Back to Journals » Vascular Health and Risk Management » Volume 18
VTE Prophylaxis Therapy: Clinical Practice vs Clinical Guidelines
Authors Abukhalil AD , Nasser A , Khader H , Albandak M , Madia R, Al-Shami N , Naseef HA
Received 14 July 2022
Accepted for publication 31 August 2022
Published 2 September 2022 Volume 2022:18 Pages 701—710
DOI https://doi.org/10.2147/VHRM.S382050
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Pietro Scicchitano
Abdallah Damin Abukhalil, Alisse Nasser,* Hadeel Khader,* Miral Albandak,* Raed Madia, Ni’meh Al-Shami, Hani A Naseef
Pharmacy Department, Faculty of Pharmacy, Nursing and Health Professions, Birzeit University, Birzeit, West Bank, Palestine
*These authors contributed equally to this work
Correspondence: Abdallah Damin Abukhalil; Raed Madia, Pharmacy Department, Faculty of Pharmacy, Nursing and Health Professions, Birzeit University, Birzeit, West Bank, Palestine, Tel +970-598204036 ; +970-5114848, Fax +970-2-2982017, Email [email protected]; [email protected]
Introduction: Venous thromboembolism (VTE) is the most preventable complication in hospitalized patients. The main objective of this study was to evaluate the adherence of current clinical practice to the established guidelines at a Palestinian teaching hospital.
Methods: This cross-sectional, retrospective, observational study was conducted at a Palestinian Teaching Hospital. The medical records of patients admitted to the medical floor over 18 years of age and hospitalized for longer than 24 hours between January 1 and May 31, 2019, were included. Patients taking anticoagulants with incomplete or duplicated medical records were excluded from the study. A data collection sheet was developed, and clotting and bleeding risks were assessed using the Padua and IMPROVE risk assessment models (scores). The data were analyzed using IBM SPSS (version 25).
Results: In total, 408 patients were included in the study, 222 of whom received thromboprophylaxis (54.4%). Of the hospitalized patients, 112 (27.5%) had a high risk of developing VTE (Padua score ≥ 4), and 73 patients were eligible for VTE pharmacological prophylaxis; however, only 44 (60.3%) received the appropriate prophylaxis. In addition, 296 patients had low Padua scores, indicating that pharmacological prophylaxis was not indicated. However, 144 (48.6%) patients received prophylaxis. The mean Padua and IMPROVE risk scores were 2.25 ± 2.08 and 4.44 ± 2.72, respectively. Among the patients, 17.6% had a high risk of bleeding (IMPROVE score ≥ 7).
Conclusion: VTE prophylaxis among hospitalized medically ill patients was mostly inappropriate; 80.18% of the patients received inappropriate prophylaxis, and only 60.3% of eligible patients received appropriate prophylaxis. Adapting assessment models or checklists in clinical practice based on clinical guidelines for VTE risk stratification is a practical and effective method to improve VTE prophylaxis management and select the appropriate therapy to prevent toxicity or complication.
Keywords: VTE, VTE prophylaxis, medical patients, Padua score, improve score, VTE assessment
A Letter to the Editor has been published for this article.
A Response to Letter by Dr Güven has been published for this article.
Introduction
Venous thromboembolism (VTE) is a thromboembolic disorder that includes deep vein thrombosis (DVT) and pulmonary embolism (P.E.), which is the most preventable complication in hospitalized patients and a major cause of morbidity and mortality.1–3 DVT affects the body’s deep veins, particularly in the legs, hips, or knees, and occurs when a blood clot (thrombus) clogs them, whereas P.E. affects the lungs.4 Several risk factors have been associated with VTE, including patients with acute medical illness or those who underwent a surgical procedure that includes general anesthesia for more than 30 minutes, obese people, prolonged bed rest or long traveling hours, patients who had major orthopedic surgery or trauma such as hip, pelvis or leg fractures, pregnancy, hypercoagulable disorders, malignancy and patients taking estrogen therapy, tamoxifen, cancer therapy, or heparin; which could cause heparin-induced thrombocytopenia.5
Hospitalized patients are prone to developing VTE and other complications. The absolute risk of VTE among hospitalized patients is 10%–20%.6 Hospitalized patients have more than one risk factor for VTE.7,8 It is estimated that 50% of VTEs occur due to current or recent hospital admission for surgery or acute medical illness.7 In addition, hospitalized patients are at risk for hypercoagulability, venous stasis, and endothelial damage causing VTE.9 Inappropriate assessment of VTE risk leads to a tremendous burden on the healthcare system and the economy due to VTE. Its incidence worldwide is 1 in 1000 each year, costing approximately $20,000 for each patient annually.10
Healthcare organizations have established clinical guidelines for the assessment and prophylaxis of VTE risk. These guidelines include patient risk factor assessment, therapy options, appropriate dosage, and prophylaxis duration. Furthermore, some institutions have implemented a clinical decision support system for VTE prophylaxis prevention assessment during hospitalization to increase health care providers’ adherence to clinical guidelines.11 Appropriate therapy selection is based on a thorough patient assessment and risk stratification using multiple risk factors for either coagulation or bleeding. For example, the American College of Chest Physicians (ACCP) recommends the Padua and IMPROVE bleeding scores to guide decision-making.12 Furthermore, the American Society of Hematology (ASH) guidelines recommend for management of venous thromboembolism also recommend that health care providers should integrate VTE and bleeding risk assessments in the decision-making process of VTE prophylaxis Therapy.7
Prophylaxis options for VTE include pharmacotherapy and non-pharmacotherapy, such as compression stockings, ambulation, vena cava filters, leg elevation, and intermittent pneumatic compression. Pharmacotherapy options include anticoagulants such as low molecular weight heparin (LMWH), betrixaban, rivaroxaban, and fondaparinux.13
ACCP guidelines and the Scottish Intercollegiate Guidelines Network (SIGN) guidelines recommend initiating prophylaxis treatment with anticoagulants if the Padua score is more than or equal to four and the IMPROVE bleeding score is less than seven. Appropriate prophylaxis treatment selection, duration, and dosage are essential to prevent complications, such as pulmonary embolism and chronic venous insufficiency. In addition, it will impact patients’ quality of life, decrease hospital stays and reduce healthcare costs.14 Unfortunately, despite developing clinical guidelines, many hospitals lack a checklist for patient assessment to prevent VTE, and many hospitalized patients do not get appropriate VTE prophylaxis.15 Although, in the United States, hospitals are given financial incentives for low rates of VTE in hospitalized patients, hospitals that have a very high rate of VTE will not get reimbursed for their services.15
To our knowledge, no studies have been conducted in Palestine to assess the usual practice of VTE prophylaxis and anticoagulant use among hospitalized patients. Therefore, the main objective of this study was to evaluate the adherence of current clinical practice to established guidelines at a Palestinian teaching hospital.
Methods
Study Design and Sample
A cross-sectional retrospective observational study was conducted to assess the adherence of current clinical practice to the established VTE prophylaxis guidelines at the Palestinian medical complex - in Ramallah, Palestine, between January 1 to May 31, 2019. All medical records of patients admitted to the medical floor over 18 years of age and hospitalized for longer than 24 h were included in the assessment. Patients diagnosed with ischaemic stroke or VTE on admission, those taking anticoagulants before admission, and those taking anticoagulants for VTE treatment were excluded from the study. Patients with incomplete or duplicated medical records were excluded from this study. Of the 665 medical records reviewed, 408 were eligible for the study according to the inclusion and exclusion criteria.
Data Collection and Management
A data collection form was developed by reviewing several study articles and literature reviews related to VTE prophylaxis.13 The data were collected by three 5th-year PharmD students and reviewed by two multidisciplinary professionals from the Faculty of Pharmacy, Nursing, and Health Professions at Birzeit University. In addition, all collected data were double-checked to ensure accuracy and completeness.The data collection form included multiple patient-related criteria divided into four sections. The first section, demographic information, consisted of patient I.D., age, sex, obesity, and admission diagnosis. In the second section - the Padua score consists of several criteria with specific points from 0 to 3 given for each criterion according to its contribution to the thromboembolism process, and in the third section – IMPROVE bleeding score consists of multiple criteria with points from 0 to 4.5 given to each criterion according to its contribution to the bleeding process.14
The Padua and IMPROVE scores were used to assess patients’ risk for VTE according to the ACCP guidelines, especially in patients hospitalized for more than 24 hours.14
Statistical Analysis
All patient-related criteria were added to an Excel sheet (version 2019) and then encoded and imported into IBM SPSS (version 25) for detailed analyses. Data analysis was performed using descriptive statistics, where the mean and standard deviation were calculated for continuous data, while frequencies and percentages were used for categorical data. Data were categorized, and admission diagnoses were grouped into different categories based on body systems. Age was categorized into three groups (18–40, 41–64, and ≥ 65 years). The Padua and IMPROVE total risk scores were calculated by adding points for each risk factor. The scores were then divided into two categories: high- and low-risk. The patient’s VTE pharmacotherapy prophylaxis assessment was classified as appropriate or inappropriate depending on their Padua and IMPROVE bleeding scores and dose, according to ACCP clinical guidelines. Furthermore, the prophylactic dose was categorized into two groups: appropriate and inappropriate. A Padua total score of 4 is considered a cutoff point for initiating prophylaxis in certain patients, indicating a high risk of developing VTE. For the IMPROVE bleeding score, a total score of 7 was considered a cutoff point for the risk of bleeding (IMPROVE Bleeding Risk Assessment Score, n.d.). Patients with a Padua score ≥ 4 and a bleeding score < 7 were candidates for VTE pharmacotherapy prophylaxis if no other contraindications were present. Based on VTE prophylaxis indications, clinical situations assessments were divided into four categories: VTE prophylaxis indicated - prescribed, not indicated - not prescribed, indicated - not prescribed, and not indicated - prescribed, as shown in Figure 1.
An appropriate prophylactic dose assessment for enoxaparin was based on the recommended dose according to clinical guidelines. The recommended dose is 40 mg subcutaneously once daily for patients with normal renal function (GFR ≥ 60 mL/ min) and 20 or 30 mg subcutaneously once daily for patients with reduced renal function (GFR < 30 mL/ min).12 Doses outside of this recommendation were considered to be inappropriate.
A Chi-square test with a 95% confidence interval was performed to investigate the associations between VTE prophylaxis appropriateness, gender, age, diagnosis categories, and Padua and IMPROVE categories. In addition, multivariable analysis was performed for all variables with a P-value lower than 0.05 to investigate the contribution of confounding factors to the significance of the results obtained from the previous Chi-square test.
Ethical Considerations
This retrospective observational study was conducted at the Palestinian Medical Complex in Palestine. The IRB committee approved the study design at Birzeit University, reference number BZUPNH2102. The requirement for informed consent from each participant was waived because this was an observational retrospective study. The patients were anonymized, and their information was nonidentifiable. The study complied with the ethical guidelines of the Declaration of Helsinki and patient data.
Results
The medical records of 665 patients were reviewed, and only 408 met the inclusion criteria for the study. Of the patients, 51.5% were male. More than half of the patients were older than 65 years. The most common diagnosis was an infectious disease, constituting 51% of all cases, while the least common diagnosis was G.I. disorder, constituting 3.2% of all cases (Table 1). For the Padua scores, the mean was 2.25 ± 2.08, total scores ranged from 0 to 9, and for IMPROVE risk, the mean was 4.44 ± 2.72, ranging from 0 to 17.
 |
Table 1 Patient Characteristics and Demographics (N= 408) |
As illustrated in Figures 2 and 3, the three most frequent risk factors for VTE were acute infection and/or rheumatologic disorders (54.7%), age > 70 years (38.5%), and heart and/or respiratory failure (21.3%). Most patients (95%) had a platelet count ≥50 × 10×9 cells/L and an international normalized ratio (INR) ≤ 1.5, reducing the risk of bleeding. Patients aged (40–84), male gender, and renal function < 30 mL/min were the most abundant risk factors for a high IMPROVE bleeding risk score.
 |
Figure 2 Padua score risk factors with corresponding percentages (N=408). *Within last month. |
 |
Figure 3 IMPROVE score for risk factors with corresponding percentages (N=408). *Within three months. |
Table 2 shows a significant association between patients’ gender, age, and diagnosis of infections or respiratory disease and the appropriateness of the administered VTE prophylaxis. Females were more likely to have appropriate prophylaxis than the males, with an OR = 1.634 (CI = 1.070–2.497), and patients aged 18–40 years were more likely to have appropriate prophylaxis than those aged ≥ 65 years with an OR = 1.965 (CI = 1.099–3.513). Furthermore, patients free of infectious and respiratory diseases were significantly more likely to receive appropriate prophylaxis than patients with these diseases (OR = 1.762 (CI = 1.120–2.772), OR = 1.929 (CI = 1.167–3.188), respectively). However, no significant association was observed between the appropriateness of prophylaxis and other disease states. In addition, no significant association was observed between VTE, bleeding risk scores, and appropriate prophylaxis.
 |
Table 2 VTE Prophylaxis (VTEPx) Assessment (N= 408) |
As shown in Table 3, of the 408 patients, 222 received thromboprophylaxis (54.4%), and only 44 received appropriate prophylaxis with the correct dose (19.82%).
 |
Table 3 Appropriateness of VTE Prophylaxis |
Figures 4 and 5 show the steps used to evaluate patients for VTE prophylaxis therapy according to their Padua and IMPROVE bleeding scores and correct dosages as recommended by the clinical guidelines. As a result, 112 (27.5%) of the total hospitalized patients (N=408) had a high risk of developing VTE, 73 patients were eligible for VTE pharmacological prophylaxis, but only 44 (60.3%) patients received the appropriate prophylaxis. In contrast, 296 (72.5%) patients had low Padua scores, indicating that pharmacological prophylaxis was not indicated; however, 144 (48.6%) patients received prophylaxis, which increased their risk of bleeding.
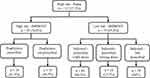 |
Figure 4 Prophylaxis administration for high Padua risk patients (N=408). |
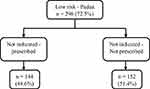 |
Figure 5 Prophylaxis administration for low Padua risk patients (N=408). |
Discussion
Patient assessment for VTE prophylaxis can be challenging to health providers and patients due to the risk of complications by the disorder or the medication used for management; therefore, risk assessment tools are essential in making appropriate decisions. This study assessed VTE prophylaxis using Padua and IMPROVE bleeding scores. The study finding illustrates over-utilization, where anticoagulants were used when not indicated and underutilization when indicated. Furthermore, subtherapeutic or high doses were prescribed to patients with indications for prophylaxis.
According to the risk assessment stratification, based on the clinical guidelines of patients enrolled in this study, only 112 patients had a high risk of developing VTE, and 73 patients (65.2%) were eligible for VTE pharmacological prophylaxis. In this study, 222 (54.4%) received anticoagulants for VTE, similar to a cross-sectional multicenter observational study conducted in Jordan and Lebanon, where 415 (58.9%) of patients enrolled in the study received prophylaxis, with 52% receiving the appropriate therapy, however, unfortunately, only 19.82% received appropriate prophylaxis in our study.16 Only 44 patients received the correct prophylaxis constituting 60.3% of the eligible patients, indicating that 29 (39.7%) of eligible patients were not given the prophylaxis or were given prophylaxis with the wrong dose. The mean age for these patients was 64.4 years, so the age factor should not be a reason for missed prophylaxis. It has been reported that up to 50% of older patients do not get anticoagulation therapy due to physician perception of a high risk of falling and associated bleeding.17 Additionally, these patients may have been exposed to unwanted VTE complications. For example, a trial called MEDENOX had shown that the incidence of developing VTE in patients who did not receive the prophylaxis when they were eligible is 9.4% higher.18 The inability to make a clinical decision on whether to initiate prophylaxis may be due to the inability to stratify patients depending on risk scores (Padua and IMPROVE) or not incorporating these assessment tools in clinical practice, as suggested by the STIME study.19,20 Furthermore, the VTE assessment rate was increased when incorporating assessment models into quality parameters, where 98.6% of hospital admitted patients were screened for initiating VTE prophylaxis.21
The risk of bleeding is a major adverse effect of anticoagulant therapy, and patient assessment for anticoagulant contraindications is necessary in clinical practice. In this study, bleeding risk was assessed using the IMPROVE score, and a high-risk score ≥ 7 was considered a contraindication to pharmacological prophylaxis, regardless of a high Padua score or the risk of clotting. In such a clinical situation, other prophylactic measures can be initiated. The total number of patients who received prophylaxis even though they have absolute contraindication (high Padua/high IMPROVE) or without an indication were 24 (61.5%) out of 39 (48.6%) and 144 out of 296, respectively, increasing their risk of bleeding complications and health care costs. This inappropriate assessment of VTE or prescribing of anticoagulants may be due to a lack of awareness, knowledge, appropriate staff, lack of practical assessment incorporated into health teamwork flow, concentrating on current diagnosis versus prophylaxis, and lack of involvement by clinical and administrative leaders.22
In this study, the most common risk factors for bleeding were median age (40–84 years), male gender, and renal insufficiency, a major contraindication to anticoagulants. A similar finding was illustrated by a Lebanese study at the American University of Beirut Medical Center, where renal insufficiency was a major risk factor for bleeding.23
The most frequent risk factors for developing VTE were acute infection and/or rheumatologic disorders (54.7%), age > 70 years (38.5%), and heart and/or respiratory failure (21.3%). These findings were consistent with a study by Asmamaw et al, which shows that acute infection and/or rheumatologic disorders 76.7% is the most common diagnosis among hospitalized patients.24 While another study by Ayalew et al showed that acute infection 51.5% and heart or respiratory disease 25.7% are the commonest.13 Furthermore, this study was conducted through the winter season, and those conditions are prevalent during that period.
The participant’s age and sex were significantly associated with the appropriateness of the administered prophylaxis. Patients aged 18–40 were more likely to receive appropriate prophylaxis than those aged ≥ 65. In contrast with two studies by Ayalew et al, there was no association.13 A prospective Malaysian study performed pre-and post-intervention among medical inpatients reported a significant association between appropriate VTE prophylaxis and age; however, middle-aged patients were more likely to receive appropriate prophylaxis.25 Another cross-sectional multicenter observational study conducted in Jordan and Lebanon showed a significant association, where patients aged 40 years or older were more likely to receive appropriate prophylaxis.16 The higher likelihood of older patients receiving appropriate prophylaxis in our study could be explained by the increased risk of clotting in this age category causing physicians to consider prophylaxis more often.26
The most common diagnosis was infection disease, accounting for 51% of the hospitalized patients. This finding is similar to Ayalew et al, which showed that 65.5% of patients were diagnosed with infectious diseases.13 Also, a significant association was detected between administering appropriate prophylaxis and not having infectious and respiratory diseases. However, a previous study showed no association between diagnosis and the appropriateness of DVT prophylaxis.13
Limitations
This study has several limitations that should be considered. For example, the study was a single-center study, mechanical prophylaxis was not assessed, incomplete patient medical records, the BMI for hospitalized patients was not available for most patients, the incidence of VTE development was not assessed after the hospital discharge, and the health care providers knowledge of current guideline was not assessed.
Conclusion
In conclusion, prophylaxis among hospitalized medically ill patients is mostly inappropriate; 80.18% of the patients received inappropriate prophylaxis, and only 60.3% of eligible patients received appropriate prophylaxis with the correct dose when indicated. Therefore, adapting assessment models or checklists in clinical practice based on clinical guidelines for VTE risk stratification is a practical and effective method to improve VTE prophylaxis management and select the appropriate therapy to prevent toxicity or complications. Furthermore, increasing healthcare providers’ knowledge and awareness and providing continuing education for VTE based on scientific evidence-based practice is essential in improving patient care and outcome in VTE prophylaxis risk assessment, prevention, and management.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Spyropoulos AC, Ageno W, Cohen AT, Gibson CM, Goldhaber SZ, Raskob G. Prevention of venous thromboembolism in hospitalized medically ill patients: a U.S. perspective. Thromb Haemost. 2020;120(6):924–936. doi:10.1055/s-0040-1710326
2. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients. Chest. 2012;141(2):e227S–e277S. doi:10.1378/chest.11-2297
3. Beckman MG, Hooper WC, Critchley SE, Ortel TL. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(4 Suppl):S495–501. doi:10.1016/j.amepre.2009.12.017
4. Hilberg T, Ransmann P, Hagedorn T. Sport and venous thromboembolism—site, accompanying features, symptoms, and diagnosis. Dtsch Arztebl Int. 2021;118(11):181–187. doi:10.3238/arztebl.m2021.0021
5. Di Nisio M, van Es N, Büller HR. Deep vein thrombosis and pulmonary embolism. Lancet. 2016;388(10063):3060–3073. doi:10.1016/S0140-6736(16)30514-1
6. Cayley WE. Preventing deep vein thrombosis in hospital inpatients. BMJ. 2007;335(7611):147–151. doi:10.1136/bmj.39247.542477.AE
7. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198–3225. doi:10.1182/bloodadvances.2018022954
8. Gerotziafas GT, Chrysanthidis M, Isaad R, et al. Prevalence of risk factors for VTE in hospitalized medical and surgical patients. Data from the comparison of methods for thromboembolic risk assessment with clinical perceptions and awareness in real life surgical and medical patients (COMPASS) study. Blood. 2010;116(21):3337. doi:10.1182/blood.V116.21.3337.3337
9. Wolberg AS, Aleman MM, Leiderman K, Machlus KR. Procoagulant activity in hemostasis and thrombosis: virchow’s triad revisited. Anesth Analg. 2012;114(2):275–285. doi:10.1213/ANE.0b013e31823a088c
10. Cushman M. Epidemiology and risk factors for venous thrombosis. Semin Hematol. 2007;44(2):62–69. doi:10.1053/j.seminhematol.2007.02.004
11. Titi MA, Alotair HA, Fayed A, et al. Effects of computerised clinical decision support on adherence to VTE prophylaxis clinical practice guidelines among hospitalised patients. Int J Qual Health Care. 2021;33(1). doi:10.1093/intqhc/mzab034
12. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2Suppl):e195S–e226S. doi:10.1378/chest.11-2296
13. Ayalew MB, Horsa BA, Zeleke MT. Appropriateness of pharmacologic prophylaxis against deep vein thrombosis in medical wards of an Ethiopian Referral Hospital. Int J Vasc Med. 2018;2018:8176898. doi:10.1155/2018/8176898
14. Skeik N, Westergard E. Recommendations for VTE prophylaxis in medically ill patients. Ann Vasc Dis. 2020;13(1):38–44. doi:10.3400/avd.ra.19-00115
15. Moffatt-Bruce SD, Hilligoss B, Gonsenhauser I. ERAS: safety checklists, antibiotics, and VTE prophylaxis. J Surg Oncol. 2017;116(5):601–607. doi:10.1002/jso.24790
16. Hajj I, Al-Masri M, Bashaireh K, et al. A cross-sectional, multicenter, observational study to assess the prophylaxis of venous thromboembolism in Lebanese and Jordanian hospitals. Thromb J. 2021;19(1):9. doi:10.1186/s12959-021-00261-2
17. Hagerty T, Rich MW. Fall risk and anticoagulation for atrial fibrillation in the elderly: a delicate balance. Cleve Clin J Med. 2017;84(1):35–40. doi:10.3949/ccjm.84a.16016
18. Turpie AG. Thrombosis prophylaxis in the acutely ill medical patient: insights from the prophylaxis in MEDical patients with ENOXaparin (MEDENOX) trial. Am J Cardiol. 2000;86(12b):48m–52m. doi:10.1016/S0002-9149(00)01481-8
19. Depietri L, Marietta M, Scarlini S, et al. Clinical impact of application of risk assessment models (Padua Prediction Score and Improve Bleeding Score) on venous thromboembolism, major hemorrhage and health expenditure associated with pharmacologic VTE prophylaxis: a “real life” prospective and retrospective observational study on patients hospitalized in a Single Internal Medicine Unit (the STIME study). Intern Emerg Med. 2018;13(4):527–534. doi:10.1007/s11739-018-1808-z
20. Ahmad HA, Geissler A, MacLellan DG. Deep venous thrombosis prophylaxis: are guidelines being followed? ANZ J Surg. 2002;72(5):331–334. doi:10.1046/j.1445-2197.2002.02402.x
21. Mahlab-Guri K, Otman MS, Replianski N, Rosenberg-Bezalel S, Rabinovich I, Sthoeger Z. Venous thromboembolism prophylaxis in patients hospitalized in medical wards: a real life experience. Medicine. 2020;99(7):e19127. doi:10.1097/MD.0000000000019127
22. Pai M, Lloyd NS, Cheng J, et al. Strategies to enhance venous thromboprophylaxis in hospitalized medical patients (SENTRY): a pilot cluster randomized trial. Implement Sci. 2013;8:1. doi:10.1186/1748-5908-8-1
23. Masroujeh R, Shamseddeen W, Isma’eel H, Otrock ZK, Khalil IM, Taher A. Underutilization of venous thromboemoblism prophylaxis in medical patients in a tertiary care center. J Thromb Thrombolysis. 2008;26(2):138–141. doi:10.1007/s11239-007-0084-y
24. Asmamaw M, Hungnaw W, Motbainor A, Kedir HM, Tadesse TA. Incidence of thromboembolism and thromboprophylaxis in medical patients admitted to specialized hospital in Ethiopia using Padua prediction score. SAGE Open Med. 2022;10:20503121221079488. doi:10.1177/20503121221079488
25. Diana Yap FS, Ng ZY, Wong CY, Muhamad Saifuzzaman MK, Yang LB. Appropriateness of deep vein thrombosis (DVT) prophylaxis use among medical inpatients: a DVT risk alert tool (DRAT) study. Med J Malaysia. 2019;74(1):45–50.
26. Lacut K, Le Gal G, Mottier D. Primary prevention of venous thromboembolism in elderly medical patients. Clin Interv Aging. 2008;3(3):399–411. doi:10.2147/CIA.S832
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.