Back to Journals » Clinical Ophthalmology » Volume 14
Visible Meibomian Gland Structure Increases After Vectored Thermal Pulsation Treatment in Dry Eye Disease Patients with Meibomian Gland Dysfunction
Authors Hura AS , Epitropoulos AT , Czyz CN , Rosenberg ED
Received 17 September 2020
Accepted for publication 15 November 2020
Published 7 December 2020 Volume 2020:14 Pages 4287—4296
DOI https://doi.org/10.2147/OPTH.S282081
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Arjan S Hura,1 Alice T Epitropoulos,2 Craig N Czyz,3,4 Eric D Rosenberg5
1Department of Ophthalmology University of Cincinnati, Cincinnati, OH, USA; 2Ophthalmic Surgeons & Consultants of Ohio Inc., Columbus, OH, USA; 3Ophthalmology, Section Oculofacial Plastic and Reconstructive Surgery, Ohio University/OhioHealth Doctors Hospital, Columbus, OH, USA; 4Ophthalmology, Oral and Maxillofacial Surgery, Grant Medical Center, Columbus, OH, USA; 5New York Presbyterian Hospital – Cornell Campus, New York, NY, USA
Correspondence: Arjan S Hura
University of Cincinnati, Department of Ophthalmology, 231 Albert Sabin Way, 5th Floor, Cincinnati, OH 45267, USA
Tel +1 513 558-5151
Email [email protected]
Purpose: To assess the effect of vectored thermal pulsation treatment (VTP) on visible meibomian gland structure (VGS) in patients with meibomian gland dysfunction (MGD).
Setting: Private group practice (A.T.E.).
Design: Retrospective, single-blinded cohort study.
Methods: Visible meibomian gland structure was evaluated at baseline and at 1-year in treatment (30 patients, 48 eyes) and control (13 patients, 22 eyes) groups. Meibography images were captured using dynamic meibomian imaging. Images were assessed using a novel morphometric analysis technique and analyzed for change in area of VGS (pixels). Additional outcomes measured include tear break up time, corneal staining, tear osmolarity, matrix metalloproteinase-9 (MMP-9), meibography grading, and meibomian gland evaluation.
Results: As high as 69% of eyes in the treatment group showed an improvement in VGS versus 27% of eyes in the control group. As high as 31% of eyes in the treatment group showed a decline in VGS versus 73% of eyes in the control group. TBUT (p = 0.0001), corneal staining (p = 0.0063), and meibomian gland evaluation scores (p = 0.0038) all significantly improved after VTP treatment. However, SPEED scores, MMP-9, tear osmolarity, and meiboscale scores were not significantly improved 1-year post treatment.
Conclusion: A morphometric analysis protocol of meibography provides clinically meaningful information that is undetectable with the standard semiquantitative method of grading meibomian gland structure. This is the first report indicating that gland structure may increase post-VTP relative to untreated controls, thus presenting significant implications regarding benefits and timing of VTP therapy. The described protocol is currently more appropriate for research than for clinical practice.
Keywords: meibomian gland dysfunction, meibomian gland pixelar analysis, meibomian gland regeneration, vectored thermal pulsation
Introduction
Dry eye disease (DED) is one of the most common ocular disorders and can have a substantial burden on quality of life, including loss of productivity at work, mental health problems, contact lens intolerance, and outcomes after refractive and cataract surgery.1–4 There are two broad sub-categories of DED: aqueous tear deficiency (ATD) and meibomian gland dysfunction (MGD). It is common to have a combination of both types. The literature indicates that 86% of patients with dry eye have MGD as a primary or contributory cause of DED.5,6 Given that MGD is the most prevalent contributor to DED, the International Workshop on Meibomian Gland Dysfunction, sponsored by The Tear Film & Ocular Surface Society (TFOS), and most recently the American Society of Cataract and Refractive Surgeons (ASCRS) Cornea Clinical committee recommend that clinicians routinely assess the lids, meibomian glands (MG), and lipid layer in patients – especially those undergoing cataract or refractive surgery.6,7 There are many different modalities and options for treating MGD. A recent abstract showing measurable acinar regeneration secondary to pharmacological treatment in atrophied MG in a mouse model,8 a report from Arita et al that topical diquafosol may lead to overall increased visible MG area,9 a paper showing assumed growth of MG tissue after intraductal MG probing,10 and lastly a paper suggesting that reversibility of MG dropout might be possible.11 Despite the compelling nature of these results, all these studies have very small sample sizes and there have been no studies that demonstrate that non-visible or atrophied MGs in humans can be re-activated and observed as “regrowth” after vectored pulsation treatment.
The Tear Film & Ocular Surface Society (TFOS) Dry Eye WorkShop (DEWS) II described MGD as a chronic and diffuse abnormality of the MGs commonly characterized by epithelial hyperkeratinization which leads to duct obstruction, stasis of meibum, cystic dilation, and eventual acinar atrophy and gland dropout.6 While the hyperkeratinization hypothesis remains debatable,12,13 the importance of healthy meibomian gland function is well-accepted. Meibomian glands are present in both the upper and lower eyelids and secrete the lipid component of the tear film that is responsible for preventing premature tear film evaporation and maintaining a healthy ocular surface.14–17 When a diagnosis of dry eye is made, the specific cause(s) and contributing factors should be assessed prior to advancing the treatment protocol. Further, TFOS Workshop recommends treating MGD at every stage of severity, including Stage 1.16 Failure to diagnose and manage MGD in its earliest and most treatable stages can lead to chronically compromised health of the ocular surface.18
Multiple variables have been shown to contribute to MGD. Modern lifestyles involve spending long hours on digital devices resulting in poor blinking habits with subsequent compromise in the lipid layer and chronic exposure to evaporative stress.4,19 Other risk factors include contact lens wear, glaucoma drops, cosmetics, smoking, and low dietary intake of omega-3 fatty acids.20–22 Most recently, the ASCRS Cornea Clinical Committee developed and published a consensus-based practical diagnostic ocular surface disease (OSD) algorithm to aid in the diagnosis and treatment of visually significant (and often asymptomatic) OSD prior to any form of ocular surgery. The committee consensus was that proactively treating OSD pre-operatively should help to achieve the high-quality visual outcomes patients expect. This recommendation was made so that MGD and DED can be identified and treated prior to ocular surgery.7
Traditional first-line treatments for MGD including daily application of heat to the external eyelids, lid hygiene (including microblepharoexfoliation, debridement, and the use of tea tree oil/hypochlorous acid),23,24 omega-3 fatty acid supplementation,22 and other adjunctive therapies are likely to be more effective once the underlying gland obstruction has been addressed.18 Warm compresses alone are largely ineffective because of the limitations in transferring heat from the outer to the inner eyelid, as well as the lack of patient compliance when daily home care is needed.25 Furthermore, co-existing eyelid conditions, such as blepharitis (including from Demodex infestation), should also be treated.18
The Vectored LipiFlow Thermal Pulsation System (“vectored thermal pulsation”, “VTP”; Johnson & Johnson Vision, Santa Ana, CA) is a well-established treatment for MGD. It is an automated procedure designed to simultaneously heat and evacuate upper and lower eyelid MG contents in a single, in-office, 12-minute treatment. Based on what is known about the pathophysiology of MGD, it is understood that gland obstruction is the primary cause of reduced gland function and a cause of MG truncation, dilation, and ultimately, gland atrophy with drop out.17 The TFOS DEWS II Management and Therapy report recommends VTP as a treatment of choice for early-stage DED and MGD.18 The ASCRS Corneal Clinical Committee describes VTP as a first-line treatment choice for MGD in pre-surgical patients, and VTP is reported to be the favored pre-operative MGD procedural treatment for over 80% of the ASCRS Corneal Clinical Committee members.7
It is well documented that VTP is highly effective at improving MG function,26–30 and while it is reasonable to presume that treating obstruction will decrease the progression of MGD and gland atrophy, there are no peer-reviewed studies showing an increase in gland structure post-VTP. It can also be difficult to accurately interpret infrared imaging of the MGs. In one report, it was suggested that the reflected material observed in infrared imaging of the MGs may be an indicator of acini activity.31 If correct, these images may have the potential to reveal increased visible gland structure (VGS) in treated glands, suggesting restored acini activity. This finding would be a departure from the previously held assumption in the literature that a true increase in VGS over time is unlikely, if not impossible. Given the consistency of data demonstrating improvement of gland function after treatment with VTP, we hypothesized that, with an appropriate method to quantify VGS, an increase in visible meibomian gland structure may be possible in patients who received VTP treatment.
Materials and Methods
This retrospective, single-blinded cohort study compared dry eye disease markers and dynamic meibomian imaging (“DMI”, LipiView II, Johnson & Johnson Vision, Santa Ana, CA) between patients who had undergone VTP (Treatment Group) and those with MGD who had received a recommendation for VTP but elected not to proceed with the procedure (Control Group). The study was performed at a single investigational site in accordance with the Declaration of Helsinki following approval by an Institutional Review Board (Mount Carmel, Columbus, OH, USA). Patient informed consent to review medical records was not required by the IRB as this was a retrospective study, all patient information was confidential, and no personal identifiers were used for data analysis. All patients included in the study were over 18 years of age with DED and MGD. Patients in both the Treatment and Control Groups had tried multiple therapeutic options, including artificial tears, Restasis, Xiidra, warm compresses, lid scrubs, and omega-3-fatty acids, prior to considering VTP treatment. Dry eye disease markers and high-quality DMI were measured at the patient’s initial visit and at 1-year post-VTP treatment; or, in the case of the Control Group, at the patient’s initial and 1-year visit. Patients were excluded from the study if they had a history of any eye injuries, ocular surgery within the past 3 months, herpes infection of the eye or eyelid, chronic recurrent eye inflammation within the 3 months prior to intended VTP treatment, current eye infection or inflammation, eyelid abnormality affecting eyelid function, or eye surface abnormality that might affect the integrity of the ocular surface. Patients were also excluded from the study if they lacked follow-up DMI at 1 year or high-quality DMI at either the initial or follow-up visits. Most patients included in the study had a single, bilateral VTP treatment, although some patients elected to initially treat one eye and then subsequently treat the other eye.
Standard Patient Evaluation of Eye Dryness (SPEED) scores,32 Tear Break Up Time (TBUT), Inflammadry (MMP-9, Quidel),33 tear osmolarity (TearLab), corneal staining, and meibomian gland evaluation measurements were collected at the patient’s first visit and at 1-year post-VTP treatment. In the Control Group, the data were collected at the initial evaluation and a 1-year follow-up. Inflammadry (Quidel, San Diego, CA) is a point-of-care device that measures MMP-9 (matrix metalloproteinase-9), an inflammatory marker that has been demonstrated to be elevated in the tear film of patients with DED. A positive MMP-9 value was graded as “1” and a negative value was graded as “0”. Tear osmolarity (TearLab, San Diego, CA) is another in-office point-of-care measurement that is found to be abnormal in patients with DED. A 50nL tear film sample with >308 mOsm/L or an inter-eye difference of greater than 8 mOsm/L signifies an abnormal tear osmolarity value. An abnormal tear osmolarity value was graded as “1” and a normal tear osmolarity value was graded as “0”. Corneal staining can be evaluated by instilling a drop of fluorescein dye onto the ocular surface and then visualizing the cornea using the cobalt blue filter on the slit lamp biomicroscope to evaluate for punctate epithelial keratopathy (PEK). The presence of PEK was given a grade of “1” and the absence of PEK was graded as “0”. For MMP-9, tear osmolarity, and corneal staining, clinical observations were converted to binary values, as previously detailed, to facilitate statistical analysis. Pushing on the lower lid margin allows for assessment of the meibomian glands to assess the quality, quantity, and flow of meibum.7 This can be accomplished using a cotton-tipped applicator, finger, or device such as a meibomian gland evaluator (MGE) (Johnson & Johnson Vision, Santa Ana, CA) which applies approximately 1.2g/mm of force to simulate the natural expression of meibum during a deliberate blink.31 Specifically in this study, an MGE was used to assess the quality of meibum expressible from the lower lid as well as the number of functional glands present. The meibum quality of each gland was scored from three to zero points (3: clear liquid secretion; 2: cloudy liquid secretion; 1: cloudy particulate fluid; 0: inspissated, similar to toothpaste). The number of expressible glands present in the temporal, central, and nasal portions of the lower lid was counted, ranging from zero to five (a score of zero indicates that a gland was not expressible). The total meibomian gland evaluation score was the sum of the products of the quality of meibum and the number of glands expressed for each lower lid sector. Gland expression is especially useful in identifying patients with nonobvious MGD, a form of obstructive MGD where the typical inflammatory signs are absent.
Vectored Thermal Pulsation and Dynamic Meibomian Imaging
For patients undergoing the VTP treatment in the study, an activator was placed directly over the eye, vaulting the cornea. The eyelids were then engaged over the eye cup and the activator position was stabilized. Once treatment was initiated, VTP energy was applied to the eyelids for 12 minutes. The VTP technology uniquely applies heat and simultaneous, directional, peristaltic motion to the eyelids to improve MG function in patients with MGD by removing stagnant gland content and gland obstructions. VTP is automated once treatment is initiated. All patients in the treatment group received the same regimen. Prior to VTP, patients also received microblepharoexfoliation (BlephEx, London, UK) to clean the lid margin and remove lash debris. After the treatment, patients were advised to continue their previous dry eye therapeutic regimen, which varied in combinations of artificial tears, warm compresses, punctal plugs, Restasis, Xiidra, omega-3 fatty acid supplements, and lid scrubs.
Dynamic meibomian imaging was collected for all patients at their initial visits and 1-year post-VTP or post-evaluation. Dynamic meibomian imaging acquired by the LipiView II unit utilizes a combination of transillumination and surface infrared light energy to reveal the MG structure in the eyelids. All DMI was graded via a masked reviewer, utilizing a semiquantitative grading scale (meiboscale),6,31,34 as well as an additional more precise morphometric analysis technique that we termed meibomian gland pixelar analysis (MGPA). Automated objective analysis was not available for use in the United States at the time of the study.
Meibomian Gland Pixelar Analysis
The current grading scales (i.e., meiboscale) use a semiquantitative method of grading MG images and are too broad to detect small changes in gland structure. We developed MGPA as a highly refined and granular method of analyzing DMI using Adobe Photoshop CC software (Adobe, San Jose, CA). Dynamic meibomian images were loaded into the software and an initial comparison of baseline and follow-up images was conducted using specific lower lid characteristics to ensure that the images included comparable areas of the lower lid. Analysis included evaluating the structural shape, positioning, size, and atrophy of meibomian glands, as well as other unique identifying characteristics such as palpebral conjunctival blood vessels. Utilizing these unique identifying characteristics, the largest high-quality contiguous area of lower lid could accurately be delineated and used for comparative assessment between the two DMI. In order to be analyzed, the DMI being compared had to have comparable sectors of lower lid and a similar amount of degree of lid eversion. Due to this criterion, an entire lower eyelid was never able to be used for analysis. Images with low exposure, poor resolution or clarity, or significantly different degrees of lid eversion rendered accurate comparison impossible and were excluded and not analyzed.
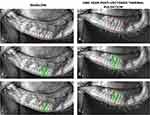
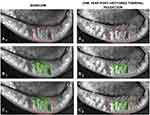
Morphometric analysis was conducted in Photoshop software which has similar functionality to ImageJ (National Institute of Health and the Laboratory for Optical and Computational Instrumentation, Bethesda, MD; Madison, WI), a popular image analysis software that is widely used in medical research. The total sector of lower lid identified for comparison was manually delineated at a pixelar level using a combination of the software’s “Magnetic Lasso Tool” and “freehand selection”. Using the software’s “Measurement Log”, the delineated area was then recorded as a numeric measurement in unit pixels. A visible colored outline was then applied to the selected area using the software’s “Stroke” effect (Figure 1A1 and A2 and 2A1 and A2). A duplicate image was then created and analyzed for MG atrophy. With the assistance of the “Magic Wand” tool and freehand manipulation, the total area of MG atrophy as well as interglandular space was identified and then given a colored outline as well (Figure 1B1 and B2 and 2B1 and B2). This second delineated area was then noted in the Measurement Log, a value we refer to as “area of atrophy”. In contrast to the area of atrophy, we refer to the remaining area with observable MG structure as VGS. Throughout the morphometric analysis, the images were magnified and shrunken to constantly ensure that the most accurate area was being delineated. No programmed functions or mathematical functions were algorithmically applied to best-estimate any area; all areas were manually and meticulously selected by a single trained, masked observer (A.S.H.). Prior to evaluating the images for the study, sample images were analyzed several times over several weeks and then those measurements were compared to make sure they were consistent.
Statistical Analysis
All data points were then transferred into an Excel (Microsoft, Redmond, WA) spreadsheet and de-identified. The following numeric calculations were then determined in Excel using the delineated total sector area and the area of atrophy measurements for both the initial and post-treatment visits in the treatment group, and initial and 1-year visit for the control group: percentage of atrophy (total area of atrophy/total area of sector x 100), percentage of difference in amount of atrophy (percentage of atrophy post-VTP – percentage of atrophy pre-VTP; percentage of atrophy at subsequent visit – percentage of atrophy at initial visit [for control group]), percentage of change in the difference of percentage of atrophy (percentage of difference in amount of atrophy/percentage of atrophy either post-VTP or at subsequent visit x 100).
The data were analyzed using SPSS version 20.0 (IBM Corporation, Summers, NY). The Mann–Whitney test was used to analyze SPEED scores and meibomian gland evaluation. A chi-square analysis was performed on MMP-9, tear osmolarity, and corneal staining values. An unpaired t-test was used to compare TBUT, meiboscale, and lid atrophy between groups. All analysis was conducted at the 0.05 alpha level with two-tailed p values reported. It was determined multiple comparison correct methods were not required. Post power analysis was conducted for all non-significant results.
Results
A total of 43 patients and 70 eyes met the inclusion criteria and completed the study. The treatment group encompassed 48 eyes which received VTP treatment. There were 22 eyes assigned to the non-treatment control group. The morphometric MGPA of DMI indicated that of the 48 eyes treated with VTP, 100% (48 eyes) displayed a change in VGS post-therapy. When the entire data set was analyzed, 69% of eyes (33 eyes) treated with VTP showed an improvement in VGS from baseline at 1-year post-treatment (Figures 1 and 2), whereas only 27% (6 eyes) in the control group improved. In contrast, 73% of the control eyes (16 eyes) showed an overall decline in VGS approximately 1 year after baseline (Table 1) compared to 31% (15 eyes) of the treatment eyes.
 |
Table 1 Results from Morphometric Meibomian Gland Pixelar Analysis of Dynamic Meibomian Imaging for Treatment and Control Groups |
The specific changes to VGS in the treatment and control groups are shown in Table 1. Comparison of the two groups demonstrated statistical significance for the following DED markers that were measured at baseline and 1 year after VTP treatment or in the case of the control group, 1 year after baseline measurements: TBUT increased by 4 seconds (p = 0.0001; 95% CI = −5.7 to −2.6), there was an improvement in corneal staining (p = 0.0063; 95% CI = 0.18 to 0.92), and MG function evaluated at the slit lamp improved by 7 points (p = 0.0038; 95% CI = −10.0 to −2.0). No statistical significance was demonstrated for SPEED scores, MMP-9, tear osmolarity, or the semiquantitative grading (meiboscale) of DMI for gland atrophy (Table 2), and post-hoc power analysis showed these to be underpowered.34
 |
Table 2 Statistical Analysis of Dry Eye Disease Markers for Treatment and Control Groups |
Discussion
The current standard, semiquantitative method of grading MG atrophy, for example, the meiboscale, limits the identification of structural compromise to broad categories of atrophy (e.g., 25–33% increments), depending on the grading system selected. While it is understood that MGD is a chronic and progressive disease, it is also understood that the generalized changes to gland structure typically occur at a relatively slow (i.e., on the order of years) and incremental rate. Mild, incremental changes in gland structure are typically undetectable using any of the current semiquantitative grading systems. The morphometric MGPA protocol described in this study facilitates the identification of these incremental structural changes over time that are essentially undetectable using the traditional grading systems.
The MGPA protocol revealed that 69% of eyes treated with VTP showed an improvement in VGS at 1 year compared to baseline. In contrast, 73% of the control group eyes showed an overall decline in VGS at 1 year compared to baseline. The data indicates that new methods of grading MG structure with finer granularity are necessary in order to capture incremental changes in MG structure over time. This data analysis also suggests that the absence of VGS when imaged with infrared light may not always indicate absolute atrophy or loss of function; it may indicate loss of activity. The results imply activity may increase following VTP treatment, suggesting gland re-activation.
The implication that MG structure can increase after therapeutic intervention with VTP cannot be over-stated. The current consensus in the literature is that once MGs have atrophied, they cannot be re-activated or re-generated. The results of this study show that this commonly held belief may require further investigation to confer validity. If MGs are capable of regeneration and/or reactivation, then this represents a significant opportunity for clinicians to intervene therapeutically to possibly improve a patient’s MGD and DED. Reactivation or regeneration of MGs could have clinically significant impacts on quality of life, symptomatic dry eye, refraction, contact lens tolerance, pre-operative measurements, and post-operative outcomes after refractive and cataract surgery. If validated in prospective studies, the trend shown in this patient population could significantly alter the management paradigm of MGD and DED.
Although the morphometric MGPA protocol facilitates the identification of incremental structural changes over time, the method is time-consuming. Users must understand the basic principles of morphometric analysis and be proficient in using imaging analysis and modification software. Consistent and meticulous lid eversion technique is crucial to render high-quality images appropriate for the analysis. These factors may make the protocol described in this study more appropriate for research purposes than for implementation into general clinical practice at this time.
The morphometric MGPA protocol used in this study requires manual delineation of DMI and is thus subjective. Multiple reports over the years have been published detailing objective and automated methods of analyzing DMI.35–38 Although these proposed methods are objective, by their nature of automation, they use mathematical algorithms and functions of imaging software to best estimate meibomian gland architecture. While more accurate than the standard meiboscale, they are not as accurate as manual delineation which by definition, does not estimate meibomian gland architecture. MGPA in particular involves a level of pixelar granularity in analysis. The increase in VGS after VTP noted in this study, and the increase in MG structure reported in previous studies after non-VTP interventions is not obvious enough that it could easily be noticed without analysis or using meiboscale.8–11 In fact, the changes in VGS are small and thus easily underestimated or entirely missed using objective automated analysis that best estimates MG area. There are currently no objective automated DMI analyzers commercially available in the United States.
The study results also show that compared to pre-treatment, TBUT, corneal staining, and meibomian gland evaluation scores all showed a statistically significant improvement 1 year after VTP therapy. SPEED scores, tear osmolarity, and MMP-9 did not show a statistical sustained improvement 1-year post-VTP. There was no change in meiboscale scores after VTP therapy, as was expected given the broad and generalizing nature of the meiboscale grading system that makes it difficult to assess for small changes.
As is typical in retrospective studies, patients in both the treatment and control groups had tried multiple therapeutic options, including artificial tears, Restasis, Xiidra, warm compresses, lid scrubs, and omega-3-fatty acids. A randomized, prospective study would need to be conducted to control for all such variables. However, it may be difficult to recruit DED patients naïve to previous treatment unless they are identified early in the disease course. Additionally, it would be ideal to have a control group of similar size to the treatment group. As this was a retrospective study, many of these parameters were pre-determined; thus, this study presents real-world data. It is also important to note that while a few prior studies have demonstrated an increase in visible gland structure post non-VTP MGD treatment, these studies all had very small sample sizes compared to our study.
Conclusions
The data presented in this study present a compelling need for improved quantitative methods of analysis and grading MG structure. The results from this study also raise the possibility that absence of VGS may not indicate absolute atrophy or loss of function but may suggest loss of activity that improves with treatment, indicating gland reactivation. This is the first report presenting quantitative data that gland structure may increase after VTP therapy relative to untreated controls. Additional, prospective studies will be required to further validate these findings which may have significant implications regarding the benefits and timing of VTP therapy for MGD.
Disclosure
This study was supported by Johnson & Johnson Vision through investigator-initiated grant support. Dr. Hura received investigator-initiated grant support from Johnson & Johnson Vision. Dr. Epitropoulos received investigator-initiated grant support from Johnson & Johnson Vision. Dr. Epitropoulos is a consultant for Johnson & Johnson Vision, a speaker/consultant for PRN and Blephex, and has a patent for EpiGlare Tester with royalties paid to EPICO. No other author has a financial or proprietary interest in any material or method mentioned. The abstract of this paper was presented at the 2019 ASCRS-ASOA Annual Meeting in San Diego, CA as an oral presentation with interim findings. Early preliminary results were also presented at the 2018 ARVO Annual Meeting in Honolulu, HI as a poster presentation and the abstract was published in Investigative Ophthalmology and Visual Science in July of 2018. https://iovs.arvojournals.org/article.aspx?articleid=2694239. The authors report no other conflicts of interest for this work.
References
1. van Tilborg MM, Murphy PJ, Evans KS. Impact of dry eye symptoms and daily activities in a modern office. Optom Vis Sci. 2017;94(6):688–693. doi:10.1097/OPX.0000000000001086
2. Zheng Y, Wu X, Lin X, Lin H. The prevalence of depression and depressive symptoms among eye disease patients: a systematic review and meta-analysis. Sci Rep. 2017;12:46453. doi:10.1038/srep46453
3. Epitropoulos AT, Matossian C, Berdy GJ, et al. Effect of tear osmolarity on repeatability of keratometry for cataract surgery planning. J Cataract Refract Surg. 2015;41(8):1672–1677. doi:10.1016/j.jcrs.2015.01.016
4. Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15(3):334–365. doi:10.1016/j.jtos.2017.05.003
5. Lemp MA, Crews LA, Bron AJ, et al. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472–478. doi:10.1097/ICO.0b013e318225415a
6. Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52:1917–2085. doi:10.1167/iovs.10-6997
7. Starr CE, Gupta PK, Farid M, et al. An algorithm for the preoperative diagnosis and treatment of ocular surface disorders. J Cataract Refract Surg. 2019;45:669–684. doi:10.1016/j.jcrs.2019.03.023
8. Reneker LW, Yang X, Zhong X, et al. Meibomian Gland (MG) Acinar Regeneration from Atrophy in a Fgfr2 Conditional Knockout Mouse Model. ARVO; 2019.
9. Arita R, Suehiro J, Haraguchi T, et al. Topical diquafosol for patients with obstructive meibomian gland dysfunction. Br J Ophthalmol. 2013;97:725–729. doi:10.1136/bjophthalmol-2012-302668
10. Maskin SL, Testa WR. Growth of meibomian gland tissue after intraductal meibomian gland probing in patients with obstructive meibomian gland dysfunction. Br J Ophthalmol. 2018;102(1):59–68. doi:10.1136/bjophthalmol-2016-310097
11. Yin Y, Gong L. Reversibility of gland dropout and significance of eyelid hygiene treatment in meibomian gland dysfunction. Cornea. 2017;36(3):332–337. doi:10.1097/ICO.0000000000001042
12. Jester JV, Parfitt GJ, Brown DJ. Meibomian gland dysfunction: hyperkeratinization or atrophy? BMC Ophthalmol. 2015;15(Suppl S1):156. doi:10.1186/s12886-015-0132-x
13. Hwang HS, Parfitt GJ, Brown DJ, Jester JV. Meibocyte differentiation and renewal: insights into novel mechanisms of meibomian gland dysfunction (MGD). Exp Eye Res. 2017;163:3745.
14. Butovich IA, Millar TJ, Ham BM. Understanding and analyzing meibomian lipids—a review. Curr Eye Res. 2008;33(5–6):405–420. doi:10.1080/02713680802018419
15. Millar TJ, Schuett BS. The real reason for having a meibomian lipid layer covering the outer surface of the tear film – a review. Exp Eye Res. 2015;137:125–138. doi:10.1016/j.exer.2015.05.002
16. Wilcox MDP, Argüeso P, Georgiev GA, et al. TFOS DEWS II tear film report. Ocul Surf. 2017;15:366–403.
17. Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438–510. doi:10.1016/j.jtos.2017.05.011
18. Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575–628. doi:10.1016/j.jtos.2017.05.006
19. Wang MTM, Tien L, Han A, et al. Impact of blinking on the ocular surface and tear film parameters. Ocul Surf. 2018;16:424–429. doi:10.1016/j.jtos.2018.06.001
20. Miljanović B, Trivedi KA, Dana MR, et al. Relation between dietary n−3 and n−6 fatty acids and clinically diagnosed dry eye syndrome in women. Am J Clin Nutr. 2005;82(4):887–893. doi:10.1093/ajcn/82.4.887
21. Macasai MS. The role of omega-3 dietary supplementation in blepharitis and meibomian gland dysfunction (an AOS thesis). Trans Am Ophthalmol Soc. 2008;106:336–356.
22. Epitropoulos AT, Donnenfeld ED, Shah ZA, et al. Effect of oral re-esterified omega-3 nutritional supplementation on dry eyes. Cornea. 2016;35(9):1185–1191. doi:10.1097/ICO.0000000000000940
23. Korb DR, Blackie CA. Debridement-scaling: a new procedure that increases meibomian gland function and reduces dry eye symptoms. Cornea. 2013;32(12):1554–1557. doi:10.1097/ICO.0b013e3182a73843
24. Stroman DW, Mintun K, Epstein AB, et al. Reduction in bacterial load using hypochlorous acid hygiene solution on ocular skin. Clin Ophthalmol. 2017;11:707–714. doi:10.2147/OPTH.S132851
25. Pang S-P, Chen Y-T, Tam K-W, et al. Efficacy of vectored thermal pulsation and warm compress treatments in meibomian gland dysfunction: a meta-analysis of randomized controlled trials. Cornea. 2019;38(6):690–697. doi:10.1097/ICO.0000000000001907
26. Blackie CA, Carlson AN, Korb DR. Treatment for meibomian gland dysfunction and dry eye symptoms with a single-dose vectored thermal pulsation: a review. Curr Opin Ophthalmol. 2015;26:306–313. doi:10.1097/ICU.0000000000000165
27. Blackie CA, Coleman CA, Holland EJ. The sustained effect (12 months) of a single-dose vectored thermal pulsation procedure for meibomian gland dysfunction and evaporative dry eye. Clin Ophthalmol. 2016;26:1385–1396. doi:10.2147/OPTH.S109663
28. Blackie CA, Coleman CA, Nichols KK, et al. A single vectored thermal pulsation treatment for meibomian gland dysfunction increases mean comfortable contact lens wearing time by approximately 4 hours per day. Clin Ophthalmol. 2018;12:169–183. doi:10.2147/OPTH.S153297
29. Hagen KB, Bedia R, Blackie CA, et al. Comparison of a single-dose vectored thermal pulsation procedure with a 3-month course of daily oral doxycycline for moderate-to-severe meibomian gland dysfunction. Clin Ophthalmol. 2018;12:161–168.
30. Epitropoulos AT, Goslin K, Bedi R, et al. Meibomian gland dysfunction patients with novel Sjögren’s syndrome biomarkers benefit significantly from a single vectored thermal pulsation procedure: a retrospective analysis. Clin Ophthalmol. 2017;11:701–706. doi:10.2147/OPTH.S119926
31. Korb DR, Blackie CA. Meibomian gland diagnostic expressibility: correlation with dry eye symptoms and gland location. Cornea. 2008;27(10):1142–1147. doi:10.1097/ICO.0b013e3181814cff
32. Ngo W, Situ P, Keir N, et al. Psychometric properties and validation of the standard patient evaluation of eye dryness questionnaire. Cornea. 2013;32(9):1204–1210. doi:10.1097/ICO.0b013e318294b0c0
33. Sambursky R, Davitt WF
34. Pult H, Riede-Pult B. Comparison of subjective grading and objective assessment in meibography. Cont Lens Anterior Eye. 2013;36(1):22–27. doi:10.1016/j.clae.2012.10.074
35. Reiko A, Suehiro J, Haraguchi T, et al. Objective image analysis of the meibomian gland area. Br J Ophthalmol. 2014;98:746–755. doi:10.1136/bjophthalmol-2012-303014
36. Celik T, Lee HK, Petznick A, et al. Bioimage informatics approach to automated meibomian gland analysis in infrared images of meibography. J Optom. 2013;6(4):194–204. doi:10.1016/j.optom.2013.09.001
37. Koprowski R, Tian L, Olczyk P. A clinical utility assessment of the automatic measurement method of quality of meibomian glands. Biomed Eng Online. 2017;16:82. doi:10.1186/s12938-017-0373-4
38. Llorens-Quintana C, Rico-del-Viejo L, Syga P, Madrid-Costa D, Iskander DR. A novel automated approach for infrared-based assessment of meibomian gland morphology. Transl Vis Sci Technol. 2019;8(4):17. doi:10.1167/tvst.8.4.17
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.