Back to Journals » Clinical Optometry » Volume 14
Wear Experience of a Water Surface Daily Disposable Contact Lens in Existing Silicone Hydrogel Planned Replacement Lens Wearers
Authors Rutschilling R , Fogt JS
Received 25 December 2021
Accepted for publication 10 February 2022
Published 4 March 2022 Volume 2022:14 Pages 27—34
DOI https://doi.org/10.2147/OPTO.S353666
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Mr Simon Berry
Ryan Rutschilling, Jennifer Swingle Fogt
College of Optometry, The Ohio State University, Columbus, OH, USA
Correspondence: Jennifer Swingle Fogt, College of Optometry, The Ohio State University, 338 W. 10th Ave, Columbus, OH, 43210, USA, Tel +1 614 292 0882, Email [email protected]
Purpose: The health benefits of silicone hydrogel lens materials and a daily replacement modality have been demonstrated in previous studies; however, existing planned replacement lens wearers may resist changing to a new lens replacement schedule. The purpose of this study is to evaluate the wear experience of satisfied planned replacement silicone hydrogel wearers when refit into a silicone hydrogel daily disposable lens.
Patients and Methods: In this open-label, non-comparison study, satisfied wearers of two week planned replacement contact lenses were evaluated for inclusion criteria and refit with optimized prescriptions in their habitual lenses. At a follow-up visit one week later, participants were refit with the study daily disposable lenses and completed visual analog scale (VAS) surveys of initial quality of vision, comfort, and satisfaction. Participants returned for a final visit after two weeks of wearing the study daily disposable lenses. At the final visit, VAS surveys for both overall and end of day (EOD) vision, comfort, and dryness were completed. Overall median and interquartile range (IQR) were assessed for all surveys in the study.
Results: Thirty individuals completed the study (29.1 ± 7.8 years old; 19 female). Median (IQR) results for the initial impression VAS surveys were 92.50(11.75) for quality of vision, 92.50(18.00) for comfort, and 93.00(18.00) for satisfaction. Final VAS survey results revealed median scores of 87.50(25.00) for EOD quality of vision and 82.50(51.25) for EOD comfort. The median overall quality of vision was 91.00(17.00) and overall comfort was 93.00(28.50). Median (IQR) overall dryness was 28.50(49.00) and median EOD dryness was 30.50(64.25).
Conclusion: The findings of this study suggest that providers can successfully refit satisfied wearers of early generation silicone hydrogel planned replacement lenses into a new generation silicone hydrogel daily disposable lens while maintaining satisfaction.
Keywords: soft contact lens, vision, comfort, wear experience
Corrigendum for this paper has been published
Introduction
Daily disposable contact lens options have continuously expanded since their introduction in 1994.1 These daily replacement lenses offer numerous benefits to contact lens wearers. Wearers do not require additional supplies such as multipurpose solutions and storage cases, nor do they require the time and effort required to clean and store lenses after removal. Since the lenses are replaced daily, there is a lower chance that tear proteins can build up on the lenses to reduce comfort.2 Additionally, the frequent replacement of daily disposable lenses makes infections related to contact lens wear less likely.3 This is largely related to the decrease in non-compliance also known as misuse, whether intentional or unintentional, of contact lenses and their respective products (ie solutions, storage cases, etc.) when using daily disposable contact lenses.4 Improvements in contact lens compliance decreases the risk for ocular complications such as dryness, giant papillary conjunctivitis, sterile inflammatory ulcers, stromal opacities, CIEs, and microbial keratitis (ie bacterial, fungal, protozoan).5–8 Not only can these complications result in discomfort while wearing contact lenses, some may result in permanent loss of visual acuity.
Contact lens comfort remains an important aspect of successful contact lens wear. Discomfort is the leading cause of contact lens discontinuation, also known as lens wear “drop out”.9 A recent review of soft contact lens materials, designs, and fitting characteristics found that surface properties affect the coefficient of friction of a lens and therefore play a large part in lens comfort.10 Surface treatments can include modifications of the lens material, addition of wetting agents, or various structural changes at the outer surface of the lens.10 A study of non-invasive tear breakup time found that a lens with a high water surface which encapsulated a silicone core had a statistically significant decrease in tear breakup time when compared to other daily disposable silicone hydrogel lenses.11 Comfort of different soft contact lenses can also vary due to other factors such as edge shape and stiffness of the material,12 and water content and salt diffusivity of the lens material;13 these factors make it possible to have a different wear experience with different soft contact lenses.
Despite the fact that labels on contact lenses may show similar or identical sizes, powers and/or base curves, every contact lens design and material can perform differently on an eye.14 Evaluation of fitting characteristics and subjective evaluations of comfort and vision are important when considering refitting an existing contact lens wearer. This information is of particular interest for patients that are wearing certain established early generation planned replacement silicone hydrogel lens materials that are then switched to newer generation silicone hydrogel daily disposable lens materials. Practitioners are often hesitant to refit these wearers despite the benefits of daily disposable wear due to the possibility that patients may be resistant to change or that a satisfied lens wearer may not be satisfied with a different lens material.
The purpose of this study was to determine if patients who attest to being satisfied with their current silicone hydrogel planned replacement lenses can be refit into a daily disposable lens and continue to be satisfied with their contact lens wear experience.
Materials and Methods
Study Design
This study was an interventional, non-randomized clinical trial, approved by The Ohio State University Institutional Review Board, registered on clinicaltrials.gov (NCT04296877) and carried out in accordance with the Declaration of Helsinki. The study consisted of three visits, with the habitual lenses being refit to optimize vision at Visit 1 before refitting the participants into the daily disposable study lenses at Visit 2. The study end points were the subjective assessment of the daily disposable lens by surveys, including a Visual Analog Scale (VAS) survey of comfort, vision, and dryness, the Contact Lens Dry Eye Questionnaire – 8 (CLDEQ-8),15 and a wear experience questionnaire. A diagram of the study design can be found in Figure 1.
 |
Figure 1 Study design. |
Study Materials
In this study, satisfied wearers of a widely used planned replacement lens (Acuvue® Oasys®; Johnson and Johnson Vision, Jacksonville, FL, USA) after being refit into a daily disposable contact lens with a 51% water silicone hydrogel core with a thin hydrogel surface containing over 80% water (PRECISION1®; Alcon, Fort Worth, TX, USA).16
Eligibility Criteria
Current habitual wearers of the planned replacement lenses who were 18 years of age or older were recruited. Participants with 20/25 visual acuity or better with habitual lenses, who were in good general health, defined by no active inflammatory disease and no changes in medications in the past month, were included in the study. Exclusion criteria included extended wear of habitual lenses, the presence of ocular inflammation or ocular infection as determined by the study investigator, and pregnancy or lactation.
Study Assessments and Procedures
Prescreening of potential participants included verification of their current contact lens parameters and required an affirmative response to the question, “Are you satisfied with your current contact lenses?” At Visit 1, participants completed the informed consent process. Wearing their habitual current planned replacement lenses, participants completed entering LogMAR visual acuity testing using a standardized ETDRS visual acuity chart on a light box positioned at 4 meters. Participants then removed their contact lenses for an anterior segment ocular health evaluation using biomicroscopy. If inclusion and exclusion criteria were met, the participant was refit with a new pair of their habitual planned replacement lenses to assure the lens fit and prescription was optimized before the refitting at Visit 2. Participants were instructed to continue their current cleaning and care regimen, reminded not to sleep while wearing the lenses, and were scheduled for a second study visit in one week.
Participants returned for a follow-up visit (Visit 2) in one week and arrived wearing the optimized habitual lenses. After confirmation that participants were still satisfied planned replacement contact lens wearers and assessments of ocular health were within normal limits, participants were fit with the daily disposable study lenses. Equivalent powers to the planned replacement lens were inserted and allowed to settle for ten minutes prior to evaluation and over-refraction. If lenses had acceptable movement and centration, the prescription was optimized if necessary, and LogMAR visual acuities were assessed. Participants then completed a Visual Analog Scale (VAS) survey via REDCap (Research Electronic Data Capture)17 using their smart phone. This survey used a slider button on the screen that could be adjusted along a 100 point scale with the participant’s finger to correspond with the score given. For example, the question about quality of vision said, “Set the slider to the position that best describes your initial Quality of Vision.” The slider scale was anchored with “POOR Quality” on one side and “EXCELLENT Quality” on the opposite end. See Figure 2 for survey topics and their anchoring scores.
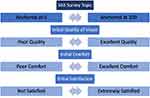 |
Figure 2 Initial visual analog scale (VAS) surveys presented and text describing each anchor, as presented to participants. |
Participants were told that the new lenses were daily replacement lenses and should be worn as long as they typically wear lenses throughout the day before being thrown away each night. Enough study lenses were dispensed so that the participant could wear lenses every day until the final study visit in two weeks (± 3 days).
At the final visit (Visit 3), participants completed a final evaluation of LogMAR visual acuity, lens fit, and ocular health. Participants completed REDCap surveys about their wear experience with the daily disposable study lenses that included: VAS surveys of overall vision, comfort and dryness, and end of day vision, comfort and dryness (Figure 3), CLDEQ-8, and a lens experience survey.
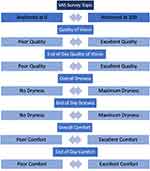 |
Figure 3 End of study visual analog scale (VAS) surveys presented and text describing each anchor, as presented to participants. |
The sample size of 30 participants was chosen for this pilot study and was based upon a previous contact lens study of lens comfort which also used VAS surveys.12 This study was exploratory and was not designed to prove superiority. All calculations were performed in Microsoft Excel (2016). Descriptive statistics were calculated for anterior segment and visual acuity findings. To minimize the impact of outliers on the dataset, median and interquartile range were calculated for the CLDEQ-8, VAS surveys, and lens wear experience surveys.
Results
Thirty participants (11 male and 19 female) completed all three visits and the average age was 29.1 ± 7.8 years (mean ± standard deviation). Twenty-five individuals identified as White, three as Asian, and two as more than one race; 27 were not Hispanic/Latinx, two were Hispanic/Latinx, and one was unknown/not reported. Throughout the study, no adverse events were contact lens related.
Evaluation of bulbar and palpebral conjunctiva, tarsal plate, and cornea by slit lamp with and without sodium fluorescein were clinically insignificant findings throughout the study and median values for all slit lamp findings were zero. Visual acuity with the daily disposable lenses remained high from initial (beginning of wear) to final (end of wear) acuity. Initial median (interquartile range) LogMAR acuities when wearing the study lenses were −0.18(0.10), −0.16(0.08), and −0.22(0.08) for OD, OS, and OU respectively. Final LogMAR acuities were −0.16(0.12), −0.12(0.13), and −0.20(0.14) for OD, OS, and OU, respectively.
VAS surveys were taken initially after insertion of the daily disposable study lenses (Visit 2) and at the end of the study (Visit 3). Median (interquartile range) scores for the initial impressions of the study lenses were 92.50(11.75) for quality of vision, 92.50(18.00) for initial comfort, and 93.00(18.00) for initial satisfaction. The final VAS surveys after 2 weeks of wearing the study lens revealed median scores for end of day quality of vision [87.50(25.00)] and end of day comfort [82.50(51.25)]. Median VAS score of overall quality of vision was 91.00(17.00), and overall comfort was 93.00(28.50).
Contact lens related dryness was evaluated through with VAS surveys and the CLDEQ-8. Median (interquartile range) overall dryness was 28.50(49.00) and end of day dryness while wearing the contact lens was 30.50(64.25). CLDEQ-8 scores revealed a median (interquartile range) of 9.50(11.75).
At the end of the study, wear experience surveys on a scale of 1–10 found median (interquartile range) scores for convenience [10.00(1.00)]; ease of use [10.0(1.00)], and satisfaction [7.50(2.75)].
Discussion
It is unsurprising that slit lamp findings were unremarkable; when daily disposable lenses are worn compliantly, they have a lower likelihood of causing complications than planned replacement lenses.3,7,18,19 The daily replacement frequency improves patient contact lens compliance, although it does not totally mitigate it.20 Visual acuity remained exceptional throughout the study, with initial median (interquartile range) LogMAR acuities of −0.22(0.08) OU and final LogMAR acuities of −0.20(0.14) OU. These scores are equivalent to approximately 20/12.5 Snellen visual acuity. This acuity is also reflected in the high VAS scores of subjective initial and overall quality of vision.
In addition to excellent acuity, comfort is imperative for successful soft contact lens wear. Participants reported high levels of comfort after wearing the lenses initially in office, and comfort scores remained high for end of day comfort and overall comfort after wearing the study lenses for 2 weeks. More variability among end of day comfort scores is not that surprising given that contact lens comfort is known to decrease as soft lenses are worn during the day.21 Participants were instructed to wear the contact lenses as they normally wore lenses, so the large variability may be a reflection of the varied length of wear time that each participant considered the “end of day” while wearing the study lenses. Regardless, the high level of comfort provided by the daily disposable lens throughout this study is promising since contact lens discomfort, especially end of day discomfort, remains a major cause for the discontinuation of contact lens wear.9,22
A common cause for contact lens discomfort is ocular dryness and contact lens wear has been identified as a risk factor for developing dry eye.23 Both the VAS dryness surveys (overall and end of day dryness) indicated minimal dryness, although some variability was present. The median (interquartile range) CLDEQ-8 score of 9.50(11.75) was less than 12. Research has shown that CLDEQ-8 scores less than 12 were minimal and would likely not require intervention during lens wear.24 Since participant wear time and environment were not controlled in this study, it is possible that these factors played a role in the variability seen between wearers.
Convenience and ease of use scores of 10(1.00) after wearing the study lens for two weeks are likely a reflection of the participants appreciation for not having to clean and store lenses daily. The median satisfaction scores of 7.50(2.75) were especially impressive since these participants attested to also being satisfied with their habitual lenses. This should provide evidence to clinicians that even individuals who express satisfaction with their planned replacement lenses (particularly older generation silicone hydrogel), would likely also be satisfied when switched to new generation silicone hydrogel daily disposable lenses.
Although satisfaction with the study lens was rated high with a 7.50 median score, the wide interquartile range (2.75) of the response may be related to the lack of additional information given to these wearers. Factors such as cost of the lenses or mitigation of the environmental impacts of daily disposable lenses, such as recycling programs, were not discussed as part of this study. Future studies should consider adding qualifiers to questions that may be perceived to be related to other factors. An additional limitation is the open-labelled nature of the study, which may have introduced some bias into the responses. The high scores reflected in the wear experience survey questions at the end of this study may reflect the fact that many wearers find vision and comfort to be important factors in contact lens wear, regardless of cost or environmental impacts. Additionally, it should be noted that all participants in this study wore the same habitual planned replacement lenses and were fit into the same daily disposable lenses since this study was designed to be exploratory in nature. The habitual lenses chosen for this study were selected because of their wide usage in the United States; due to the smaller sample size of this exploratory study, additional planned replacement lenses were not included. More studies would need to be conducted to determine if these results can be found with other habitual planned replacement wearers and with other silicone hydrogel daily disposable lenses.
Comparing to historical comfort scores, the scores for comfort [93.00(28.50)] and end of day comfort [82.50(51.25)] found in this study population is very promising. A previous study by Maissa et al found that mean comfort VAS scores of various planned replacement lenses ranged from 85.8 to 90.3 for four different silicone hydrogel planned replacement lenses.12 The same study found evening comfort scores for the silicone hydrogel lenses ranged from 64.2 to 81.4.12 The similarity in the findings of the current study to those of previous findings further demonstrate that soft contact lens wearers can find similar comfort in silicone hydrogel daily disposable and planned replacement lenses. While refitting contact lens patients who report problems with their current lenses are often refit in order to find lenses which increase comfortable wear time and decrease dryness,25 there can be less motivation of patients and practitioners to refit satisfied contact lens wearers. The high scores for vision and comfort and the positive responses to the lens wear questionnaire in this study suggest that it is possible to refit satisfied planned replacement lens wearers with daily disposable lenses, which have been shown to have fewer complications,6–8 and continue to have satisfied wearers.
Conclusion
In this study, participants who were satisfied planned replacement soft lens wearers gave high rankings to their experience when refit into a daily disposable lens with water surface technology. Providers should continually advocate for their patient’s ocular health, and discuss the benefits of daily disposable lenses, even with those patients who are established wearers of planned replacement lenses. The high median satisfaction, comfort, and quality of vision scores with the daily disposable lenses suggest that eye care practitioners can successfully refit satisfied planned replacement wearers of early generation silicone hydrogel materials into a new generation silicone hydrogel daily disposable contact lens.
Data Sharing Statement
Data reported in this manuscript are available within the article. Study-level data including the study protocol are available. To request access to the data, the researcher must sign a data use agreement. All proposals should be directed to [email protected] for up to 36 months following article publication.
Acknowledgments
Funding for this investigator initiated trial was provided by Alcon, Inc., Fort Worth, TX, USA. The project described was supported in part by Award Number Grant UL1TR002733 from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Disclosure
R. Rutschilling reports a travel grant from CooperVision to attend the American Academy of Optometry meeting in 2019. J.S. Fogt reports research funding from Nevakar, Eyenovia, Alcon, Unicon, Bausch & Lomb; consulting for Contamac and Alcon. The authors report no other conflicts of interest in this work.
References
1. Efron N, Morgan PB, Helland M, et al. Daily disposable contact lens prescribing around the world. Cont Lens Anterior Eye. 2010;33(5):225–227. doi:10.1016/j.clae.2010.06.003
2. Gellatly KW, Brennan NA, Efron N. Visual decrement with deposit accumulation of HEMA contact lenses. Am J Optom Physiol Opt. 1988;65(12):937–941. doi:10.1097/00006324-198812000-00003
3. Chalmers RL, Keay L, McNally J, Kern J. Multicenter case-control study of the role of lens materials and care products on the development of corneal infiltrates. Optom Vis Sci. 2012;89(3):316–325. doi:10.1097/OPX.0b013e318240c7ff
4. Dumbleton K, Richter D, Woods C, Jones L, Fonn D. Compliance with contact lens replacement in Canada and the United States. Optom Vis Sci. 2010;87(2):131–139. doi:10.1097/OPX.0b013e3181ca32dc
5. Bui TH, Cavanagh HD, Robertson DM. Patient compliance during contact lens wear: perceptions, awareness, and behavior. Eye Contact Lens. 2010;36(6):334–339. doi:10.1097/ICL.0b013e3181f579f7
6. Alipour F, Khaheshi S, Soleimanzadeh M, Heidarzadeh S, Heydarzadeh S. Contact lens-related complications: a review. J Ophthalmic Vis Res. 2017;12(2):193–204. doi:10.4103/jovr.jovr_159_16
7. Steele KR, Szczotka-Flynn L. Epidemiology of contact lens-induced infiltrates: an updated review. Clin Exp Optom. 2017;100(5):473–481. doi:10.1111/cxo.12598
8. Rueff EM, Wolfe J, Bailey MD. A study of contact lens compliance in a non-clinical setting. Cont Lens Anterior Eye. 2019;42(5):557–561. doi:10.1016/j.clae.2019.03.001
9. Dumbleton K, Woods CA, Jones LW, Fonn D. The impact of contemporary contact lenses on contact lens discontinuation. Eye Contact Lens. 2013;39(1):93–99. doi:10.1097/ICL.0b013e318271caf4
10. Stapleton F, Tan J. Impact of contact lens material, design, and fitting on discomfort. Eye Contact Lens. 2017;43(1):32–39. doi:10.1097/ICL.0000000000000318
11. Wolffsohn JS, Mroczkowska S, Hunt OA, Bilkhu P, Drew T, Sheppard A. Crossover evaluation of silicone hydrogel daily disposable contact lenses. Optom Vis Sci. 2015;92(11):1063–1068. doi:10.1097/OPX.0000000000000706
12. Maissa C, Guillon M, Garofalo RJ. Contact lens-induced circumlimbal staining in silicone hydrogel contact lenses worn on a daily wear basis. Eye Contact Lens. 2012;38(1):16–26. doi:10.1097/ICL.0b013e31823bad46
13. Kim YH, Nguyen T, Lin MC, Peng CC, Radke CJ. Protection against corneal hyperosmolarity with soft-contact-lens wear. Prog Retin Eye Res. 2021;101012. doi:10.1016/j.preteyeres.2021.101012
14. Barr JT. “Generic” Soft Contact Lenses: Scientific, Clinical, and Regulatory Matters. Contact Lens Spectrum; 2021:37–40.
15. Koh S, Chalmers R, Kabata D, Shintani A, Nishida K. Translation and validation of the 8-item Contact Lens Dry Eye Questionnaire (CLDEQ-8) among Japanese soft contact lens wearers: the J-CLDEQ-8. Cont Lens Anterior Eye. 2019;42(5):533–539. doi:10.1016/j.clae.2019.03.002
16. Cummings S, Giedd B, Pearson C. Clinical performance of a novel daily disposable soft contact lens. J Contact Lens Res Sci. 2020;4(2):23–30. doi:10.22374/jclrs.v4i1.39
17. Harris PA, Taylor R, Thielke R, Payne J, Gonzales N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi:10.1016/j.jbi.2008.08.010
18. Insua Pereira E, Lira M. Comfort, ocular dryness, and equilibrium water content changes of daily disposable contact lenses. Eye Contact Lens. 2018;44(Suppl 2):S233–S240. doi:10.1097/ICL.0000000000000441
19. Carnt N, Jalbert I, Stretton S, Naduvilath T, Papas E. Solution toxicity in soft contact lens daily wear is associated with corneal inflammation. Optom Vis Sci. 2007;84(4):309–315. doi:10.1097/OPX.0b013e318046551b
20. Mickles CV, Kinoshita BT, Lam D, et al. The contact lens risk survey to assess risk of soft contact lens-related inflammatory events. Cont Lens Anterior Eye. 2021;44(1):35–41. doi:10.1016/j.clae.2020.11.013
21. Jones L, Brennan NA, Gonzalez-Meijome J, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the contact lens materials, design, and care subcommittee. Invest Ophthalmol Vis Sci. 2013;54(11):TFOS37–70. doi:10.1167/iovs.13-13215
22. Nichols KK, Redfern RL, Jacob JT, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. 2013;54(11):TFOS14–9. doi:10.1167/iovs.13-13074
23. Gomes JAP, Azar DT, Baudouin C, et al. TFOS DEWS II iatrogenic report. Ocul Surf. 2017;15(3):511–538. doi:10.1016/j.jtos.2017.05.004
24. Chalmers RL, Keay L, Hickson-Curran SB, Gleason WJ. Cutoff score and responsiveness of the 8-item Contact Lens Dry Eye Questionnaire (CLDEQ-8) in a large daily disposable contact lens registry. Cont Lens Anterior Eye. 2016;39(5):342–352. doi:10.1016/j.clae.2016.04.005
25. Hickson-Curran S, Spyridon M, Hunt C, Young G. The use of daily disposable lenses in problematic reusable contact lens wearers. Cont Lens Anterior Eye. 2014;37(4):285–291. doi:10.1016/j.clae.2014.03.002
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
