Back to Journals » Clinical Ophthalmology » Volume 16
Visual Outcomes of an Enhanced UV Protected Light Adjustable Lens Using a Novel Co-Managed, Open-Access Methodology
Received 16 June 2022
Accepted for publication 26 July 2022
Published 4 August 2022 Volume 2022:16 Pages 2413—2420
DOI https://doi.org/10.2147/OPTH.S378525
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
David V Folden,1– 3 Jennifer R Wong4
1Twin Cities Eye Consultants, Edina, MN, USA; 2North Suburban Eye Specialists, Coon Rapids, MN, USA; 3North Metro Surgery Center, Blaine, MN, USA; 4Praxis Vision, Edina, MN, USA
Correspondence: David V Folden, Twin Cities Eye Consultants, 3777 Coon Rapids Blvd NW, Coon Rapids, MN, 55433, USA, Tel +1 763-421-7420, Fax +1 763-421-0730, Email [email protected]
Purpose: To report on the safety and visual acuity (VA) outcomes using a co-managed, open-access methodology with a second-generation (ActivShieldTM) Light Adjustable Lens (LAL 2.0).
Patients and Methods: This retrospective observational case series of consecutive patients implanted with the LAL 2.0 choosing an emmetropic target in at least one eye were included in the study. All patients were co-managed with light treatments occurring at an open-access facility. Exclusion criteria included pathology of the macula and/or cornea with reduced best corrected visual acuity (BCVA). The primary outcome measures were uncorrected distance visual acuity (UDVA), spherical equivalent (SE), and residual cylinder for emmetropic goal eyes at the final 3- to 9-month postoperative visit.
Results: Thirty-three patients (62 eyes) were included in the study and implanted with the LAL 2.0. Thirty-three (53.2%) eyes had previous corneal refractive treatment(s) with 22 (66.7%) having no original historical refractive records available. Thirty-six (58.1%) total eyes and 20 (32.3%) postrefractive eyes had an emmetropic refractive target. Of all the emmetropic goal eyes, 35 (97.2%) saw 20/20 or better and 36 (100%) were within ± 0.50 D SE of plano and had a mean cylinder of − 0.15 ± 0.26 D. Of the postrefractive emmetropic goal eyes, 19 (95%) saw 20/20 or better, 20 (100%) were within ± 0.50 D SE of plano and had a mean cylinder of − 0.17 ± 0.28 D.
Conclusion: A co-managed, open-access methodology using the LAL 2.0 was safe and efficacious even in challenging postrefractive clinical scenarios.
Keywords: ActivShieldTM, light adjustable lens, co-manage, open-access, refractive cataract surgery
Introduction
Light Adjustable Lens (LAL; RxSight, Inc.) is an intraocular lens (IOL) that enables correction of refractive error and vision customization after implantation and ocular healing has occurred. The LAL is made of a special photosensitive material (macromers) that can be adjusted in response to ultraviolet (UV) light through a series of noninvasive treatments using a Light Delivery Device (LDD) in the weeks following IOL implantation.1,2 Light treatments commonly begin at 3 to 4 weeks postoperatively, and end at 5 to 6 weeks postoperatively as the patient secures refractive stability. Patients receive up to 3 light treatments for refractive adjustment and 2 light treatments for “lock-in” resulting in full polymerization of the macromers, preventing any further refractive change to the IOL. To help prevent uncontrolled changes to the LAL from ambient environmental UV light, patients are instructed to wear RxSight UV protective glasses until 24 hours after the last “lock-in” treatment.
A second-generation IOL from RxSight (LAL 2.0) was FDA approved and commercially available in 2021. The LAL 2.0 incorporates an enhanced UV absorber (ActivShieldTM) in the silicone matrix, providing redundant protection against UV exposure.3 This additional UV protection may aid in safely extending the final treatments and “lock-in” with less concern for the uncontrolled refractive effects of ambient environmental UV light exposure.
Postoperative visits involving LDD treatment are comprehensive and typically include the following: (1) discussion of the patient’s current refractive state and adjustment requests to improve their vision goals; (2) manifest refraction (MRx); (3) loose lens trial(s) showing the intended adjustment based on patient-directed requests; (4) full 6.5–7.0 mm pupil dilation exposing the full LAL optic; and (5) refractive adjustments using the LDD. Up to 5 postoperative treatments are required for refractive adjustments and “lock-in”. These visits can consume a 90-minute block of time for the physician’s schedule. The additional postoperative commitment required for patients choosing the LAL can impact the surgeon’s typical scheduling patterns.
The purpose of this paper is to report on the safety and visual acuity (VA) outcomes using a novel care delivery model for LAL technology. To our knowledge, this is the first study to introduce a co-managed, open-access methodology for this technology and the first to report visual outcomes of the LAL 2.0 in the peer-reviewed literature.
Patients and Methods
Study Design and Participants
This retrospective observational case series assessed the VA and safety outcomes of a consecutive series of patients after cataract extraction and IOL implantation with the LAL 2.0 in a private practice setting between July 2021 and January 2022. All patients were de-identified with no PHI patient-specific data being used in the analysis. The study was registered with the ISRCTN Registry (ISRCTN55809023; https://www.isrctn.com/), approved by an institutional review board (SALUS IRB, Austin, TX) and performed in accordance with the tenets of the Declaration of Helsinki. Requirement for written consent was waived by the IRB due to the study’s retrospective design, in which only “standard of care” pre and postoperative protocols/procedures for the FDA approved technologies were employed. Patients with reduced best corrected visual acuity (BCVA) related to retinal/macular pathology and/or corneal abnormalities including scarring and ectasia were excluded from the study.
Patients undergoing cataract surgery and choosing the LAL with an emmetropic target (±0.25 D) in at least one eye were included in the study. All surgeries were performed in Blaine, Minnesota, by a single surgeon (DVF). Postoperatively, through a co-managed arrangement at an open-access facility, all LDD treatments were performed by a single co-managing physician (herein referred to as an LDD Specialist) (JRW) in Edina, Minnesota. This open-access facility currently employs 2 LDD Specialists providing LAL postoperative management for 16 surgeons. This facility’s co-managed arrangements are solely for the purpose of LAL postoperative management and LDD treatments, with physicians that provide continuous communication with referring surgeons throughout the patient’s LDD treatment process.
All patients had a complete baseline ophthalmic examination prior to surgery. Biometry was achieved using an IOLMaster 500, and topography imaging was obtained using a Pentacam HR/AXL or Galilei G4 for all patients. IOL powers were calculated using the Barrett Universal II, SRK/T, and Holladay 2 formulas in non-postrefractive cataract patients. The Post-Refractive IOL Calculator at the American Society of Cataract and Refractive Surgery (ASCRS) was used for eyes that had previous LASIK, PRK, and/or RK with emphasis on the Masket, Modified Masket, and Barrett True K in patients with historical refractive records, and Barrett True K No History in patients with no historical refractive records.
Postimplantation refractive targets were based on patient’s estimated preoperative refractive goals. A goal of +0.25 to +0.50 manifest refraction spherical equivalent (MRSE) was targeted for emmetropic-goal eyes, and plano MRSE was targeted in myopic-goal eyes for patients wanting customized monovision. Patients were allowed to adjust their refractive target to fit their lifestyle postoperatively during LDD exams and adjustments, with a wide range of near targets being selected.
The primary outcome measures for this study were monocular uncorrected distance visual acuity (UDVA), monocular mean spherical equivalent (SE), and monocular mean residual cylinder for emmetropic-goal eyes at least 3 months postoperatively. For patients that rescheduled and/or had continued management of other conditions, including dry eye and posterior capsule opacification beyond their scheduled 3-month postoperative exam, visual outcome data of longest postoperative duration (final exam) for each patient was used during data collection, ranging 92 to 268 days postoperatively. Safety outcomes reported include serious adverse events (AEs) occurring at the time of surgery extending out to the final postoperative examination.
Surgical Technique and Postoperative Light Adjustments
All cataract surgeries were performed with topical anesthesia using a 2.4 mm clear corneal, self-sealing temporal incision, and widened to 2.8–3.0 mm for LAL implantation. The LENSARTM femtosecond laser was used for capsulotomy and lens fragmentation in all patients except for those with previous history of RK. Capsulotomy diameters ranged from 4.9 to 5.2 mm with centration on the optical axis to ensure full 360-degree anterior capsule overlap of the IOL optic. All patients received the LAL 2.0.
The LDD was used to adjust the LAL power by delivering targeted UV light in a precisely programmed pattern to correct spherical and cylindrical refractive error, facilitating optimization of the lens to achieve the patient’s desired vision. LDD treatments provided a custom prescription formulated on refractive measurements and lifestyle requirements of the patient. After the desired vision was achieved and the refraction verified, the entire lens was exposed to UV light to “lock in” the IOL power, preventing any further refractive change to the optic. Patients were instructed to wear RxSight UV protective glasses during all waking hours from the time of lens implantation until 24 hours after the last “lock-in” treatment was completed.
The first LDD treatment for all patients occurred 17 to 21 days post LAL implantation when corneal edema and anterior chamber inflammation were resolved. This was confirmed by slit-lamp examination. Additional LDD treatments were separated by at least 72 hours. If refractive targets were stable from the previous LDD treatment goal and patients did not request additional time to evaluate their vision, treatments were performed every 72 hours until final “lock-in”. Patients wanting to evaluate their vision longer, or those with an unstable refraction from the target of the previous LDD treatment, extended the duration between treatments until further patient direction and/or refractive stability. The range of total LDD refractive treatments was 1 to 3 and “lock-in” treatments was 1 to 2 for all patients. Final “lock-in” occurred between 25 and 67 days in all patients. Patients with a history of previous corneal refractive surgery (history of LASIK, PRK, and/or RK) had longer duration from surgery to final “lock-in” to help ensure refractive stability and good long-term uncorrected visual acuity (UCVA).
At all postoperative visits, clinical examinations included slit-lamp biomicroscopy, intraocular pressure, MRx with corrected distance visual acuity (CDVA), and monocular/binocular uncorrected distance visual acuity (UDVA) and uncorrected near visual acuity (UNVA). Distance VA testing was achieved using a computer calibrated Snellen chart under photopic conditions. Near VA testing was assessed using a Jaeger chart under photopic conditions. The near VA goal was a fluid process in all patients and based on functionality with adjustments to the original goal based on patient experience and feedback during LDD treatments. Patients were instructed to hold the Jaeger card at their preferred reading distance (range: 14–20 inches), MRx was then completed to bring the desired near point into focus, and LDD treatments were performed to adjust accordingly. A standard phoropter was used to determine the subjective MRx rounded to steps of 0.25 D. All testing was “standard of care” and there were no “study specific” tests performed.
Results
Sixty-two eyes from 33 patients with a mean age of 61.5 years (range: 49–72) were implanted with the LAL 2.0. Both bilateral emmetropia and customized monovision refractive goals were based on patient feedback during LDD treatments. Thirty-six (58.1%) eyes had an emmetropic “distance vision goal” refractive target. Twenty-six (41.9%) eyes had a myopic “near vision goal” refractive target (≥-0.50 D) in those patients requesting a binocular monovision outcome.
Table 1 shows patient demographics and baseline characteristics. Thirty-three (53.2%) eyes had ≥1 corneal refractive treatment(s) (LASIK, PRK, and/or RK) with 22 (66.7%) having no original historical refractive records available.
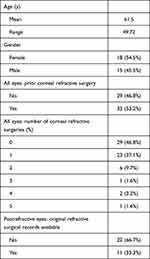 |
Table 1 Patient Demographics and Baseline Characteristics |
At the final 3- to 9-month postoperative examination, UDVA for all emmetropic goal eyes was 20/20 or better in 97.2% (35/36), with a mean SE of 0.02 ± 0.22 D and mean cylinder of −0.15 ± 0.26 D [range 0.00, −1.00 D]. In postrefractive emmetropic goal eyes, UDVA was 20/20 in 95% (19/20), with a mean SE of 0.03 ± 0.21 D and a mean cylinder of −0.17 D ± 0.28 D [range 0.00, −1.00 D]. Following all the postoperative LDD refractive adjustments and final “lock-in” treatment, LAL patients experienced a low mean level of residual refractive error, as shown in Tables 2 and 3.
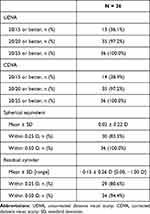 |
Table 2 All Emmetropic Goal Eyes at Final Exam |
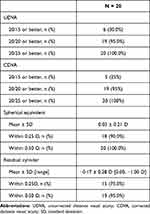 |
Table 3 Postrefractive Emmetropic Goal Eyes at Final Exam |
Twenty-nine patients received binocular implantation of the LAL, of which 26 (89.7%) chose to customize a monovision goal optimized for their practical, real-world binocular near vision needs during LDD treatments. In patients who chose monovision, 25 (96.2%) had binocular UDVA of 20/20 or better and binocular UNVA of J2. Fifteen (57.7%) had binocular UDVA of 20/20 or better and binocular UNVA of J1 (Table 4). The average myopic defocus for all “near vision goal” eyes was −0.88 ± 0.38 D.
 |
Table 4 Patients with Monovision Goal at Final Exam |
Five eyes of 3 patients were excluded from the study and data set because of reduced BCVA unrelated to the methodology or IOL. One eye was excluded from previous history of macula-off retinal detachment. Two eyes from one patient were excluded because of corneal haze related to previous history of Automated Lamellar Keratoplasty (ALK). One patient experienced bilateral cystoid macular edema (CME) after uneventful surgery noted at the one-week postoperative examination but deemed unrelated to the IOL or methodology. There were no explants, refractive enhancements, AEs related to the LAL or methodology, and no patient lost BCVA.
Discussion
The safety and effectiveness of the LAL has been previously reported in cataract patients with and without a history of corneal-based refractive surgery.4–12
In patients without a previous refractive surgical history who have received a nonadjustable IOL, pseudophakic postoperative SE predictions have improved but are still limited to around 80% within ±0.5 D of refractive target.13 Using the LAL in a similar patient population, Hengerer et al showed 98% of eyes were within ±0.50 D of refractive target, 97% within ±0.25 D of target and mean SE refraction of 0.03 ± 0.17 D.4 Villegas et al showed 80% of their LAL patients had a SE of 0.50 D or lower and 85% had astigmatism of 0.75 D or lower. Their UDVA was better than 0.9 (20/22) in 70% of study eyes.5 Phase 3 FDA study data showed those receiving the LAL had 92% accuracy within 0.50 D of target MRSE and were approximately twice as likely to achieve uncorrected 20/20 vision or better at 6 months compared with nonadjustable monofocal IOL patients (70.1% vs 36.3%).6
Patients with a history of previous refractive surgery pose problems with preoperative biometry and IOL calculations with subsequent postoperative refractive error and reduced UCVA following cataract surgery. This population of patients is particularly well suited for LAL technology.
Wang and Koch summarize refractive outcomes in postrefractive pseudophakic patients with nonadjustable IOL implants. Patients with a history of myopic LASIK/PRK typically do not exceed 75% accuracy within ±0.5 D of target and are slightly worse with a history of hyperopic LASIK/PRK. Refractive outcomes in patients with a history of RK show even lower accuracy compared with LASIK/PRK, with 29% to 62% of eyes within ±0.5 D of target. Even in studies utilizing intraoperative wavefront aberrometry, postrefractive pseudophakic patients range from 67% to 75% accuracy within ±0.5 D of target.14 Using the LAL in a similar patient population, Brierley reported on 16 eyes, 94% of which were within ±0.50 D of intended refraction and 75% of eyes within ±0.25 D.7 Brierley later reported on 34 eyes, 97% of which were within ±0.50 D of target and 74% of which were within ±0.25 D of target.8 Additional case reports of the LAL used in refractively challenging patient populations have been reported, including post-RK (pseudophakic UDVA of 20/25),9 post-traumatic cataract with LASIK flap dislocation (pseudophakic UDVA of 20/20),10 and post-DMEK (pseudophakic UDVA 20/20 or better).11
The current report evaluates a new methodology for offering LAL technology in cataract surgery, employing a co-managed arrangement at an open-access facility. In light of this methodology, we assessed safety and effectiveness in both patients with and without a history of refractive surgery, including highly complex patients with combinations of LASIK, PRK, RK, and/or limbal relaxing incisions (LRIs) and provide data using a second-generation LAL (LAL 2.0). Near vision goals with the LAL are a fluid process and based on patient feedback and real-world experience during the LDD treatment period. Due to this variability, the present study focused on eyes with an emmetropic goal of assessing the effectiveness and safety of the reported methodology.
Of the 62 operated eyes, 33 (53.2%) had previous corneal refractive surgery, of which 22 (66.7%) were without any original historical refractive records. Ten eyes (16.1%) had 2 or more refractive surgical procedures. One eye had a history of 5 previous corneal refractive surgeries, including 2 LASIK, 2 PRK, and paired 60° LRIs achieving a pseudophakic UDVA of 20/20−1 and MRx of −0.25+0.25x45.
Of all emmetropic goal eyes, 97.2% (35/36) saw 20/20 or better. All eyes (36/36) were within ±0.50 D SE of plano, 83.3% (30/36) were within ±0.25 D SE of plano, and 94.4% (34/36) were within ±0.50 D of residual cylinder. Of all postrefractive emmetropic goal eyes, 95% (19/20) saw 20/20 or better. All postrefractive eyes (20/20) were within ±0.50 D SE of plano, 90% (18/20) were within ±0.25 D SE of plano, and 95% (19/20) were within ±0.5 D of residual cylinder.
The pseudophakic refractive advantages, particularly in a postrefractive patient population, are compelling applications for LAL technology. Pseudophakic refractive error requiring corneal-based refractive procedures can pose challenges in an older patient population and in those with previous refractive surgery. Importantly, the pseudophakic refractive error with the LAL allows refractive adjustments that avoid the cornea and have a reported refractive stability of ~6 times better than after corneal refractive procedures.12
One of the obstacles to implanting the LAL is the logistical challenges posed to the surgeon due to the number of time-consuming follow-up LDD treatment visits. A co-managed arrangement helps remedy these challenges by allowing surgeons to focus on their surgical role and allowing a second physician (the LDD Specialist) to focus on the postoperative skills and techniques necessary for accurate LDD treatments and refractive outcomes. Moreover, an open-access arrangement allows the LDD Specialist to work with multiple surgeons, further increasing their efficiencies, experience, and expertise with postoperative LAL management. From a practical perspective, this methodology provides opportunities to streamline workflow and improve offerings to well-suited patients. Given the separate facilities and co-managed arrangement of this proposed methodology, communication between providers is essential for maintaining safety and achieving the patient’s refractive goals.
This report provides proof of principle that a methodology employing a co-managed, open-access arrangement with the LAL 2.0 is safe and efficacious and can provide advantages even in challenging postrefractive clinical scenarios.
Conclusion
The LAL provides refractive advantages in cataract surgery. Offering LAL technology can be prohibitive because of the logistical demands of a single surgical provider. A co-managed arrangement at an open-access facility can improve the postoperative management efficiencies and provide an opportunity to streamline workflow. This strategy was safe and efficacious using a second-generation LAL (LAL 2.0) even in challenging postrefractive clinical scenarios.
Acknowledgments
We thank John Vukich, MD, for his study design ideas and manuscript review, Hannah Schoenecker for assistance with data collection, and Terri Flom for providing research guidance. No public or private support was provided for this study.
Disclosure
Jennifer R. Wong is a paid consultant for RxSight. The authors report no other conflicts of interest in this work.
References
1. Schwartz DM. Light-adjustable lens. Trans Am Ophthalmol Soc. 2003;101:417–436.
2. Schwartz DM, Sandstedt CA, Chang SH, Kornfield JA, Grubbs RH. Light-adjustable lens: development of in vitro nomograms. Trans Am Ophthalmol Soc. 2004;102:
3. RxSight® PMA P160055 S015: Change Design/Components/Specifications/Material; 2021. Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?ID=P160055S015. Accessed Augest 1, 2022.
4. Hengerer FH, Dick HB, Conrad-Hengerer I. Clinical evaluation of an ultraviolet light adjustable intraocular lens implanted after cataract removal: eighteen months follow-up. Ophthalmology. 2011;118:2382–2388. doi:10.1016/j.ophtha.2011.05.030
5. Villegas EA, Alcon E, Rubio E, Marín JM, Artal P. Refractive accuracy with light-adjustable intraocular lenses. J Cataract Refract Surg. 2014;40(7):1075–1084. doi:10.1016/j.jcrs.2013.10.046
6. RxSight® PMA P160055: FDA Summary of Safety and Effectiveness Data; 2017. Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?ID=P160055. Accessed Augest 1, 2022.
7. Brierley LA. Precision of IOL refractive power adjustment of the Light Adjustable Lens (LAL) in post-refractive surgery patients.
8. Brierley L. Refractive results after implantation of a light-adjustable intraocular lens in postrefractive surgery cataract patients. Ophthalmology. 2013;120(10):1968–1972. doi:10.1016/j.ophtha.2013.03.010
9. Moshirfar M, Duong AA, Shmunes KM, Castillo-Ronquillo YS, Hoopes PC. Light adjustable intraocular lens for cataract surgery after Radial Keratotomy. J Refract Surg. 2020;36(12):852–854. doi:10.3928/1081597X-20201002-01
10. Rocha G, Mednick ZD. Light-adjustable intraocular lens in post-LASIK and post-traumatic cataract patient. J Cataract Refract Surg. 2012;38(6):1101–1104. doi:10.1016/j.jcrs.2012.03.017
11. Eisenbeisz HC, Bleeker AR, Terveen DC, Berdahl JP. Descemet membrane endothelial keratoplasty and light adjustable lens triple procedure. Am J Ophthalmol Case Rep. 2021;25(22):101061. doi:10.1016/j.ajoc.2021.101061
12. Chayet A, Sandstedt C, Chang S, et al. Correction of myopia after cataract surgery with a light-adjustable lens. Ophthalmology. 2009;116:1432–1435. doi:10.1016/j.ophtha.2009.02.012
13. Pantanelli SM, Lin CC, Al-Mohtaseb Z, et al. Intraocular lens power calculation in eyes with previous excimer laser surgery for myopia: a report by the American Academy of Ophthalmology. Ophthalmology. 2021;128(5):781–792. doi:10.1016/j.ophtha.2020.10.031
14. Wang L, Koch DD. Intraocular lens power calculations in eyes with previous corneal refractive surgery: review and expert opinion. Ophthalmology. 2021;128(11):e121–e131. doi:10.1016/j.ophtha.2020.06.054
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
