Back to Journals » Clinical Ophthalmology » Volume 17
VIsion Salvage Using Intra-Ophthalmic Arterial Alteplase Combine with Nimodipine in Central Retinal Artery Occlusion (VISION)
Authors Kobkitsuksakul C , Namphol N , Sirilert B, Kritfuangfoo T , Chanthanaphak E, Apirakkan M, Somboonnithiphol K, Boonyakarnkul S , Lueangapapong P, Thongborisuth T, Sujirakul T
Received 7 February 2023
Accepted for publication 4 April 2023
Published 25 April 2023 Volume 2023:17 Pages 1215—1222
DOI https://doi.org/10.2147/OPTH.S407617
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Chai Kobkitsuksakul,1 Nasathapot Namphol,1 Bandit Sirilert,1 Thanaporn Kritfuangfoo,2 Ekachat Chanthanaphak,1 Mungkorn Apirakkan,1 Kittiphop Somboonnithiphol,1 Surawan Boonyakarnkul,1 Peerapong Lueangapapong,3 Thitiporn Thongborisuth,2 Tharikarn Sujirakul2
1Division of Interventional Neuroradiology, Department of Radiology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; 2Department of Ophthalmology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; 3Department of Neurosurgery, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Correspondence: Tharikarn Sujirakul, Department of Ophthalmology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, 270 Thanon Rama VI, Thung Phaya Thai, Ratchathewi, Bangkok, 10400, Thailand, Tel +6622011526, Fax +6622012729, Email [email protected]
Purpose: To investigate the efficacy and safety of selective intra-ophthalmic arterial combined nimodipine and alteplase infusion in patients with central retinal artery occlusion (CRAO).
Design: Non-randomized, prospective interventional study.
Methods: All patients with CRAO who presented at our institute within 24 hours from CRAO onset from August 2020 to July 2022 were included. Intra-arterial nimodipine and alteplase were given selectively into the ophthalmic artery. Visual acuity was recorded during and after the procedure. Change in best corrected visual acuity (BCVA) 1 month post-treatment, relative to baseline, was set as the primary outcome measure. Significant improvement in vision and adverse events are reported as secondary outcomes.
Patients: Nine patients with non-arteritic CRAO were enrolled.
Results: A total of nine patients with CRAO underwent selective intra-ophthalmic arterial nimodipine and alteplase injection. Overall, BCVA had statistically significantly improved by 0.78 logarithm of the minimum angle of resolution (logMAR) at 1 month compared with baseline (95% confidence interval: (− 1.24, − 0.31), p-value = 0.001). Seven (77.8%) patients had significant visual improvement (≥ 0.3 logMAR) at 1-month post-treatment. There were minor adverse events during administration of the nimodipine, including chemosis and headache, which resolved after the discontinuation of nimodipine. There were also asymptomatic thromboembolic events in 2 patients (22.2%) after the intervention procedure, without any morbidity or mortality.
Conclusion: The use of selective intra-ophthalmic arterial combined nimodipine and alteplase was efficacious in improving BCVA at 1 month for patients with non-arteritic CRAO presenting between 24 hours from onset, with minor adverse events but no serious adverse events.
Keywords: central retinal artery occlusion, thrombolysis, intra-ophthalmic arterial thrombolysis
Introduction
Central retinal artery occlusion (CRAO) is a rare condition with incidence of 1.8–2.5 per 100,000 person-years1–3 which can result in permanent visual loss, less than 20% of affected patients regaining functional visual acuity (VA).4,5
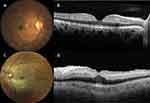
CRAO can be categorized into three different stages: incomplete CRAO (stage 1), subtotal CRAO (stage 2) and total CRAO (stage 3), based on clinical assessment, fundoscopy and fluorescein angiography (FA). Incomplete CRAO is characterized by partial diminution of VA and minimal visible macular edema. FA shows blood flow that is delayed but not completely interrupted. Subtotal CRAO usually presents with severely reduced VA, prominent retinal edema with a typical cherry-red spot, and FA characterized by a marked delay in retinal arterial circulation, particularly involving the perimacular arterioles. In total CRAO or CRAO with choroidal infarction, there can be visual loss to no light perception, with massive retinal edema without cherry-red spot; absent blood flow in the perimacular arterioles with additional interruption of choroidal circulation.5,6
With optical coherence tomography (OCT), distinctive features of the different CRAO stages can be identified, including hyperreflectivity of the inner retinal layers, thickening of the retinal layer and loss of the organized layered structure.7 Subtotal CRAO is the most common stage, occurring in 73% of all patient, following with incomplete CRAO presented in 21.9% of patients and total CRAO presented in 5.1% of patients.6
The prognosis for CRAO is dependent on the stage of CRAO, with incomplete CRAO carrying the best prognosis. CRAO patients treated with standard therapies showed an improvement in vision to better than 20/200 in 15.4% of incomplete CRAO, but no change was found in subtotal or total CRAO.8,9
Although CRAO is now considered a minor stroke, there is still no definitive standard treatment for acute CRAO, unlike acute cerebral stroke. Since an embolus is the most common cause of CRAO,10 theoretically the goal of acute CRAO treatment is to dislodge or lyse the embolus before irreversible retinal damage occurs.
A meta-analysis of intravenous fibrinolytic therapy in CRAO found that the treatment can significantly improve vision (defined as the improvement of VA from 20/200 or worse at presentation to 20/100 or better) in up to 50% in patients presenting within 4.5 hours after the onset.4 To lessen systemic side effects, intra-arterial thrombolytic therapy (IAT) with direct infusion and lowered total thrombolytic dose has been used with several studies showed promising results of IAT in terms of visual improvement6,8,9,11–13, despite The European Assessment Group for Lysis in the Eye (EAGLE) trial, the only randomized control trial of IAT, was terminated before the study was completed, due to a higher rate of adverse events, although minor, in the IAT group, with similar efficacy in the IAT and conservative management groups.14
Various vasodilators such as sublingual isosorbide dinitrate and intravenous glyceryl trinitrate have been used to increase retinal blood flow, to dislodge the embolus and allow it to migrate more peripherally. Nimodipine, a vasodilator, is a calcium channel blocker that has been used intra-arterially for treatment and prevention of cerebral vasospasm after subarachnoid hemorrhage. It has been shown to be effective, with a good safety profile.15,16
Other than thrombolysis, vasodilators might be a potential treatment option, either isolated or in conjunction with IAT. The aim of this study was to investigate the efficacy and safety outcomes of IAT with combined nimodipine and alteplase for the treatment of patients with acute non-arteritic CRAO.
Materials and Methods
This non-randomized prospective study was conducted at Ramathibodi Hospital, Bangkok, Thailand from August 2020 to July 2022. The study was conducted followed with the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) in Mahidol University and Human Research Ethics Committee, Faculty of Medicine Ramathibodi Hospital, Mahidol University (COA. MURA2020/1081) in June 2020. Written informed consent was obtained from all enrolled patients after providing information about the procedure including possible complications, such as adverse events from nimodipine and alteplase.
Patients
Patients were aged 18 years or older and had been diagnosed with acute non-arteritic CRAO. Only patients who presented with vision worse than 20/60 and a time of onset to the predicted time of treatment of less than 24 hours were enrolled in the study.
All patients underwent complete ocular examinations by ophthalmologists and retinal subspecialists. Once the diagnosis of acute CRAO was made, CRAO “fast-track” was activated. The duration from patient presentation to treatment time at our institute was about 2 hours. At presentation, multimodal imaging was performed as available, including color fundus photography, OCT, OCT angiography and FA, without delaying treatment. CRAO staging was based on fundus and OCT findings in all cases (Figure 1) and on additional FA in some cases.
Patients with concurrent neurological deficits were excluded from the study and further treated according to stroke guidelines. Patients with a high risk of bleeding were excluded, to minimize the risk of adverse events from intra-arterial alteplase. The details of inclusion and exclusion criteria are presented in Table 1.
 |
Table 1 Inclusion and Exclusion Criteria |
Treatment Protocol
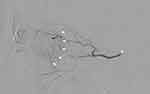
After the consent process, intra-arterial cannulation was into the ophthalmic artery of the patient’s affected side (Figure 2). Nimodipine in a concentration of 0.13 mg/mL was infused at the rate of 2 mg in 15–20 minute via microcatheter into the ophthalmic artery, for a total dose of up to 5 mg. Nimodipine infusion was stopped if the patient reported immediate visual improvement and it was confirmed by an ophthalmologist that VA had improved to the level of equal to or better than 20/60 (log of the minimum angle of resolution (logMAR) ≤ 0.5) using a near chart with corrected reading glasses.
After nimodipine infusion, BCVA was measured, and a fundoscopic examination was performed by an ophthalmologist. After that, alteplase was infused via microcatheter into the ophthalmic artery in 3 divided dose 15mg, 15mg and 15mg for a dose of up to 45 mg. The dose was applied from EAGLE study, previous retrospective IAT reports.4,14 The dose of alteplase was reduced if an ophthalmologist confirmed that the patient’s vision immediately improved to BCVA better equal to or better than 20/60 (logMAR) ≤ 0.5). Alternatively, the infusion was stopped prematurely if the patient experienced intolerable adverse events. After alteplase infusion, BCVA was measured, and the fundoscopic examination was repeated. BCVA, fundoscopic examination and adverse events were recorded on day 1, day 3 and again at 1 month.
The primary outcome was the change in BCVA from baseline to 1 month after IAT. Secondary outcome measures were significant visual improvement, defined as an improvement of ≥0.3 logMAR (double visual angle improvement), and adverse events from IAT.
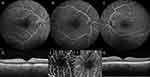
Additional imaging was performed at day 1 after treatment, including color fundus photography, OCT and OCT angiography (Figure 3). Post-procedural brain imaging was used to detect parenchymal hemorrhage after the administration of intra-arterial alteplase, and as the part of the standard ischemic stroke evaluation scheme in CRAO.
Statistical Analysis
Baseline characteristics are described using the mean and standard deviation for continuous data. VA results were converted to logMAR for analyses. Approximate values were assigned for very low vision.17
Statistical analyses in this study were performed using the STATA/MP version 17 program. Visual outcomes were analyzed using a mixed-effect model with a p-value of less than 0.05 considered to be statistically significant for all analyses.
Results
Of the total of 9 patients, 5 (55.6%) were male. The mean age was 54.7 ± 19.23 years. The mean baseline VA was 1.86 ± 0.47 logMAR. Six patients (66.7%) presented with incomplete CRAO and 3 (33.3%) with subtotal CRAO. The mean time from onset to treatment was 13.1 ± 4.5 hours. The most common underlying diseases were hypertension and dyslipidemia, present in 7 (77.8%) patients, as shown in (Table 2). Six (66.7%) patients received 45 mg of alteplase, and 3 patients received 15 mg of alteplase due to significant improvement of visual acuity during the infusion.
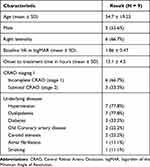 |
Table 2 Demographic Data of CRAO Patients Treated with Selective Intra-Ophthalmic Arterial Combined Nimodipine and Alteplase |
Assessment at 1 month post-treatment showed a significant improvement in mean BCVA compared with baseline (−0.78 logMAR; 95% confidence interval (CI) −1.24 to −0.31 logMAR; P = 0.001). When comparing baseline VA with that at different time points, a significant mean improvement in BCVA was detected right after alteplase infusion (−0.71 logMAR; 95% CI −1.18 to −0.25 logMAR; P = 0.003) and remained significant at days 1 through and 3, and 1 month after treatment. However, the visual improvement was not significant after nimodipine infusion alone (−0.23, logMAR; 95% CI −0.69 to 0.24 logMAR; P = 0.34).
Details on CRAO staging, duration from onset to treatment and VA at different time points for each patient are shown in Table 3. At 1 month post-treatment, doubling or more of visual angle (≥0.3 logMAR) was found in 7 (77.8%) patients out of 9, of whom 6 were in the incomplete-CRAO subgroup (stage 1 CRAO) whereas only 1 of the 3 patients with subtotal CRAO (stage 2 CRAO) had visual improvement ≥0.3 logMAR (from light perception to hand motion). Surprisingly, 5 (83.3%) out of the 6 patients with incomplete CRAO had a final BCVA at 1 month better than or equal to 20/50.
 |
Table 3 Stage of CRAO, Onset of CRAO and Visual Acuity at Different Time Points of All Patients |
During the treatment, 5 (55.6%) patients reported a headache during nimodipine infusion; headaches were reduced after slowing the rate of infusion. Mild conjunctival injection with minimal chemosis after nimodipine infusion was found in all patients; this improved spontaneously without treatment.
No immediate adverse events were observed during alteplase infusion, but delayed hemorrhage was observed in three patients. One patient had an asymptomatic, small acute hematoma in the right cerebellum, which was detected in routine magnetic resonance imaging (MRI) follow-up, and two patients had small optic nerve head hemorrhages that did not affect their vision. During routine MRI follow-up after treatment, scattered lacunar infarctions were found in two patients, without any neurological symptoms. We do not know if the infarctions were preexisting or were procedure-related since there was no MRI prior to the treatment, for comparison.
Discussion
Emboli are thought to be the major cause of CRAO.10 Conventional treatments for CRAO, eg, sublingual isosorbide dinitrate, ocular massage or ocular pressure-lowering agents, are utilized to dilate the vessels and dislodge the embolic particle. With the advent of modern neuro-intervention devices, which can directly target the ophthalmic artery, local thrombolytic infusion was introduced as a potential treatment for CRAO. However, fibrin accounts for only 15.5% of visible emboli; the rest are cholesterol and calcium emboli,10 which cannot be dissolved by the thrombolytic agent.
The use of the adjunctive intra-arterial vasodilating agent in the present study was based on the hypothesis that nimodipine may have a direct vasodilating effect on ophthalmic and retina arteries, which might improve retinal perfusion and dislodge emboli to more-peripheral areas. However, there was no solid evidence of distal propagation of retinal emboli to support the hypothesis.
This study showed that combined intra-arterial nimodipine and alteplase infusion is very effective in treating patients with acute CRAO. Significant improvements in BCVA were observed immediately after intra-arterial alteplase injection and persisted throughout 1 month of follow-up. The mean improvements in BCVA, compared with baseline, were 0.71 logMAR (95% CI −1.18 to −0.21 logMAR; p = 0.003) and 0.78 logMAR (95% CI −1.24 to −0.31 logMAR; p = 0.001), immediately after intra-arterial alteplase injection and at 1 month post-treatment, respectively.
Doubling of the visual angle occurred in 77.8% of all patients. The treatment benefit was more pronounced in the patients with incomplete CRAO, with 83.3% of them having a final BCVA better than or equal to 20/50. The percentage of patients with very good visual outcome in our study was much higher than the 22% of patients with spontaneous visual improvement that one could expect in CRAO according to studies of the natural course of the disease.4,5 The higher rate of overall visual improvement in our study could partly be the result of the high proportion of patients with incomplete CRAO, which usually carries a better prognosis than do the other stages.5,9
In the study of intra-arterial thrombolysis alone by Ahn et al, at 1 month post treatment, significant visual improvement was reported in 76.9% of incomplete CRAO subgroup, with BCVA better than 20/200 in 46.2% of patients.9 By comparison, our combined-therapy study showed a higher proportion (100%) of patients with incomplete CRAO who had significant visual improvement at 1 month follow-up, with up to 83.3% showing BCVA of 20/50 or better. For subtotal CRAO subgroup, our study showed 33% (1 of 3 patients) had significant visual improvement compared with 14.3% in Ahn et al. It is unclear, however, whether our result is actually better than theirs because of the very few patients in that subgroup.
Our results contradict the results from the EAGLE study, the only randomized controlled trial in intra-arterial thrombolysis in CRAO, which did not support the use of intra-arterial thrombolysis. However, the inclusion criteria of the EAGLE trial were more broad and, more importantly, did not differentiate between patients concerning the initial severity or stage of arterial occlusion.14
Recent studies have also reported that the completeness of retinal artery occlusion is a major factor related to visual outcome, with patients with incomplete CRAO having a more-favorable visual outcome compared with others.8,9 Significant visual improvement after IAT has been reported in patients with incomplete CRAO, despite the onset of CRAO 7 days prior to treatment.9 Therefore, the staging of CRAO is crucial in considering the candidates who potentially will most benefit from the treatment. We believe that our study illustrates the benefit of selective intra-arterial thrombolysis in treating acute CRAO, particularly in incomplete CRAO, with potentially superior results when combining nimodipine and alteplase infusion.
Regarding the side effects of IAT, headache was the most common adverse event during nimodipine infusion. However, after lowering the rate of infusion to 15–20 minutes, the headaches were substantially reduced. The other minor adverse events were conjunctival injection and chemosis, which recovered spontaneously in a few days. Therefore, we recommend that the rate of nimodipine infusion should not be faster than 15–20 minutes to avoid such complications. There were no symptomatic intracranial hemorrhages in our study, although there were three asymptomatic bleeding events as described earlier.
In this study, we used 45 mg of alteplase, which was a full standard intravenous dose for a 50-kg patient (0.9 mg/kg of total body weight). In a study comparing low-dose versus standard-dose intravenous alteplase in acute ischemic stroke, low-dose alteplase (0.6 mg/kg) significantly reduced the risk of symptomatic cerebral hemorrhage compared with the standard dose (0.9 mg/kg), without affecting the 90-day morbidity and mortality.18 Hence, the effective dose of local intra-arterial alteplase infusion for CRAO is possibly lower, concerning the size of affected artery, and the route of administration.
There were two (22.2%) asymptomatic lacuna infarctions found our patients during routine MRI after the treatment, the incidence was comparable to the incidence of silent infarctions from overall diagnostic cerebral angiography and intervention for other condition.19 We do not know if the infarctions were present before the CRAOs or were procedure-related, since there was no pre-treatment MRI to compare against. Imaging before and after intervention is recommended, to distinguish between silent ischemic stroke and the effects of the procedure. Thromboembolic events are common adverse events of intra-arterial diagnostic and interventional procedures; most are asymptomatic, so are worth taking into account but should not discourage this interventional procedure.
This prospective study is limited by the small sample size, with most of the population being in the incomplete-CRAO subgroup, resulting in optimal outcome. Further study of both subtotal CRAO, which is the most common subgroup, and total CRAO is necessary to investigate the efficacy of combined nimodipine and alteplase IAT in these CRAO groups. Finally, additional larger studies, including well-designed randomized controlled trials, are needed before using combined intra-arterial nimodipine and alteplase as the standard of care.
Conclusion
Selective intra-ophthalmic arterial combined nimodipine and alteplase treatment showed promising results in patients with non-arteritic CRAO, especially those with incomplete CRAO treated within 24 hours from the onset. The use of nimodipine combined with alteplase potentially has an added positive effect compared with alteplase alone. Though mostly minor and asymptomatic, the potential for adverse reactions resulting from the procedure should be considered prior to this treatment.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Leavitt JA, Larson TA, Hodge DO, Gullerud RE. The incidence of central retinal artery occlusion in Olmsted County, Minnesota. Am J Ophthalmol. 2011;152(5):820–3.e2. doi:10.1016/j.ajo.2011.05.005
2. Park SJ, Choi NK, Seo KH, Park KH, Woo SJ. Nationwide incidence of clinically diagnosed central retinal artery occlusion in Korea, 2008 to 2011. Ophthalmology. 2014;121(10):1933–1938. doi:10.1016/j.ophtha.2014.04.029
3. Kido A, Tamura H, Ikeda HO, Miyake M, Hiragi S, Tsujikawa A. Nationwide incidence of central retinal artery occlusion in Japan: an exploratory descriptive study using the National Database of Health Insurance Claims (2011–2015). BMJ Open. 2020;10(9):e041104. doi:10.1136/bmjopen-2020-041104
4. Schrag M, Youn T, Schindler J, Kirshner H, Greer D. Intravenous fibrinolytic therapy in central retinal artery occlusion: a patient-level meta-analysis. JAMA Neurol. 2015;72(10):1148–1154. doi:10.1001/jamaneurol.2015.1578
5. Hayreh SS. Ocular vascular occlusive disorders: natural history of visual outcome. Prog Retin Eye Res. 2014;41:1–25. doi:10.1016/j.preteyeres.2014.04.001
6. Schmidt DP, Schulte-Mönting J, Schumacher M. Prognosis of central retinal artery occlusion: local intraarterial fibrinolysis versus conservative treatment. AJNR Am J Neuroradiol. 2002;23(8):1301–1307.
7. Furashova O, Matthé E. Retinal changes in different grades of retinal artery occlusion: an optical coherence tomography study. Invest Ophthalmol Vis Sci. 2017;58(12):5209–5216. doi:10.1167/iovs.17-22411
8. Schmidt D, Schumacher M. Stage-dependent efficacy of intra-arterial fibrinolysis in central retinal artery occlusion (CRAO). Neuro-Ophthalmology. 1998;20(3):125–141. doi:10.1076/noph.20.3.125.4155
9. Ahn SJ, Kim JM, Hong JH, et al. Efficacy and safety of intra-arterial thrombolysis in central retinal artery occlusion. Invest Ophthalmol Vis Sci. 2013;54(12):7746–7755. doi:10.1167/iovs.13-12952
10. Arruga J, Sanders MD. Ophthalmologic findings in 70 patients with evidence of retinal embolism. Ophthalmology. 1982;89(12):1336–1347. doi:10.1016/s0161-6420(82)34626-6
11. Page PS, Khattar NK, White AC, et al. Intra-arterial thrombolysis for acute central retinal artery occlusion: a systematic review and meta-analysis. Front Neurol. 2018;9:76. doi:10.3389/fneur.2018.00076
12. Donaldson L, Nicholson P, Margolin E. Visual recovery in 2 cases of central retinal artery occlusion treated with prompt intra-ophthalmic artery fibrinolysis. J Neuroophthalmol. 2023;10. doi:10.1097/WNO.0000000000001785
13. Raber FP, Gmeiner FV, Dreyhaupt J, et al. Thrombolysis in central retinal artery occlusion: a retrospective observational study. J Neurol. 2023;270(2):891–897. doi:10.1007/s00415-022-11439-7
14. Schumacher M, Schmidt D, Jurklies B, et al. Central retinal artery occlusion: local intra-arterial fibrinolysis versus conservative treatment, a multicenter randomized trial. Ophthalmology. 2010;117(7):1367–75.e1. doi:10.1016/j.ophtha.2010.03.061
15. Biondi A, Ricciardi GK, Puybasset L, et al. Intra-arterial nimodipine for the treatment of symptomatic cerebral vasospasm after aneurysmal subarachnoid hemorrhage: preliminary results. AJNR Am J Neuroradiol. 2004;25(6):1067–1076.
16. Bashir A, Andresen M, J B Jr, Cortsen M, Eskesen V, Wagner A. Intra-arterial nimodipine for cerebral vasospasm after subarachnoid haemorrhage: influence on clinical course and predictors of clinical outcome. Neuroradiol J. 2016;29(1):72–81. doi:10.1177/1971400915626429
17. Lange C, Feltgen N, Junker B, Schulze-Bonsel K, Bach M. Resolving the clinical acuity categories “hand motion” and “counting fingers” using the Freiburg Visual Acuity Test (FrACT). Graefes Arch Clin Exp Ophthalmol. 2009;247(1):137–142. doi:10.1007/s00417-008-0926-0
18. Anderson CS, Robinson T, Lindley RI, et al. Low-dose versus standard-dose intravenous alteplase in acute ischemic stroke. N Engl J Med. 2016;374(24):2313–2323. doi:10.1056/NEJMoa1515510
19. Bendszus M, Koltzenburg M, Burger R, Warmuth-Metz M, Hofmann E, Solymosi L. Silent embolism in diagnostic cerebral angiography and neurointerventional procedures: a prospective study. Lancet. 1999;354(9190):1594–1597. doi:10.1016/S0140-6736(99)07083-X
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.