Back to Journals » Clinical Ophthalmology » Volume 13
Vitrectomy for macular edema due to retinal vein occlusion
Authors Kumagai K, Ogino N, Fukami M, Furukawa M
Received 28 January 2019
Accepted for publication 10 May 2019
Published 13 June 2019 Volume 2019:13 Pages 969—984
DOI https://doi.org/10.2147/OPTH.S203212
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Kazuyuki Kumagai,1 Nobuchika Ogino,2 Marie Fukami,1 Mariko Furukawa1
1Kami-iida Daiichi General Hospital, Aichi, Japan; 2Shinjo Ophthalmologic Institute, Miyazaki, Japan
Purpose: To determine the long-term outcomes of vitrectomy for the macular edema associated with a retinal vein occlusion (RVO).
Methods: This was a retrospective, consecutive, interventional case series. The intraoperative procedures included internal limiting membrane peeling, arteriovenous sheathotomy, radial optic neurotomy, and intravitreal triamcinolone acetonide injection at the end of the surgery. The main outcome was the best-corrected visual acuity (BCVA).
Results: Eight hundred and fifty-four eyes of 854 patients were studied. The eyes consisted of 602 with branch RVO (BRVO), 74 with hemi-central RVO (hemi-CRVO), 87 with nonischemic central retinal vein occlusion (CRVO), and 91 with ischemic CRVO. The mean follow-up period was 68.6 months with a range of 12 to 262 months. The mean BCVA was significantly improved at the final visit (P<0.0001 to 0.0016). The final BCVA improved in 74.4% of the BRVO eyes, in 58.1% of the hemi-CRVO eyes, in 57.4% of the nonischemic CRVO eyes, and in 51.6% of the ischemic CRVO eyes. Multiple regression analysis showed there was no significant relationship between the intraoperative combined procedures and the final BCVA.
Conclusions: The results indicate that the type of RVO is significantly associated with the final BCVA, and vitrectomy is a treatment option to improve and maintain BCVA for a long term.
Keywords: retinal vein occlusion, vitrectomy, macular edema, internal limiting membrane
Introduction
A retinal vein occlusion (RVO) is the second most common vision-threatening retinal vascular disorder after diabetic retinopathy. Approximately 16 million people have RVO from population studies in the United States, Europe, Asia, and Australia.1 Patients with RVO are at risk of vision reduction from complications such as vitreous hemorrhage, epiretinal membrane formation, and tractional retinal detachment. Signs indicating a development of these complications are indications for vitrectomy.
Macular edema is another common cause of visual reduction in eyes with a RVO, and vitrectomy has been performed for the macular edema since 1994.2–8 The results of many studies on the effectiveness of vitrectomy with and without intraoperative combined procedures have been published.9–39 In combined vitrectomy, the vitrectomy is combined with internal limiting membrane (ILM) peeling, arteriovenous sheathotomy, radial optic neurotomy, intravitreous triamcinolone acetonide (IVTA) at the completion of surgery, and endovascular cannulation. However, the role of vitrectomy for macular edema is uncertain,40 and there is limited evidence from well-conducted randomized studies indicating that a specific type of surgical intervention for RVO is superior to the others.41,42
In recent years, the treatment of macular edema secondary to RVO has shifted to the intravitreal injection of anti-vascular endothelial growth factor (VEGF) agents.43–45 However, a recurrence of the macular edema is often observed, and patients require close monitoring and the repeated injections increase the risk of endophthalmitis, adverse systemic events,46 and the financial burdens.47 In addition, the long-term outcomes of anti-VEGF treatments and the rate of reoperations48 after the anti-VEGF treatments have not been definitively determined.
Thus, the purpose of this study was to determine the long-term outcomes of vitrectomy performed by a single surgeon on the macular edema associated with a RVO.
Methods
Patients
We reviewed the medical records of all patients who had undergone vitrectomy for macular edema due to a RVO by a single surgeon (NO) between September 1994 and November 2011. The inclusion criteria were the presence of a RVO and macular edema associated with a decrease in the visual acuity and foveal hemorrhages that were detected by fluorescein angiography. The exclusion criteria included eyes with vitreous hemorrhage, severe cataract, vitreomacular traction, presence of an epiretinal membrane, the intravitreal anti-VEGF agents used, the intra- or periocular use of TA, previous vitreoretinal surgery, previous macular grid laser photocoagulation, uncontrolled glaucoma, and other ocular diseases that could cause a reduction in vision. This study did not include cases with insufficiently controlled hypertension and diabetics. Duration of symptoms was defined as the time from onset to surgery.
All patients had signed an informed consent for the surgery, data collection, and the use of the data for research studies. The Ethics Committee of the Kami-iida Daiichi General Hospital approved the procedures used in this study, and the procedures conformed to the tenets of the Declaration of Helsinki.
All of the patients had a complete ophthalmic examination including measurements of the best-corrected visual acuity (BCVA) with a visual acuity chart, slit-lamp biomicroscopy with a contact lens, indirect ophthalmoscopy, fundus photography, and fluorescein angiography. Foveal thickness was determined by OCT 3 (Carl Zeiss Meditec, Dublin, CA), and images were obtained from 307 eyes preoperatively and postoperatively.
An ischemic central retinal vein occlusion (CRVO) was determined to be present when a retinal nonperfusion area >10 disk diameters that involved the periphery and/or the macula was present.
Patients were examined preoperatively and at 1 day, 1 week, 1, 2, 3, and 6 months post-treatment. The patients were examined every 3 to 6 months thereafter.
Surgical procedures
All surgeries were performed by one experienced surgeon (NO). Phacovitrectomy with intraocular lens implantation was performed in all phakic patients who were >40-years-of-age to avoid cataract progression. Standard three-port pars plana vitrectomy was performed. A separation of the posterior hyaloid from the optic disk and posterior retina was performed when a posterior vitreous detachment was not present. The retinal periphery was carefully inspected microscopically with scleral depression. Argon laser photocoagulation was applied to any tears or suspicious retinal hemorrhages or thinning.
Intraoperative laser treatment was performed for the nonperfused areas except in cases of BRVO to prevent postoperative neovascular glaucoma.
The intraoperative combined procedures included ILM peeling, cystotomy,39 arteriovenous sheathotomy, radial optic neurotomy, drainage of subretinal fluid, and a 10 mg IVTA injection at the end of surgery.
ILM peeling was started in March 1998. The early stage indications were eyes with dense hemorrhage and/or large cysts. Later, the indication of ILM peeling expanded to all patients and during generally performed vitrectomy. Cystotomy consisted of the incision of the cyst wall or excision of the cyst wall, and this was performed for cysts >1/3 disc diameter. Radial optic neurotomy was performed from April 2002 to December 2003. Indications for radial optic neurotomy were large cysts, severe macular ischemia, and recent onset of the macular edema in both nonischemic and ischemic CRVO. The drainage of subretinal fluid with a subretinal cannula was performed for subretinal fluid with dense hemorrhage. IVTA injection at the end of surgery was performed from December 2002 to December 2006.
Additional treatments after surgery
Postoperative laser treatments were performed when iris neovascularization developed or nonperfused area enlarged or when severe fluorescein leakage was observed.
A persistent macular edema was defined as a macular edema that was sustained for at least 3 months at ≥300 µm or a macular edema deemed to be affecting the patient’s visual acuity based on the investigator’s evaluation. A recurrent macular edema was defined as an increase of foveal thickness >30% after an initial decrease or a worsening of the BCVA by >0.2 logMAR units after an initial improvement.
Additional treatments, including IVTA and ILM peeling, were performed when the eye had persistent or recurrent macular edema and when the patients agreed to the retreatments. For this, 10 mg of IVTA injections was performed from December 1998 to September 2007. Vitrectomy with ILM peeling was performed if the ILM was preserved at the initial surgery.
Subclass analyses on the time period and the duration
We divided the time period into an early period (from 9/1994 to 6/2003; n=446) and the late period (from 7/2003 to 11/2011; n=408) to evaluate the effect of the time period of surgery.
We divided all cases by the duration of symptoms into acute eyes (duration≤3 mon) and chronic eyes (duration>3 mon) to evaluate the long-term results including the BCVA, postoperative adverse events, and need of additional postoperative procedures.
Statistical analyses
The decimal visual acuities were converted to the logarithm of minimum angle resolution (logMAR) units. The paired t tests were used to determine the significance of the differences in the BCVA, and chi-square tests were used to determine the significance of the differences in the ratios of the BCVA. The differences in the measured values among the groups were compared by ANOVA with posthoc comparisons tested by the Scheffe procedure. The significance of the difference between the final BCVA and foveal thickness at last visit was evaluated by Spearman’s rank correlation tests. An improvement or worsening of the visual acuity was defined as changes that were greater or lesser than 0.2 logMAR units. A P<0.05 was accepted as statistically significant. Statistical analyses of data were carried out with the Statview 5.0 software (SAS Institute Inc., Cary, North Carolina).
Results
Eight hundred eighty-two eyes of 882 patients met our selection criteria. Twenty-eight patients were excluded from the statistical analysis because they had not been followed for at least 12 months. Therefore, the analyses were performed on 854 eyes of 854 patients.
Four hundred seventy-seven patients (55.9%) were women, 377 (44.1%) were men. The mean age of the patients was 65.9 years with a range of 31 to 95 years. The mean age of the women was 67.4±9.7 years and that of the men was 64.0±11.3 years (P<0.0001).
The duration of the symptoms was 1 to 60 months with a mean of 4.3 months and a median of 3.0 months. One hundred seventy eyes (19.9%) had a duration of ≤1 month, 550 eyes (64.4%) had a duration of ≤3 fewer months, and 109 eyes (12.8%) had a duration of >6 months.
The follow-up period ranged from 12 to 262 months with a mean of 68.6 and a median of 53.0 months. The follow-up period of 708 eyes (82.9%) was at least 2 years, 633 eyes (74.1%) was at least 3 years, and 371 eyes (42.1%) was ≥5 years, and 140 eyes (16.4%) was ≥10 years of follow-up
For all eyes, 602 had a branch RVO (BRVO), 74 had a hemi-central RVO (hemi-CRVO), 87 had nonischemic CRVO, and 91 had ischemic CRVO. The patients were further subdivided into; 51 with macular RVO, 305 with first branch RVO, and 246 with second branch RVO.
The preoperative characteristics of the patients are shown in Table 1. There were significant differences in the sex distribution, the BCVA, incidence of glaucoma, prior scatter or panretinal photocoagulation, incidence of posterior vitreous detachment, presence of foveal cysts, hemorrhage in the foveal cyst, subfoveal hemorrhage, and subfoveal detachment but not for the age, lens status, duration of symptoms, and follow-up duration among the four types of BRVO.
 | Table 1 Preoperative characteristics of the patient (n=854) |
The combined procedures are shown in Table 2. ILM peeling was performed in 564 eyes (66.0%) that included indocyanine green-assistance in 220 eyes (39.0%), triamcinolone-assistance in 216 eyes (38.3%), and no assistance in 128 eyes (22.7%).
 | Table 2 Combined procedures (n=854) |
Cystotomy was performed in 77 eyes (9.0%) and combined with ILM peeling in 48 eyes. Drainage of subretinal fluid was performed in 50 eyes (5.9%) of all eyes. Arteriovenous sheathotomy was performed in 121 eyes (20.1%) with BRVO.
Radial optic neurotomy was performed on 16 eyes (18.4%) with nonischemic CRVO and 16 eyes (17.6%) with ischemic CRVO. Radial optic neurotomy was performed on 16 of 18 eyes with nonischemic CRVO and 16 of 18 eyes with ischemic CRVO from April 2002 to December 2003.
Ninety-seven patients received IVTA during the follow-up period. The interval from surgery to first injections ranged from 1 to 77 months with a mean of 8.0 months. Twenty-nine eyes received a second IVTA. The interval from the surgery to the second injection ranged from 2 to 54 months with a mean of 16.2 months. Ten patients received a third IVTA. The interval from surgery to the third injection ranged from 9 to 38 months with a mean of 19.2 months. Two patients received a fourth IVTA, and the interval from surgery to fourth injections was 14 months in both patients.
The procedures by the study periods are shown in Table 3.
 | Table 3 Procedures by study period |
The time course of the changes in the BCVA for the four types of RVOs is shown in Figure 1. The visual prognosis was better in the eyes with a BRVO followed by eyes with hemi-CRVO, nonischemic CRVO, and ischemic CRVO.
The time course of the changes in the foveal thickness for the four types of RVOs is shown in Figure 2. There was a significant difference between the eyes with a BRVO and ischemic CRVO at preoperatively (P=0.0008)
The time course of the changes in the BCVA for the subtypes of BRVO is shown in Figure 3. The visual prognosis was better in the eyes with a macular RVO followed by eyes with a second branch RVO and with a first branch RVO.
The results of the comparisons of the BCVA at each time point are shown in Table 4. There was a significant difference between the preoperative BCVA and that at all postoperative examination times for all types of RVO. In BRVO, there was a significant difference in the BCVA at 1 year and that at the final visit (P<0.0001), and there was a significant difference between the BCVA at 1 year and that at 2 years (P<0.0001) but not between 2 years and 3 years (P=0.072).
 | Table 4 Statistical comparison of visual acuity between each time points |
A summary of visual outcomes at 12 months postoperatively and at the final visit is presented in Table 5. In eyes with a BRVO with an initial BCVA of ≥20/40, a final BCVA of ≥20/20 was found in 119 of the 175 eyes (68.0%) and a final BCVA of ≥20/40 was found in 168 of the 175 eyes (96.0%). The incidence of BRVO eyes with a preoperative BCVA ≤20/40 (n=490) and a BCVA ≥20/40 and a BCVA ≥20/20 at 12 months after the surgery was 68.6%, 24.7%, respectively.
 | Table 5 Visual outcomes at 12 months postoperatively and the final visit |
The postoperative adverse events and classification by acute and chronic eyes are shown in Tables 6 and 7, respectively.
 | Table 6 Postoperative adverse events (n=854) |
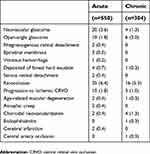 | Table 7 Postoperative adverse events in acute or chronic eyes (n=854) |
The additional postoperative surgeries and additional postoperative procedures in acute or chronic eyes acute and chronic eyes are shown in Tables 8 and 9, respectively. Postoperative adverse events and the postoperative additional procedures were more often in the acute eyes than the chronic eyes.
 | Table 8 Additional postoperative surgeries (n=854) |
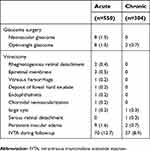 | Table 9 Additional postoperative procedures in acute or chronic eyes (n=854) |
The effects of IVTA on the rate of re-occlusion and development of neovascular glaucoma are shown in Table 10. Re-occlusion and neovascular glaucoma were frequently observed in the eyes treated by IVTA. In eyes with a BRVO without a history of glaucoma, an IVTA injection increased the incidence of these complications significantly. In ischemic CRVO without a history of glaucoma, IVTA increased the rate of neovascular glaucoma significantly.
 | Table 10 The effects of IVTA on re-occlusion and neovascular glaucoma |
The outcomes of multiple regression analyses on the associations between the occurrence of re-occlusion and several clinical factors are shown in Table 11, and the outcomes on the associations between the occurrence of neovascular glaucoma and several clinical factors are shown in Table 12. The results showed that the use of IVTA was significantly associated with re-occlusion (r =0.13, P=0.0004) and neovascular glaucoma (r=0.14, P=0.0002).
 | Table 11 Multiple regression analyses for re-occlusion |
 | Table 12 Multiple regression analyses for neovascular glaucoma |
The results of multiple regression analyses for the final BCVA for the different BRVO groups are shown in Table 13. An improvement of the final BCVA was associated with a younger age, obstruction located peripherally, better preoperative BCVA, and absence of hemorrhage in the cysts. IVTA at the end of surgery and during follow-up were not significant factors for the final BCVA; however, IVTA at the end of surgery was significant at 1 month (r=−0.11, P=0.0005), 2 months (r=−0.088, P=0.011), and 3 months (r=−0.075, P=0.035), but not at 6 months (r=−0.049, P=0.17).
 | Table 13 Multiple regression analyses for the final BCVA for the BRVO (n=602) |
The outcomes of multiple regression analyses for the final BCVA for the CRVO groups are shown in Table 14. A better final BCVA was significantly associated with a younger age, peripheral obstruction site, and better preoperative BCVA. IVTA at the end of surgery and during follow-up were not a significant factors associated with the final BCVA; however, IVTA at the end of surgery was significant at 1 month (r=−0.14, P=0.0068), 2 months (r=−0.13, P=0.016), and 3 months (r=−0.11, P=0.040), but not at 6 months (r=−0.046, P=0.47).
 | Table 14 Multiple regression analyses for the final BCVA for the CRVO (n=178) |
The time course of the changes in the BCVA for the subgroups of RVO by the acute (duration ≤3 months, n=550) and chronic (duration >3 months, n=304) eyes is shown in Figure 4. The improvement was better in the acute eyes than the chronic eyes. There was a significant difference between two groups at 9 months (P=0.020), at 12 months (P=0.0095), and at the final visit (P=0.012).
The time course of the changes in the BCVA for the subgroups of RVO by the duration of symptoms is shown in Figure 5. There was a significant difference between the preoperative BCVA and the post-treatment BCVA at all visits in all subgroup of duration (all P<0.0001). The subgroup with a duration ≦1 month, 2–3 months, and 3–6 months had a similar time course. The subgroup with a duration >6 months had the worst time course. There was a significant difference between a duration>6 months and a duration of 2–3 months at 9 months (P=0.0037), at 12 months (P=0.0046), and at the final visit (P=0.0070).
The time course of the changes in the BCVA for the subgroups of RVO by the early period (from 9/1994 to 6/2003; n=446) and late period (7/2003 to 11/2011; n=408) is shown in Figure 6. There was a significant difference between the two groups at 1 month (P=0.048). The two groups had a similar time course.
Scatter plots showing the relationship between the final visual acuity (logMAR units) and the foveal thickness at the final visit in BRVO (n=214) are shown in Figure 7. The final BCVA ≥20/20 was observed in 13 (35.1%) of 37 eyes with a foveal thickness ≥300 µm.
Discussion
The results showed that the BCVA was significantly improved at the end of the first postoperative year, and the BCVA was maintained for a long period thereafter in all types of RVOs. Recently, intravitreal injections of anti-VEGF agents are very common treatment for ME secondary to RVOs. However, our present findings indicate that there are several advantages of vitrectomy.
RVOs occurred more frequently in men than women except for the major BRVOs.49 Men tended to develop an RVO at a younger age than women.49 CRVOs were significantly associated with glaucoma.50 Ischemic CRVOs occurred more frequently in women. However, our results are most consistent with the reports by Hayreh et al,49,50 and they indicated that the patients in our cohort have the general characteristics of RVO.
Hemorrhages in the foveal cysts and subfoveal hemorrhages were frequently observed in eyes with a BRVO. Foveal cysts and serous foveal detachments were frequently observed in eyes with ischemic CRVO. Because there was no significant difference in the age and duration of the symptoms, the biomicroscopically observed pathological foveal changes appear to be characteristic of the RVO types. These changes did not significantly affect the final BCVA; however, further studies are needed to determine the associations between optically observed foveal changes and functional outcomes.
The time course of the changes in the BCVA for the 4 types of RVOs showed that the improvements of the BCVA were greater during the early postoperative period and lesser and slower thereafter. This is consistent with our previous findings that the visual gain curves after vitrectomy for different macular diseases can be well fit by a hyperbolic function.51 The continued visual improvement after vitrectomy is in contrast to the early improvement after anti-VEGF therapy.8
The time course of the changes in the BCVA indicates that the type of RVO determines the long-term BCVA. Hemi-CRVO and nonischemic CRVO had similar changes which are not unexpected because the pathogenesis of hemi-CRVO is quite similar to that of CRVO.52 The statistical comparisons showed the BCVA improved during the first postoperative year, and the BCVA was maintained for a long time in all types of RVO. In BRVO, the improvement continued postoperatively even after one year. There was a significant improvement from 1 year to 2 years postoperatively. These findings suggest that the outcomes on the BCVA after vitrectomy for BRVO require longer follow-up times of at least 2 years.
The BRVOs consisted of three subtypes, and the degree of improvement of the BCVA was in the following order; macular RVO, second branch RVO, and first branch RVO. A final BCVA of ≥20/40 was obtained in 70% to 94% of the patients, and a final BCVA of ≥20/20 was obtained in 37% to 63%. These findings suggest that the evaluation of the BCVA after vitrectomy for a BRVO needs to be classified by the subtypes. In addition, these findings indicate that the major and macular RVOs are two distinct clinical entities.53,54
Rogers et al reported that the visual acuity improved in eyes with BRVO without intervention although significant improvements >20/40 were not common.55 In our cohort, a final BCVA of ≥20/40 was obtained in 78.9%. Quinlan et al56 studied the natural course of 107 nonischemic CRVO eyes and 61 ischemic CRVO eyes with a mean follow-up duration of 22 months (range, 6 months to 6 years). In the nonischemic CRVO eyes, the final BCVA improved in 15% and worsened in 31%, and a final BCVA was ≤20/200 in 50% of the patients. In ischemic CRVO, an improvement of the final BCVA was found in 28% and a worsening in 38%. The final BCVA was ≤20/200 in 93%, counting fingers or worse in 54%, and hand motions or worse in 36%. In our cohort of nonischemic CRVO, the final BCVA improved in 57% and worsened in 18%, and the final BCVA was ≤20/200 in 32%. In ischemic CRVO, the final BCVA improved in 52% and worsened in 23% of the eyes, and the final VA was ≤20/200 in 77%, counting fingers or worse in 10%, and hand motions or worse in 9%. Our present findings indicate that vitrectomy is not inferior to natural course of RVO.
Our results showed that a faster visual recovery was present in patients treated with an IVTA at the end of surgery. However, IVTA was not a significant factor for the final BCVA. This is consistent with one report that there was no significant difference in the improvement of BCVA between vitrectomy alone group and vitrectomy with IVTA group at 12 months postoperatively.26 Moreover, we found that IVTA can increase the risk of glaucoma and re-occlusion. However, we could not explain the mechanism, further examinations are needed.
It is believed that early treatment for macular edema achieves better BCVAs. In our study, the acute eyes had better visual outcomes compared to the chronic eyes. In the subdivided study, the BCVA improvements in patients with duration of ≤1 month, 2 to 3 months, and 3 to 6 months had similar BCVA and had better BCVA than eyes with duration longer than 6 months. The duration of the symptoms was not a factor that significantly affected the final BCVA. It was somewhat surprising that the outcomes did not differ for symptom duration of 1 to 3 months. One clue for this might be that the results were not stratified into treatment-naïve and pre-treated patients. Macular edema occasionally resolves during the natural course, and the duration of symptoms and signs for this course is uncertain. Further studies are needed to determine appropriate timing of treatments including anti-VEGF treatment.
It is clear that a depression of the VEGF levels leads to excellent short-term outcomes in patients with RVO.57 However, there have been only a few reports on the long-term outcomes of anti-VEGF therapy.57–59 In patients with BRVO, a pre-treatment of eyes with a BCVA of ≥20/40 in the VIBRANT,59 HORIZON,58 and this study was 25.2%, 70.2%, and 29.1% at the baseline and 84.6%, 61.6%, and 73.4% at 12 months, respectively. The incidence of a BCVA of ≤20/200 in the HORIZON58 and this study was 2.9% and 23.6% at the baseline and 2.7% and 6.3% at 12 months, respectively. In eyes with a CRVO including an ischemic CRVO, the incidence of eyes with a BCVA of ≥20/40 in the HORIZON58 and this study was 51.0% and 12.9% at the baseline and 41.2% and 28.7% at 12 months, respectively. The incidence of eyes with a BCVA of ≤20/200 in the HORIZON58 and this study was 8.2% and 59.6% at baseline and 5.9% and 47.2% at 12 months, respectively. The comparisons of the outcomes to the anti-VEGF therapy need to be done more accurately with an inclusion of the postoperative treatments of the patients. Then, we cannot compare the effectiveness of vitrectomy alone to that of anti-VEGF therapy.
The RETAIN study57 reported on the outcomes of intravitreal ranibizumab injections for RVO, and the results showed that one-half of the patients had a resolution of edema, and the other half still needed repeated injections even in the fourth year. The mean number of injections for macular edema due to CRVO was 10.9 in the aflibercept group and 14.4 in the ranibizumab group with a follow-up period of 18 months.60 In addition, serial anti-VEGF therapy did not prevent the developments of neovascular abnormalities,61,62 and an increase in the size of the nonperfused area.63 These findings suggest that serial anti-VEGF therapy still requires repeat injections and treatments for neovascular events during long-term follow-ups. Smiddy64 concluded that the relative cost and benefits of anti-VEGF therapy should also be considered when considering the treatment strategies. Vitrectomy was reported to be a useful method in terms of the relative costs and benefits for diabetic macular edema64 and proliferative diabetic retinopathy.65 We believe that vitrectomy is a useful therapy for macular edema due to RVO in terms of the relative costs and benefits.
Sato et al35 performed microincision vitrectomy with ILM peeling on 101 eyes with macular edema secondary to BRVO. The preoperative mean BCVA in their cohort increased from 0.52±0.43 logMAR units to 0.25±0.41 logMAR units at 12 months, and the mean preoperative foveal thickness decreased from 489±224 µm to 274±135 µm at 12 months postoperatively. The percentage of patients with BCVA ≤20/40 at the baseline and ≥20/40 at 12 months after the vitrectomy was 63.4%. Their findings were similar to ours. The reasons for the favorable visual outcomes in our study were not determined. However, the consecutive case series by one surgeon might be one reason.
IVTA was used in 97 (11.4%) of 854 eyes with persistent macular edema, but its use was not significantly correlated with the final BCVA. Thus, IVTA during the follow-up period has not been performed recently. If the ILM is not peeled at the initial surgery, we performed ILM peeling as an additional procedure for persistent macular edema. If persistent macular edema was observed in ILM peeled eyes, most eyes had good BCVA as our OCT data showed. Further studies are needed for the additional treatments such as anti-VEGF injection and direct photocoagulation.
The time course of the changes in the BCVA raises doubts about the effectiveness of surgery in eyes with ischemic CRVO. The BCVAs and foveal thicknesses were significantly improved after the surgery. The final BCVA was also improved in 51.6% and unchanged in 25.3% of the patients. The number of eyes with a preoperative BCVA ≥20/40 was only 1 (1.1%), and it was 13 eyes (14.3%) at the final visit. However, final BCVA worsened in 23.1% and the 64 (83.1%) of the 77 eyes with a preoperative BCVA ≤20/200 had a final BCVA of ≤20/200. In addition, neovascular glaucoma developed postoperatively in 12 eyes (13.2%), and 6 of these eyes required glaucoma surgery. Some patients had favorable visual outcomes after the surgery. However, the prognosis of visual outcomes generally remained poor, and the incidence of postoperative complications was high. The indications for ischemic CRVO need to be examined in more detail.
In treatment-naïve patients, we recommended vitrectomy as the initial treatment for patients who requested surgery and eyes with cataracts, vitreomacular traction, and chronic macular edema. In the pre-treated patients with recurrent or persistent macular edema, we recommended vitrectomy, as have many other authors. In patients with ischemic CRVO, vitrectomy might also be considered after panretinal photocoagulation and anti-VEGF injection. Recently, surgical procedures have been combined with ILM peeling. However, the necessity of ILM peeling for acute and treatment-naïve patients has not been definitively determined.
The exact mechanism for the effectiveness of vitrectomy was not determined. It is known that the release of traction, removal of angiogenic agents, and improving oxygenation to the retina66–68 may play important roles. In this study, the final BCVA was not associated with PVD. Thus, we believe that the main reason may be improving oxygenation to the retina. Further analysis is needed to explain the effectiveness of vitrectomy.
Several authors have reported that the vitreous may play an important role in the development of macular edema.69–72 In addition, the degree of uveitis decreased73 and the retinal oxygen concentration improved74 after the vitreous surgery. On the basis of these reports, we first used vitrectomy in eyes with diffuse diabetic macular edema.75 We considered the possibility that vitrectomy would also be effective for macular edema associated with other conditions. At that time, the standard treatment for macular edema due to a BRVO was laser photocoagulation; however, the treatment benefits were limited. In 1994, we started performing vitrectomy for patients with marked retinal thickening and severe hemorrhage in the macular area that conventional laser photocoagulation treatment of macular edema, as outlined in the BVOS study, could not be performed. In 1996, we expanded the indications for vitrectomy to eyes with macular edema due to a CRVO.
There are several limitations in this study. The absence of a control group that underwent observations only or had undergone anti-VEGF treatments. Systemic risk factors such as hypertension and diabetes mellitus were not examined extensively. There were the effects of the time when the surgery was performed and the changes in the surgical techniques and instruments, surgical strategy including indication for surgery, and the additional treatments. The postoperative standard therapy and the need for treatment escalation were not established. The reported treatment success was not achieved only by surgery because some patients received preoperative and postoperative treatments. The positive aspects of this study include a large sample size, phacovitrectomy by the same surgeon, longer follow-up period, and the use of BCVA. In addition, all eyes except the clear phakic eyes were pseudophakic at the last visit so a worsening of nuclear sclerotic cataracts did not influence the final BCVA.
In conclusion, the BCVA improves and is maintained for a long postoperative period after vitrectomy for macular edema due to RVO. The type of RVO is significantly associated with the final BCVA. The effects of several attempts to release the obstruction site seem invalid. Vitrectomy can be a treatment option and can reduce the financial and time burden for patients and physicians.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rogers S, McIntosh RL, Cheung N, et al. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117(2):313–9.e1. doi:10.1016/j.ophtha.2009.07.017
2. Tachi N, Hashimoto Y, Ogino N. Vitrectomy for macular edema combined with retinal vein occlusion. Doc Ophthalmol. 1999;97(3–4):465–469.
3. Kumagai K, Ogino N, Furukawa M, et al. [Vitreous surgery for macular edema in branch retinal vein occlusion]. Nippon Ganka Gakkai Zasshi. 2002;106(11):701–707.
4. Furukawa M, Kumagai K, Ogino N, Uemura A, Larson E. Long-term visual outcomes of vitrectomy for cystoid macular edema due to nonischemic central retinal vein occlusion. Eur J Ophthalmol. 2006;16(6):841–846. doi:10.1177/112067210601600609
5. Kumagai K, Furukawa M, Ogino N, Larson E, Uemura A. Long-term visual outcomes after vitrectomy for macular edema with foveal hemorrhage in branch retinal vein occlusion. Retina. 2007;27(5):584–588. doi:10.1097/01.iae.0000249576.98520.25
6. Kumagai K, Furukawa M, Ogino N, Uemura A, Larson E. Long-term outcomes of vitrectomy with or without arteriovenous sheathotomy in branch retinal vein occlusion. Retina. 2007;27(1):49–54. doi:10.1097/01.iae.0000221996.77421.69
7. Kumagai K, Furukawa M, Ogino N, Larson E. Possible effects of internal limiting membrane peeling in vitrectomy for macular vein occlusion. Jpn J Ophthalmol. 2010;54(1):61–65. doi:10.1007/s10384-009-0750-z
8. Kumagai K, Ogino N, Furukawa M, Larson E. Three treatments for macular edema because of branch retinal vein occlusion: intravitreous bevacizumab or tissue plasminogen activator, and vitrectomy. Retina. 2012;32(3):520–529. doi:10.1097/IAE.0b013e31822529e2
9. Osterloh MD, Charles S. Surgical decompression of branch retinal vein occlusions. Arch Ophthalmol. 1988;106(10):1469–1471.
10. Opremcak EM, Bruce RA. Surgical decompression of branch retinal vein occlusion via arteriovenous crossing sheathotomy: a prospective review of 15 cases. Retina. 1999;19(1):1–5.
11. Shah GK, Sharma S, Fineman MS, Federman J, Brown MM, Brown GC. Arteriovenous adventitial sheathotomy for the treatment of macular edema associated with branch retinal vein occlusion. Am J Ophthalmol. 2000;129(1):104–106.
12. Opremcak EM, Bruce RA, Lomeo MD, Ridenour CD, Letson AD, Rehmar AJ. Radial optic neurotomy for central retinal vein occlusion: a retrospective pilot study of 11 consecutive cases. Retina. 2001;21(5):408–415.
13. Weiss JN, Bynoe LA. Injection of tissue plasminogen activator into a branch retinal vein in eyes with central retinal vein occlusion. Ophthalmology. 2001;108(12):2249–2257.
14. Mester U, Dillinger P. Vitrectomy with arteriovenous decompression and internal limiting membrane dissection in branch retinal vein occlusion. Retina. 2002;22(6):740–746.
15. Cahill MT, Kaiser PK, Sears JE, Fekrat S. The effect of arteriovenous sheathotomy on cystoid macular oedema secondary to branch retinal vein occlusion. Br J Ophthalmol. 2003;87(11):1329–1332. doi:10.1136/bjo.87.11.1329
16. Weizer JS, Stinnett SS, Fekrat S. Radial optic neurotomy as treatment for central retinal vein occlusion. Am J Ophthalmol. 2003;136(5):814–819. doi:10.1016/S0002-9394(03)00698-6
17. Garcia-Arumii J, Boixadera A, Martinez-Castillo V, Castillo R, Dou A, Corcostegui B. Chorioretinal anastomosis after radial optic neurotomy for central retinal vein occlusion. Arch Ophthalmol. 2003;121(10):1385–1391. doi:10.1001/archopht.121.10.1385
18. Williamson TH, Poon W, Whitefield L, Strothidis N, Jaycock P. A pilot study of pars plana vitrectomy, intraocular gas, and radial neurotomy in ischaemic central retinal vein occlusion. Br J Ophthalmol. 2003;87(9):1126–1129. doi:10.1136/bjo.87.9.1126
19. Mason J
20. Mandelcorn MS, Nrusimhadevara RK. Internal limiting membrane peeling for decompression of macular edema in retinal vein occlusion: a report of 14 cases. Retina. 2004;24(3):348–355.
21. Yamamoto S, Saito W, Yagi F, Takeuchi S, Sato E, Mizunoya S. Vitrectomy with or without arteriovenous adventitial sheathotomy for macular edema associated with branch retinal vein occlusion. Am J Ophthalmol. 2004;138(6):907–914. doi:10.1016/j.ajo.2004.06.061
22. Tsujikawa A, Fujihara M, Iwawaki T, Yamamoto K, Kurimoto Y. Triamcinolone acetonide with vitrectomy for treatment of macular edema associated with branch retinal vein occlusion. Retina. 2005;25(7):861–867.
23. Leizaola-Fernandez C, Suarez-Tata L, Quiroz-Mercado H, et al. Vitrectomy with complete posterior hyaloid removal for ischemic central retinal vein occlusion: series of cases. BMC Ophthalmol. 2005;5:10. doi:10.1186/1471-2415-5-10
24. Ma J, Yao K, Zhang Z, Tang X. 25-gauge vitrectomy and triamcinolone acetonide-assisted internal limiting membrane peeling for chronic cystoid macular edema associated with branch retinal vein occlusion. Retina. 2008;28(7):947–956. doi:10.1097/IAE.0b013e31816c683d
25. Chung EJ, Freeman WR, Koh HJ. Visual acuity and multifocal electroretinographic changes after arteriovenous crossing sheathotomy for macular edema associated with branch retinal vein occlusion. Retina. 2008;28(2):220–225. doi:10.1097/IAE.0b013e31813c69df
26. Uemura A, Yamamoto S, Sato E, Sugawara T, Mitamura Y, Mizunoya S. Vitrectomy alone versus vitrectomy with simultaneous intravitreal injection of triamcinolone for macular edema associated with branch retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 2009;40(1):6–12. doi:10.3928/15428877-20090101-19
27. DeCroos FC, Shuler RK
28. Muqit MM, Saidkasimova S, Keating D, Murdoch JR. Long-term study of vascular perfusion effects following arteriovenous sheathotomy for branch retinal vein occlusion. Acta Ophthalmol. 2010;88(3):e57–e65. doi:10.1111/j.1755-3768.2010.01877.x
29. Park DH, Kim IT. Long-term effects of vitrectomy and internal limiting membrane peeling for macular edema secondary to central retinal vein occlusion and hemiretinal vein occlusion. Retina. 2010;30(1):117–124. doi:10.1097/IAE.0b013e3181bced68
30. Harino S, Bessho K, Kida T. Prospective multicenter study of visual outcomes following three different treatments for macular edema associated with branch retinal vein occlusion: a study by the Japanese BRVO study group. Jpn J Ophthalmol. 2012;56(3):250–261. doi:10.1007/s10384-012-0121-z
31. Kadonosono K, Yamane S, Arakawa A, et al. Endovascular cannulation with a microneedle for central retinal vein occlusion. JAMA Ophthalmol. 2013;131(6):783–786. doi:10.1001/jamaophthalmol.2013.2585
32. Noma H, Shimada K, Mimura T. Visual function after pars plana vitrectomy in macular edema with branch retinal vein occlusion. Int Ophthalmol. 2013;33(3):227–236. doi:10.1007/s10792-012-9676-4
33. Yunoki T, Mitarai K, Yanagisawa S, Kato T, Ishida N, Hayashi A. Effects of vitrectomy on recurrent macular edema due to branch retinal vein occlusion after intravitreal injection of bevacizumab. J Ophthalmol. 2013;2013:415974. doi:10.1155/2013/670242
34. Yamane S, Kamei M, Sakimoto S, et al. Matched control study of visual outcomes after arteriovenous sheathotomy for branch retinal vein occlusion. Clin Ophthalmol. 2014;8:471–476. doi:10.2147/OPTH.S58681
35. Sato S, Inoue M, Yamane S, Arakawa A, Mori M, Kadonosono K. Outcomes of microincision vitrectomy surgery with internal limiting membrane peeling for macular edema secondary to branch retinal vein occlusion. Clin Ophthalmol. 2015;9:439–444. doi:10.2147/OPTH.S75659
36. Shirakata Y, Fukuda K, Fujita T, et al. Pars plana vitrectomy combined with internal limiting membrane peeling for recurrent macular edema due to branch retinal vein occlusion after antivascular endothelial growth factor treatments. Clin Ophthalmol. 2016;10:277–283. doi:10.2147/OPTH.S85751
37. Nishida A, Kojima H, Kameda T, Mandai M, Kurimoto Y. Five-year outcomes of pars plana vitrectomy for macular edema associated with branch retinal vein occlusion. Clin Ophthalmol. 2017;11:369–375. doi:10.2147/OPTH.S123419
38. Shirakata Y, Fujita T, Nakano Y, Shiraga F, Tsujikawa A. Pars plana vitrectomy combined with internal limiting membrane peeling to treat persistent macular edema after anti-vascular endothelial growth factor treatment in cases of ischemic central retinal vein occlusion. Case Rep Ophthalmol. 2016;7(1):1–8. doi:10.1159/000443322
39. Tachi N, Hashimoto Y, Ogino N. Cystotomy for diabetic cystoid macular edema. Doc Ophthalmol. 1999;97(3–4):459–463.
40. Thompson JT. What is the role of vitrectomy for macular edema from branch retinal vein occlusion? Am J Ophthalmol. 2004;138(6):1037–1038. doi:10.1016/j.ajo.2004.08.063
41. McIntosh RL, Mohamed Q, Saw SM, Wong TY. Interventions for branch retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2007;114(5):835–854. doi:10.1016/j.ophtha.2007.01.010
42. Mohamed Q, McIntosh RL, Saw SM, Wong TY. Interventions for central retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2007;114(3):507–19, 524. doi:10.1016/j.ophtha.2006.11.011
43. Li J, Paulus YM, Shuai Y, Fang W, Liu Q, Yuan S. New developments in the classification, pathogenesis, risk factors, natural history, and treatment of branch retinal vein occlusion. J Ophthalmol. 2017;2017:4936924. doi:10.1155/2017/4936924
44. Ehlers JP, Kim SJ, Yeh S, et al. Therapies for macular edema associated with branch retinal vein occlusion: a report by the American academy of ophthalmology. Ophthalmology. 2017;124(9):1412–1423. doi:10.1016/j.ophtha.2017.03.060
45. Jumper JM, Dugel PU, Chen S, Blinder KJ, Walt JG. Anti-VEGF treatment of macular edema associated with retinal vein occlusion: patterns of use and effectiveness in clinical practice (ECHO study report 2). Clin Ophthalmol. 2018;12:621–629. doi:10.2147/OPTH.S163859
46. Schlenker MB, Thiruchelvam D, Redelmeier DA. Intravitreal anti-vascular endothelial growth factor treatment and the risk of thromboembolism. Am J Ophthalmol. 2015;160(3):569–580.e5. doi:10.1016/j.ajo.2015.06.011
47. Campochiaro PA, Hafiz G, Mir TA, et al. Scatter photocoagulation does not reduce macular edema or treatment burden in patients with retinal vein occlusion: the RELATE trial. Ophthalmology. 2015;122(7):1426–1437. doi:10.1016/j.ophtha.2015.04.006
48. Parke DW
49. Hayreh SS, Zimmerman MB, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrence and demographic characteristics. Am J Ophthalmol. 1994;117(4):429–441.
50. Hayreh SS, Zimmerman MB, Beri M, Podhajsky P. Intraocular pressure abnormalities associated with central and hemicentral retinal vein occlusion. Ophthalmology. 2004;111(1):133–141. doi:10.1016/j.ophtha.2003.03.002
51. Kumagai K, Ogino N, Larson E. Mathematical function describing visual gain curves following vitrectomy for different macular diseases. Jpn J Ophthalmol. 2011;55(2):89–92. doi:10.1007/s10384-010-0922-x
52. Hayreh SS, Hayreh MS. Hemi-central retinal vein occulsion. Pathogenesis, clinical features, and natural history. Arch Ophthalmol. 1980;98(9):1600–1609.
53. Joffe L, Goldberg RE, Magargal LE, Annesley WH. Macular branch vein occlusion. Ophthalmology. 1980;87(2):91–98.
54. Hayreh SS, Zimmerman MB. Fundus changes in branch retinal vein occlusion. Retina. 2015;35(5):1016–1027. doi:10.1097/IAE.0000000000000418
55. Rogers SL, McIntosh RL, Lim L, et al. Natural history of branch retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010;117(6):1094–1101.e5. doi:10.1016/j.ophtha.2010.01.058
56. Quinlan PM, Elman MJ, Bhatt AK, Mardesich P, Enger C. The natural course of central retinal vein occlusion. Am J Ophthalmol. 1990;110(2):118–123.
57. Campochiaro PA, Sophie R, Pearlman J, et al. Long-term outcomes in patients with retinal vein occlusion treated with ranibizumab: the RETAIN study. Ophthalmology. 2014;121(1):209–219. doi:10.1016/j.ophtha.2013.08.038
58. Heier JS, Campochiaro PA, Yau L, et al. Ranibizumab for macular edema due to retinal vein occlusions: long-term follow-up in the HORIZON trial. Ophthalmology. 2012;119(4):802–809. doi:10.1016/j.ophtha.2011.12.005
59. Clark WL, Boyer DS, Heier JS, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: 52-week results of the VIBRANT study. Ophthalmology. 2016;123(2):330–336. doi:10.1016/j.ophtha.2015.09.035
60. Casselholm de Salles M, Amren U, Kvanta A, Epstein DL. Injection frequency of aflibercept versus ranibizumab in a treat-and-extend regimen for central retinal vein occlusion: a randomized clinical trial. Retina. 2018;1. doi:10.1097/IAE.0000000000002171
61. DeCroos FC, Todorich B, Alshareef R, et al. Neovascular events in eyes with central retinal vein occlusion undergoing serial bevacizumab or ranibizumab intravitreal injections: a retrospective review. J Ophthalmic Vis Res. 2014;9(4):461–468. doi:10.4103/2008-322X.150825
62. Brown DM, Wykoff CC, Wong TP, Mariani AF, Croft DE, Schuetzle KL. Ranibizumab in preproliferative (ischemic) central retinal vein occlusion: the rubeosis anti-VEGF (RAVE) trial. Retina. 2014;34(9):1728–1735. doi:10.1097/IAE.0000000000000191
63. Winegarner A, Wakabayashi T, Fukushima Y, et al. Changes in retinal microvasculature and visual acuity after antivascular endothelial growth factor therapy in retinal vein occlusion. Invest Ophthalmol Vis Sci. 2018;59(7):2708–2716. doi:10.1167/iovs.17-23437
64. Smiddy WE. Economic considerations of macular edema therapies. Ophthalmology. 2011;118(9):1827–1833. doi:10.1016/j.ophtha.2010.12.034
65. Lin J, Chang JS, Yannuzzi NA, Smiddy WE. Cost evaluation of early vitrectomy versus panretinal photocoagulation and intravitreal ranibizumab for proliferative diabetic retinopathy. Ophthalmology. 2018;125(9):1393–1400. doi:10.1016/j.ophtha.2018.02.038
66. Williamson TH, Grewal J, Gupta B, Mokete B, Lim M, Fry CH. Measurement of PO2 during vitrectomy for central retinal vein occlusion, a pilot study. Graefes Arch Clin Exp Ophthalmol. 2009;247(8):1019–1023. doi:10.1007/s00417-009-1072-z
67. Stefansson E. Physiology of vitreous surgery. Graefes Arch Clin Exp Ophthalmol. 2009;247(2):147–163. doi:10.1007/s00417-008-0980-7
68. Nakano Y, Manabe K, Osaka R, et al. The effect of vitreomacular and cataract surgery on oxygen saturation in retinal vessels. Clin Ophthalmol. 2017;11:759–765. doi:10.2147/OPTH.S132392
69. Schepens CL, Avila MP, Jalkh AE, Trempe CL. Role of the vitreous in cystoid macular edema. Surv Ophthalmol. 1984;28:
70. Sebag J, Balazs EA. Pathogenesis of cystoid macular edema: an anatomic consideration of vitreoretinal adhesions. Surv Ophthalmol. 1984;28(Suppl):493–498.
71. Nasrallah FP, Jalkh AE, Van Coppenolle F, et al. The role of the vitreous in diabetic macular edema. Ophthalmology. 1988;95(10):1335–1339.
72. Roldan M, Serrano JM. Macular edema and vitreous detachment. Ann Ophthalmol. 1989;21(4):141–148.
73. Diamond JG, Kaplan HJ. Lensectomy and vitrectomy for complicated cataract secondary to uveitis. Arch Ophthalmol. 1978;96(10):1798–1804.
74. Stefánsson E, Novack RL, Hatchell DL. Vitrectomy prevents retinal hypoxia in branch retinal vein occlusion. Invest Ophthalmol Vis Sci. 1990;31(2):284–289.
75. Tachi N, Ogino N. Vitrectomy for diffuse macular edema in cases of diabetic retinopathy. Am J Ophthalmol. 1996;122(2):258–260.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







