.
The maximum AUC with the highest sensitivity, specificity, positive predictive value, negative predictive value, and accuracy rate were obtained by using a ratio of eArea of 88.06% in the present study. Comparison with the contralateral kidney would be a promising approach to screen the unilateral small kidney. Weitz et al demonstrated that relative renal volume (evaluated by three-dimensional measurements) in 85 children with primary VUR was significantly correlated with SRF, which was estimated using 99mTc-MAG3 renal scans.20 A similar finding was reported by Sargent et al, who calculated the mean difference between relative volume and SRF in 19 children with various renal disorders using 99mTc-DMSA renal scans.16 The present study demonstrated that the ratio of eVol showed the same sensitivity as the ratio of eArea and similar but slightly lower positive predictive value, negative predictive value, and accuracy rate. As only two-dimensional measurements of renal size were obtained on US evaluations in the present study due to its retrospective nature, eVol was calculated from these. This may be the reason that the ratio of eArea was slightly superior to the ratio of eVol in detecting small kidney. However, as previous studies have identified a good correlation between renal function and renal volume, as well as renal area,22,23 evaluation of eArea may be another choice for renal size measurement. Furthermore, two-dimensional measurements may be performed using only a single image on the longitudinal scan section, which is a simple procedure.
Renal abnormality on 99mTc-DMSA renal scans has been reported to be a risk factor of recurrent UTI or breakthrough UTI.24–27 Accordingly, risk stratification may be possible by detection of small kidney using screening US, even in initial febrile UTI episodes. In addition, incidence of high-grade VUR was significantly higher in patients with small kidney. High-grade VUR has also been reported as a risk factor of recurrent UTI or breakthrough UTI.27–29 Therefore, an individualized approach that includes indication of VCUG or 99mTc-DMSA renal scan, or administration of continuous antibiotic prophylaxis, may be necessary even after the first febrile UTI by screening for small kidney on US.
The limitations of the present study include the retrospective design, the small sample (n=69), and single institutional experience. To clarify our findings, a multi-institutional prospective study on a larger number of patients is necessary.
In conclusion, small kidney may be screened by two-dimensional measurements on US examination in small children. By using the cutoff value demonstrated in the current study (ratio of eArea of 74.26% or maximum renal length of 4.97 cm), risk stratification or an individualized approach may be possible.
Ethics Approval
This study was approved by the institutional review board (protocol 018-0346). The need for written informed consent was waived, because this study was conducted by retrospective chart review. Data were gathered from the institution database with permission. Patient data were anonymized and maintained with confidentiality throughout the study. This study was performed in accordance with the Declaration of Helsinki.
Author Contributions
Masafumi Kon contributed to conception and design, data analysis, and manuscript writing/editing. Michiko Nakamura collected and analyzed the data. Kimihiko Moriya was involved in conception and design, data collection, data analysis, and critical revision of the manuscript for scientific and factual content. Yoko Nishimura, Yurie Hirata, and Mutsumi Nishida collected and analyzed the data. Madoka Higuchi and Takeya Kitta critically revised the manuscript for scientific and factual content. Nobuo Shinohara supervised the study. All authors contributed to data analysis, drafting, or revising the article, have agreed on the journal to which the article will be submitted, gave final approval to the version to be published, and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests.
References
1. Stein R, Dogan HS, Hoebeke P, et al. Urinary tract infections in children: EAU/ESPU guidelines. Eur Urol. 2015;67(3):546–558. doi:10.1016/j.eururo.2014.11.007
2. Subcommittee on Urinary Tract Infection. Reaffirmation of AAP clinical practice guideline: the diagnosis and management of the initial urinary tract infection in febrile infants and young children 2–24 months of age. Pediatrics. 2016;138:6.
3. Buettcher M, Trueck J, Niederer-Loher A, et al. Swiss consensus recommendations on urinary tract infections in children. Eur J Pediatr. 2021;180(3):663–674.
4. Cendron M. Reflux nephropathy. J Pediatr Urol. 2008;4(6):414–421. doi:10.1016/j.jpurol.2008.04.009
5. Peters C, Rushton HG. Vesicoureteral reflux associated renal damage: congenital reflux nephropathy and acquired renal scarring. J Urol. 2010;184(1):265–273. doi:10.1016/j.juro.2010.03.076
6. Mackie GG, Stephens FD. Duplex kidneys: a correlation of renal dysplasia with position of the ureteral orifice. J Urol. 1975;114(2):274–280. doi:10.1016/S0022-5347(17)67007-1
7. Murawski IJ, Gupta IR. Vesicoureteric reflux and renal malformations: a developmental problem. Clin Genet. 2006;69(2):105–117. doi:10.1111/j.1399-0004.2005.00562.x
8. Gobet R, Cisek LJ, Chang B, Barnewolt CE, Retik AB, Peters CA. Experimental fetal vesicoureteral reflux induces renal tubular and glomerular damage, and is associated with persistent bladder instability. J Urol. 1999;162(3 Pt 2):1090–1095. doi:10.1016/S0022-5347(01)68078-9
9. Nakai H, Kakizaki H, Konda R, et al. Clinical characteristics of primary vesicoureteral reflux in infants: multicenter retrospective study in Japan. J Urol. 2003;169(1):309–312. doi:10.1016/S0022-5347(05)64113-4
10. Mohanan N, Colhoun E, Puri P. Renal parenchymal damage in intermediate and high grade infantile vesicoureteral reflux. J Urol. 2008;180(4 Suppl):
11. Hunziker M, Colhoun E, Puri P. Prevalence and predictors of renal functional abnormalities of high grade vesicoureteral reflux. J Urol. 2013;190(4 Suppl):1490–1494. doi:10.1016/j.juro.2013.01.068
12. Houbois C, Haneder S, Merkt M, et al. Can computed tomography volumetry of the renal cortex replace MAG3-scintigraphy in all patients for determining split renal function? Eur J Radiol. 2018;103:105–111. doi:10.1016/j.ejrad.2018.04.016
13. Siedek F, Haneder S, Dörner J, et al. Estimation of split renal function using different volumetric methods: inter- and intraindividual comparison between MRI and CT. Abdom Imaging. 2019;44(4):1481–1492. doi:10.1007/s00261-018-1857-9
14. Bonadio W, Maida G. Urinary tract infection in outpatient febrile infants younger than 30 days of age: a 10-year evaluation. Pediatr Infect Dis J. 2014;33(4):342–344. doi:10.1097/INF.0000000000000110
15. Moorthy I, Wheat D, Gordon I. Ultrasonography in the evaluation of renal scarring using DMSA scan as the gold standard. Pediatr Nephrol. 2004;19(2):153–156. doi:10.1007/s00467-003-1363-2
16. Sargent MA, Gupta SC. Sonographic measurement of relative renal volume in children: comparison with scintigraphic determination of relative renal function. AJR Am J Roentgenol. 1993;161(1):157–160. doi:10.2214/ajr.161.1.8390789
17. Luk WH, Lo AX, Au-Yeung AW, et al. Renal length nomogram in Hong Kong Asian children: sonographic measurement and multivariable approach. J Paediatr Child Health. 2010;46(6):310–315. doi:10.1111/j.1440-1754.2010.01714.x
18. Coombs PR, Lavender I, Leung MYZ, et al. Normal sonographic renal length measurements in an Australian pediatric population. Pediatr Radiol. 2019;49(13):1754–1761. doi:10.1007/s00247-019-04486-2
19. Farhat W, McLorie G, Bagli D, Khoury A. Greater reliability of neonatal ultrasonography in defining renal hypoplasia with antenatal hydronephrosis and vesicoureteral reflux. Can J Urol. 2002;9(1):1459–1463.
20. Weitz M, Licht C, Müller M, Haber P. Renal ultrasound volume in children with primary vesicoureteral reflux allows functional assessment. J Pediatr Urol. 2013;9(6 Pt B):1077–1083. doi:10.1016/j.jpurol.2013.03.007
21. Chen JJ, Pugach J, Patel M, Luisiri A, Steinhardt GF. The renal length nomogram: multivariable approach. J Urol. 2002;168(5):2149–2152. doi:10.1016/S0022-5347(05)64339-X
22. Troell S, Berg U, Johansson B, Wikstad I. Ultrasonographic renal parenchymal volume related to kidney function and renal parenchymal area in children with recurrent urinary tract infections and asymptomatic bacteriuria. Acta Radiol Diagn. 1984;25(5):411–416. doi:10.1177/028418518402500512
23. Troell S, Berg U, Johansson B, Wikstad I. Comparison between renal parenchymal sonographic volume, renal parenchymal urographic area, glomerular filtration rate and renal plasma flow in children. Scand J Urol Nephrol. 1988;22(3):207–214. doi:10.1080/00365599.1988.11690413
24. Mingin GC, Nguyen HT, Baskin LS, Harlan S. Abnormal dimercapto-succinic acid scans predict an increased risk of breakthrough infection in children with vesicoureteral reflux. J Urol. 2004;172(3):
25. Nakamura M, Moriya K, Mitsui T, Tanaka H, Nonomura K. Abnormal dimercapto-succinic acid scan is a predictive factor of breakthrough urinary tract infection in children with primary vesicoureteral reflux. J Urol. 2009;182(4 Suppl):1694–1697. doi:10.1016/j.juro.2009.03.070
26. Yamazaki Y, Shiroyanagi Y, Matsuno D, Nishi M. Predicting early recurrent urinary tract infection in pretoilet trained children with vesicoureteral reflux. J Urol. 2009;182(4 Suppl):1699–1702. doi:10.1016/j.juro.2009.03.020
27. Shiraishi K, Yoshino K, Watanabe M, Matsuyama H, Tanikaze S. Risk factors for breakthrough infection in children with primary vesicoureteral reflux. J Urol. 2010;183(4):1527–1531. doi:10.1016/j.juro.2009.12.039
28. Dias CS, Silva JM, Diniz JS, et al. Risk factors for recurrent urinary tract infections in a cohort of patients with primary vesicoureteral reflux. Pediatr Infect Dis J. 2010;29(2):139–144. doi:10.1097/INF.0b013e3181b8e85f
29. Park S, Han JY, Kim KS. Risk factors for recurrent urinary tract infection in infants with vesicoureteral reflux during prophylactic treatment: effect of delayed contrast passage on voiding cystourethrogram. Urology. 2011;78(1):170–173. doi:10.1016/j.urology.2010.12.023
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
