Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 14
Virological and Immunological Antiretroviral Treatment Failure and Predictors Among HIV Positive Adult and Adolescent Clients in Southeast Ethiopia
Authors Mamo A , Assefa T , Negash W , Takelign Y , Sahiledinigl B , Teferu Z , Mohammed M, Solomon D , Gezahegn H , Bekele K, Zenbaba D, Tasew A , Tahir A, Desta F , Regassa T, Takele A , Regassa Z , Atilaw D
Received 18 December 2021
Accepted for publication 19 February 2022
Published 26 February 2022 Volume 2022:14 Pages 73—85
DOI https://doi.org/10.2147/HIV.S354716
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Olubunmi Akindele Ogunrin
Ayele Mamo,1 Tesfaye Assefa,2 Wegene Negash,2 Yohannes Takelign,3 Biniyam Sahiledinigl,3 Zinash Teferu,3 Mesud Mohammed,1 Damtew Solomon,4 Habtamu Gezahegn,4 Kebebe Bekele,5 Demsu Zenbaba,3 Alelign Tasew,3 Anwar Tahir,2 Fikereab Desta,3 Tadele Regassa,4 Abule Takele,3 Zegeye Regassa,2 Daniel Atilaw4
1Madda Walabu University, Goba Referral Hospital, Pharmacy Department, Bale Goba, Ethiopia; 2Madda Walabu University, Goba Referral Hospital, Nursing department, Bale Goba, Ethiopia; 3Madda Walabu University, Goba Referral Hospital, Public Health Department, Bale Goba, Ethiopia; 4Madda Walabu University, Goba Referral Hospital, Biomedical Department, Bale Goba, Ethiopia; 5Madda Walabu University, Goba Referral Hospital, Surgery Department, Bale Goba, Ethiopia
Correspondence: Ayele Mamo, Tel +251913512089, Email [email protected]
Background: Antiretroviral therapy (ART) regimen failure is linked to an increased risk of disease progression and death, while early detection of ART failure can help to prevent the development of resistance. This study aimed to evaluate virological and immunological ART failure and predictors among HIV-positive adult and adolescent clients in southeast Ethiopia.
Methods: A retrospective cohort study was implemented from January 2016 to November 30, 2020; all HIV-positive nave patients on follow-up during the study period from four hospitals were included. Virological and immunological treatment failure was the primary outcome of the study. Cox proportional hazards regression models were employed for analysis. Hazard ratios with 95% confidence intervals were reported and variables with p-values < 0.05 were considered statistically significant predictors of treatment failure.
Results: A total of 641 HIV patients’ charts were reviewed, 62.6% of the study participants were females. Of the total study participants, 18.4% and 15% developed virological and immunological ART regimen treatment failure respectively. The median time to virological failure was 40 months. WHO stage IV [AHR = 4.616; 95% CI: (2.136– 9.974)], WHO stage III [AHR = 2.323; 95% CI: (1.317– 4.098)], poor adherence to HAART regimen [AHR = 3.097; 95% CI: (1.349– 7.108)], and fair adherence [AHR = 2.058; 95% CI: (1.234– 3.432)] were significantly associated with virological treatment failure among adolescent and adult study participants in southeast Ethiopia.
Conclusion: The prevalence of virological treatment failure was 18.4% (95% CI: 15.4 − 21.4) and the prevalence of immunological treatment failure was 15% (95% CI: 11.8– 18.4). WHO clinical stage III/IV and non-adherence were independent predictors of virological ART treatment failure. Early management of clinical WHO stages and improving patients’ ART regimen adherence are important to decrease the prevalence of ART regimen treatment failure.
Keywords: treatment failure, antiretroviral, predictors, HIV, Bale zone
Corrigendum for this paper has been published
Background
Human immunodeficiency virus (HIV) is one of the most predominant chronic health conditions which attacks the body’s immune system and interferes with the ability to fight infection.1 In 2020, an estimated 37.0 million people worldwide were living with HIV, with 1.5 million people newly infected and 0.68 million people dying from acquired immunodeficiency syndrome (AIDS) related causes.2 Only three nations are on track to accomplish the worldwide target of a 75% decrease in incidence. Africa accounted for 25.7 million of the total estimated 38.0 million individuals living with HIV in 2019.3
Despite making significant progress in preventing and controlling the HIV/AIDS epidemic over the years, the Sub-Saharan African region currently has the highest HIV/AIDS burden in the world.4 In 2021, 616,105 persons living with HIV/AIDS were registered in Ethiopia and almost 11,673 people die as a result of AIDS per year.5
Because of the high adult and adolescent death rates in Sub-Saharan Africa, HIV/AIDS has the potential to have a severe impact on particular societies’ socioeconomic growth. Despite advances achieved in the fight against HIV/AIDS over the last decade, the pandemic remains one of the most serious threats to global health and is expected to stay one of the world’s top causes of death and disability for years to come.6
Ethiopia is one of the low-income countries experiencing a high communicable disease burden, including human immunodeficiency virus/acquired immunodeficiency syndromes (HIV/AIDS) which accounts for 70 disability-adjusted life years per 100,000 individuals.7 In Ethiopia antiretroviral therapy (ART) began in 2003 and was scaled-up in 2005. Since then, a total of 616,105 people living with HIV were actively on ART by 2020 with 78% ART coverage and improved health and survival of patients in many parts of Ethiopia.8,9 Treatment among the population receiving ART in Ethiopia is still a public health concern.10
In HIV/AIDS patients, ART failure increases medication toxicity and resistance. According to data from the US Agency for International Development, 46% of HIV-infected individuals who failed first-line ART have a higher chance of failing again on second-line treatment.11,12 To avoid unnecessary switching from first-line to second-line treatment, confirmatory testing using a viral load test method is critical.13 Clinical and immunologic treatment failure criteria missed 42% of the failures discovered by viral load testing and were found to be ineffective in predicting ART virological failure.7 The gold standard for determining treatment success is plasma viral load monitoring.14 Virological testing is useful in bolstering earlier research in the area and assessing treatment failure that was not determined by utilizing gold standard measurement.15
ART regimens that do not achieve virologic suppression are linked to an increased risk of disease progression and death.16 Although research indicates that 20% of patients with a regimen that did not achieve virologic suppression experienced significant adverse effects by 30 months.18 While early detection of ART failure and rapid measure of therapy can help to prevent the development of resistance.
The primary goal of highly active antiretroviral therapy (HAART) is to suppress plasma viral load below the detection level and also restore and preserve immunologic function, improving the quality of life, and reducing vertical transmission.17,18 There is currently little evidence on the virological measurement of ART failure and predictors among HIV-positive adult and adolescent clients in Ethiopia and other low-income countries, particularly in Southeast Ethiopia. Research on virological treatment failure among HIV patients is crucial to determine existing HIV treatment status and determinants of treatment failure. The findings of the study will be used to identify effective measures for preventing and controlling HIV treatment failure, as well as to design effective HIV treatment failure prevention mechanisms and control programs that target health care providers. The study aims to evaluate virological and immunological ART failure and predictors among HIV-positive adolescent and adult patients in southeast Ethiopia.
Methods
Study Area and Period
This study was conducted in the Bale zone and East Bale zone, Southeast Ethiopia. They are located in Oromia regional state 430 km and 580km away from the capital city (Addis Ababa) of Ethiopia respectively. The topography of the land is composed of 14.92% highland, 21.53% midland 63.55% lowland with an altitude of 300–4377m, and annual rainfall of 900–1400mm. According to the 2018 zonal report, there are 90 health centers and 323 health posts, and 4 hospitals named Madda Walabu University Goba referral hospital (MWU-GRH), Robe hospital, Delomena hospital, and Ginnir hospital in two Bale Zones. MWU-GRH, Robe hospital, and Delomena hospital are located in Bale Zone while Ginnir hospital is in East Bale Zone. The data were collected from March 1 to 30, 2021 by retrieving the new ART client document between January 1, 2016, and November 30, 2020.
Study Design and Population
A retrospective cohort study was implemented in four Bale Zone hospitals. All HIV-positive patients who had follow-up services in the Bale Zones hospitals were the source population. Adult and adolescent clients who had HAART initiated between January 1, 2016, and November 30, 2020 in the Bale Zones hospitals and fulfilled the inclusion criteria were the study population. Age greater than or equal to ten years and clients who had complete data were included in the study whereas clients who had less than six months follow-up and patients who were transferred-in were excluded.
Sample Size Determination and Sampling Technique
We included all HIV positive patients being followed-up during the study period (641) from the four hospitals (Figure 1).
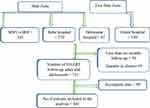 |
Figure 1 Workflow of the study participants’ inclusion criteria. |
Measurement Variables, Data Collection Tools, and Procedures
Virological and immunological treatment failure was the dependent variable of the study. Patient-related variables included age, sex, marital status, occupational status, residence, and disclosure status. Disease-related variables such as functional status, WHO stage, hemoglobin level, opportunistic infection, type of regimen, adherence, drug toxicity, and CD4 count were predictors. The standard registration logbook for ART patients developed by the federal ministry of health was used to develop data abstraction format. Data on CD4+ T-cells count which was measured at baseline and at six months interval thereafter during regular follow-up visit were collected from medical charts. Data were extracted by the data clerk BSc nurses. Data collectors were provided with intensive training on the objective of the study, contents of data abstraction format, and how to maintain privacy of the study subjects. The data collection process was supervised by principal investigators and supervisors. Time to treatment failure after starting first-line ART was calculated in months using the time between dates of treatment initiation to the date of event or date of censoring.
Data Quality, Management, and Analysis Procedure
Data were checked for completeness before EpiData entry. During data entry, EpiData double-entry validation was used to check the accuracy of data entry. Data were entered into the data entry template of EpiData 3.1 and exported to Statistical Package for the Social Sciences (SPSS) 26.0 version (IBM Corporation 2019) for data analysis. Descriptive statistics such as frequency, median, mean and standard deviation were computed for all continuous and categorical variables. Person time contributions of each study participant were calculated by comparing the duration of ART and treatment failure as an outcome variable. The Kaplan Meier curve with Log rank test was used to describe the probability of survival without treatment failure. To identify the predictors of treatment failure, bivariate and multivariable Cox proportional hazards regression models were employed. Both crude and adjusted hazard ratios with 95% confidence intervals were reported, and variables with p-values <0.05 in the Cox regression model were considered statistically significant predictors of treatment failure.
Operational Definition
Immunologic failure: CD4 count at or below 250 cells/mm3 following clinical failure or persistent CD4 levels below 100 cells/mm3.16
Virologic failure: refers to the inability to achieve or maintain viral suppression below a certain threshold. Viral failure is defined by a persistently detectable viral load exceeding 1000 copies/mL (two consecutive viral load measurements within a 3-month interval with adherence support between measurements) after at least 6 months of using ART.16
Good adherence > 95%, fair adherence 85–95% and poor adherence < 85% doses taken.19–21
Result
Socio-Demographic Characteristics of the Study Participants
A total of 641 HIV-positive patients’ charts were reviewed. The mean age of study participants was 34.24 with a standard deviation of +11.085. More than half (62.6%) were females, 37.3% of the study participants were living in the rural area of the study zones. Educational status of the participants: 28.5% had no formal education, 272 (37.8%) had primary education, and 7.2% had college education and above. Almost one-fourth 155 (24.2%) were housewives (occupation) (Table 1).
Clinically Related Data of the Study Participants
From the clinically related document reviewed data, 53 (84.1%) had working functional status, 85 (13.3%) ambulatory, and 17 (2.7%) had bedridden functional status at initiation of ART. The majority (553 (86.3%)) of study participants were clinical WHO stage I, 34 (5.3%), and 44 (6.9%) were clinical WHO stage II and WHO stage III respectively. Of the total study participants, 204 (31.8) were developing opportunistic infection whereas 434 (67.1%) did not develop an opportunistic infection. From total clients receiving ART regimens, more than 85% had good adherence to HAART regimens, 9.8% and 4.1% had fair and poor adherence to HAART regimens respectively. From the total 641 study participants, 18.4% (95% CI: 15.4 −21.4) had viral load above 1000 copies/cells and were considered as virological failure, 81.6% (95% CI: 78.6–84.6) were censored observations. Similarly, from the total (641) study participants, 15% (95% CI: 11.8–18.4) had immunological failure (Table 2). As shown in Figure 2, bacterial infection and diarrhea were commonly occurring opportunistic infections followed by zoster and pneumocystis pneumonia and toxoplasmosis was the least occurring opportunistic infections (Figure 2).
 |
Figure 2 Types of frequently occurring opportunistic infections in HIV-positive patients. |
Predictors of Virological Treatment Failure Among HIV Positive Adult Patients at Bale Zone Hospitals
The association of each independent variable was checked using Cox proportional hazards regression, those with a value less than 0.25 were selected for multivariable Cox proportional hazards regression model based on that WHO stage during treatment initiation. WHO stage during data collection and adherence to HAART regimen were predictors of virological treatment failure among adolescent and adult study participants in southeast Ethiopia.
Multivariable hazards Cox regression showed that WHO stage at the initiation of ART was a predictor of virological treatment failure based on: WHO stage IV was 4.616 more likely to develop virological treatment failure as compared to WHO stage I [AHR = 4.616; 95% CI: (2.136–9.974)]. Similarly, baseline WHO stage III was shown to be a statistical predictor of virological treatment failure [AHR = 2.323; 95% CI: (1.317–4.098)] as compared to baseline WHO stage I. Clients with poor adherence to HAART regimen were 3.097 times more likely to experience virological treatment failure as compared to clients with good adherence [AHR = 3.097; 95% CI: (1.349–7.108)]. Fairly adherent clients were found to be significantly associated with higher odds of virological treatment failure when compared to good adherence among adolescent and adult study participants in southeast Ethiopia [AHR = 2.058; 95% CI: (1.234–3.432)]. Those with WHO stage IV during data collection were 3.054 more likely to develop virological treatment failure as compared to those with WHO stage me [AHR = 3.054; 95% CI: (1.227–7.600)]. Similarly, WHO stage III during data collection was found to be significantly associated with biological treatment failure [AHR = 3.309; 95% CI: (1.884–5.813)] as compared to WHO stage I during the data collection period (Table 3).
 |
Table 3 Predictors of Virological Treatment Failure Using Bivariable and Multivariable Cox Regression Analysis Among Adolescent and Adult ART Clients in Southeast Ethiopia (n = 641) |
Discussion
The result of this study showed that the prevalence of virological treatment failure was high. WHO stage and adherence to HAART regimen were significantly associated with virological treatment failure among adolescent and adult study participants in southeast Ethiopia. The median time of ART initiation to virological failure was 40 weeks (range: 37.6–42.4 weeks) (Figure 3).
 |
Figure 3 Kaplan Meier curve analysis of virological treatment failure starting from six months of follow-up. |
This prevalence rate is comparatively similar to different studies from Ethiopia: virological ART treatment failure in Welo was 15.9%,22 a study done in Gonder showed 20.3%,20 15.7% of patients experienced immunological treatment failure in Ethiopia.23 This study is also in line with Enderis et al’s study in Arba-Minch in which 17.42% developed treatment failure,11 the study conducted in Harar which showed 21.1%,24 Nega et al’s 16.6%, and Wondie et al’s study showed 15.9% ART treatment failure respectively.22,25 When we compare the studies done in Africa, it is still in line with a study done by Rawizza et al in Nigeria which showed 21.6%18 and a study done in Tanzania which showed 19%.26 Similarly, this study was also in line with a study done in China: 13.4%27 and India: 16.5%.28
The finding of this study is lower than the finding from Adigrat, in which immunologic and virologic overall prevalence of treatment failure was 27.48%.29 The difference is that in the study done by Demsie et al, the prevalence report was both cumulative overall result of both immunologic and virologic ART treatment failure. Similarly, it is lower than that of the study conducted by Gesesew et al in South West Ethiopia which was 25.1%30 and Lenjiso et al’s study in Dire Dawa which was 22.7%.31 The finding was lower than in studies conducted in other countries: Mozambique 24.4%,32 study conducted in Canada 37.1%,33 in Peru 24.0%,34 and study done in Saudi was 23.4.35 The variation may be due to the lifestyle difference between countries. Many national recommendations now advocate either targeted or routine viral load monitoring, and governments are working to expand access to these methods. Regular access to routine viral load testing, on the other hand, remains limited, and this has been highlighted as a major explanation for lower-than-expected rates of detected treatment failure in resource-limited settings.16,36 It is important to ensure that those receiving ART remain in care and on effective therapy.13,19
The finding of the study is higher than that of the study conducted in southeast Ethiopia: 2.4%,15 in Mekelle: 11.5%,9 Asgedom et al’s study: 4.5%,37 and the study conducted in St. Luke and Tulubolo Hospitals: 6.8%38 and in Southern Ethiopia: 11.1%.39 The variation may be due to sampling size, method of analysis as well as the method of treatment failure measurement, for example, in Hailu et al’s study, Yirdaw et al’s study, Haile et al’s study,9,15,39 and Bayou et al’s study,38 prevalence of treatment failure was assessed immunologically, which has a low detection rate of treatment failure. The finding of this study is lower than the studies done in the rest of the world: such as studies done in Kenya: 6%,40 in Uganda: 11%,41 in Vietnam: 6.6%,42 and studies done in China: 11.8%.27 The discrepancy may be due to the cultural and economic differences from the people of Ethiopia, sample size, and maybe the policy difference between countries regarding start of ART regimens and patient adherence to their medication.
The median time of ART initiation to virological failure was 40 weeks (range: 37.6–42.4 weeks). The finding of ART initiation to treatment failure study done in 1.8 Years.39 The variation in the study population included in Yirdaw et al’s study was all age groups, and all patients on regimen starting from ART initiation were included.
Regarding the predictors of virological treatment failure, the finding of our study showed WHO stage IV was significantly associated with ART treatment failure. This is similar to a finding of an Adigrat study in which WHO stages III/IV were significantly associated with treatment failure in multivariable analysis.29 Likewise, in Huang et al’s study in China, patients with WHO clinical stage IV were 4.16 times more likely when compared with those with stage I.27 The severe AIDS symptoms which have been categorized under WHO stage III and IV were the predictors for ART regimen treatment failure. Early intervention and serious follow-up of opportunistic infections which are characterized by stage IV/III are crucial to minimize treatment failure.
Those with poor adherence level were three times more likely to develop virological treatment failure when compared to those with good adherence level. The finding of this study was consistent with the finding from another study, accordingly, Wendie et al’s study done in Welo showed poor adherence was significantly associated with virological failure.22 Similarly, in Asgedom et al’s study, non-adherent study participants were five times more likely to experience immunological failure compared with those who had good adherence.11,37 Also, it is similar to studies conducted in North Ethiopia,9,43 southwest Ethiopia,30 Northeast Ethiopia,44 Southeast Ethiopia,15 a study conducted in Uganda,41 in Mozambique,32 in Peru,34 and a study conducted in India.28
The median time to virological treatment failure for patients who had good adherence was 24 months and for fairly adherent patients the median time to biological failure was 25 months, and for non-adherent patients time to virological failure was less than the median time (Figure 4). Due to a lack of adherence programs, loose peer educator activities, poor adherence, and over-adherence, meaning when patients anticipate pill counts, they may remove pills without swallowing them to make their adherence appear desirable resulting in over 100% adherence,21 patients might have incomplete viral suppression leading to higher rates of ART virological and immunological treatment failure, additionally, some professionals have expressed reluctance to advise patients to stick to their regimen while initiating ART medications at various units.45,46 Unlike other studies, TB co-infection, being male, BMI and regimen type, and duration of treatment period were not statistically significant in our study.47
 |
Figure 4 Relationship between time to virological treatment failure and adherence to antiretroviral medication. |
This study has the strength of having used virological treatment failure measurement which does not underestimate treatment failure like clinical and immunological treatment failure measures, and it also has the strength of multi-center treatment settings. Even though the study has these strengths, the study has the limitation of using secondary data which miss fundamental variables which should be considered. Due to time and financial problems, we applied the retrospective cohort study design but a prospective cohort study is preferable.
Conclusion
The prevalence of treatment failure in the study area is higher when compared to the previous studies done by using clinical and immunological measurements. WHO clinical stages and non-adherence to HAART regimens are independent predictors of virological ART regimen treatment failure. ART treatment failure in Bale and East Bale zone hospitals needs attention from ART program coordinators, health care workers, and stakeholders. Early management of clinical WHO stages, reassurance regarding test and treatment, prevention and control of opportunistic infection, and improving patients’ ART regimen adherence are important to decrease the prevalence of ART regimen treatment failure.
Abbreviations
AIDS, acquired immunodeficiency syndrome; ART, antiretroviral therapy; HAART, highly active antiretroviral therapy; HIV/AIDS, human immunodeficiency virus/acquired immunodeficiency syndromes; HIV, human immunodeficiency virus; MWUGRH, Madda Walabu University Goba Referral Hospital; SPSS, Statistical Package for the Social Sciences; WHO, World Health Organization.
Data Sharing Statement
All the data sets generated and/or analyzed in this study are available from the corresponding author upon reasonable request.
Ethical Consideration and Consent to Participate
Ethical permission was obtained from the Madda Walabu University ethical review committee. A letter of cooperation was written to each hospital (Ref. No: - RDD/0099/13). Since we were going to do chart reviews of secondary data, there was no need for informed consent. Strict confidentiality was maintained during the data collection process as well as secrecy during data processing and report-writing. The research was also carried out in accordance with the Helsinki declaration.
Acknowledgments
We would like to thank Goba referral hospital, Robe Hospital, and Ginnir Hospital ART clinic staff members for providing important information during the development of our proposal.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Not applicable, except that of data collectors fee by Madda Walabu University.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Assebe LF, Negussie EK, Jbaily A, Tolla MTT, Johansson KA. Financial burden of HIV and TB among patients in Ethiopia: a cross-sectional survey. BMJ Open. 2020;10(6):e036892. doi:10.1136/bmjopen-2020-036892
2. The global HIV/AIDS epidemic 2022. Available from: https://www.hiv.gov/hiv-basics/overview/data-and-trends/global-statistics.
3. Platform WHD. Estimated number of people (all ages) living with HIV; 2020. Available from: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/estimated-number-of-people-(all-ages)-living-with-hiv.
4. Mirkuzie AH, Ali S, Abate E, Worku A, Misganaw A. Progress towards the 2020 Fast Track HIV/AIDS reduction targets in Ethiopia comparing with neighboring countries and across ages; using Global Burden of Diseases 2017 data. 2020.
5. Ethiopia country operational plan COP 2021 strategic direction summary final; 2021 Available from: https://www.state.gov/wp-content/uploads/2021/09/Ethiopia_SDS_Final-Public_Aug-11-2021.pdf.
6. Vitoria M, Granich R, Gilks CF, et al. The global fight against HIV/AIDS, tuberculosis, and malaria: current status and future perspectives. Am J Clin Pathol. 2009;131(6):844–848. doi:10.1309/AJCP5XHDB1PNAEYT
7. Assemie MA, Alene M, Ketema DB, Mulatu S. Treatment failure and associated factors among first line patients on highly active antiretroviral therapy in Ethiopia: a systematic review and meta-analysis. Glob Health Res Policy. 2019;4(1):1–10. doi:10.1186/s41256-019-0120-4
8. WHO. Ethiopia HIV country profile 2020. Available from: https://cfs.hivci.org/.
9. Hailu GG, Hagos DG, Hagos AK, Wasihun AG, Dejene TA. Virological and immunological failure of HAART and associated risk factors among adults and adolescents in the Tigray region of Northern Ethiopia. PLoS One. 2018;13(5):e0196259. doi:10.1371/journal.pone.0196259
10. Mulisa D, Tesfa M, Kassa GM, Tolossa T. Determinants of first line antiretroviral therapy treatment failure among adult patients on ART at central Ethiopia: un-matched case control study. BMC Infect Dis. 2019;19(1):1024. doi:10.1186/s12879-019-4651-6
11. Enderis BO, Hebo SH, Debir MK, Sidamo NB, Shimber MS. Predictors of time to first line antiretroviral treatment failure among adult patients living with HIV in public health facilities of Arba Minch Town, Southern Ethiopia. Ethiop J Health Sci. 2019;29(2):175–186. doi:10.4314/ejhs.v29i2.4
12. Niemeyer K, King A, Mengistu S, Hennig N. Predictors for antiretroviral therapy (ART) failure in an urban HIV/AIDS clinic in Addis Ababa, Ethiopia. Lancet Glob Health. 2016;4:S6. doi:10.1016/S2214-109X(16)30011-0
13. WHO. WHO Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Geneva, Switzerland: WHO; 2013.
14. Rutherford GW, Anglemyer A, Easterbrook PJ, et al. Predicting treatment failure in adults and children on antiretroviral therapy: a systematic review of the performance characteristics of the 2010 WHO immunologic and clinical criteria for virologic failure. Aids. 2014;28:S161–S9. doi:10.1097/QAD.0000000000000236
15. Haile D, Takele A, Gashaw K, Demelash H, Nigatu D. Predictors of treatment failure among adult antiretroviral treatment (ART) clients in Bale zone hospitals, south eastern Ethiopia. PLoS One. 2016;11(10):e0164299. doi:10.1371/journal.pone.0164299
16. Rawizza HE, Chaplin B, Meloni ST, et al. Immunologic criteria are poor predictors of virologic outcome: implications for HIV treatment monitoring in resource-limited settings. Clin Infect Dis. 2011;53(12):1283–1290. doi:10.1093/cid/cir729
17. World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. World Health Organization; 2016.
18. Misgena DK. The pattern of immunologic and virologic responses to Highly Active Antiretroviral Treatment (HAART): does success bring further challenges? Ethiop J Health Dev. 2011;25(1):61–70. doi:10.4314/ejhd.v25i1.69853
19. WHO. WHO March 2014 Supplement to the 2013 Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. WHO; 2014.
20. Ayele G, Tessema B, Amsalu A, Ferede G, Yismaw G. Prevalence and associated factors of treatment failure among HIV/AIDS patients on HAART attending University of Gondar Referral Hospital Northwest Ethiopia. BMC Immunol. 2018;19(1):37. doi:10.1186/s12865-018-0278-4
21. Okatch H, Beiter K, Eby J, et al. Brief report: apparent antiretroviral overadherence by pill count is associated with HIV treatment failure in adolescents. J Acquir Immune Deficiency Syndr. 2016;72(5):542–545. doi:10.1097/QAI.0000000000000994
22. Wendie TF, Workneh BD. Prevalence and predictors of virological failure among adults living with HIV in South Wollo Zone, Northeast Ethiopia: a retrospective cohort study. HIV/AIDS. 2020;12:393.
23. Teshome W, Tefera A. Detection of immunological treatment failure among HIV infected patients in Ethiopia: a retrospective cohort study. BMC Immunol. 2015;16(1):55. doi:10.1186/s12865-015-0120-1
24. Feleke R, Geda B, Teji Roba K, Weldegebreal F. Magnitude of antiretroviral treatment failure and associated factors among adult HIV-positive patients in Harar public hospitals, Eastern Ethiopia. SAGE Open Med. 2020;8:2050312120906076. doi:10.1177/2050312120906076
25. Nega J, Taye S, Million Y, Rodrigo C, Eshetie S. Antiretroviral treatment failure and associated factors among HIV patients on first-line antiretroviral treatment in Sekota, northeast Ethiopia. AIDS Res Ther. 2020;17(1):1–9. doi:10.1186/s12981-020-00294-z
26. Vanobberghen FM, Kilama B, Wringe A, et al. Immunological failure of first-line and switch to second-line antiretroviral therapy among HIV-infected persons in Tanzania: analysis of routinely collected national data. Trop Med Int Health. 2015;20(7):880–892. doi:10.1111/tmi.12507
27. Huang P, Tan J, Ma W, et al. Outcomes of antiretroviral treatment in HIV-infected adults: a dynamic and observational cohort study in Shenzhen, China, 2003–2014. BMJ open. 2015;5(5):e007508–e007508. doi:10.1136/bmjopen-2014-007508
28. Shet A, Neogi U, Kumarasamy N, DeCosta A, Shastri S, Rewari BB. Virological efficacy with first‐line antiretroviral treatment in India: predictors of viral failure and evidence of viral resuppression. Trop Med Int Health. 2015;20(11):1462–1472. doi:10.1111/tmi.12563
29. Demsie DG, Bantie AT, Allene MD, Alema NM, Gebrie D. Antiretroviral treatment failure among HIV-positive adults taking first-line therapy and associated risk factors at Adigrat General hospital, Adigart, Ethiopia 2019: a cross sectional study. Int J Surg Open. 2020;26:16–21. doi:10.1016/j.ijso.2020.08.001
30. Gesesew HA, Ward P, Woldemichael K, Mwanri L. Immunological failure in HIV-infected adults from 2003 to 2015 in Southwest Ethiopia: a retrospective cohort study. BMJ open. 2018;8(8):e017413. doi:10.1136/bmjopen-2017-017413
31. Lenjiso GA, Endale BS, Bacha YD. Clinical and immunological failure among HIV-positive adults taking first-line antiretroviral therapy in Dire Dawa, eastern Ethiopia. BMC Public Health. 2019;19(1):771. doi:10.1186/s12889-019-7078-5
32. Palladino C, Briz V, Bellón JM, et al. Predictors of attrition and immunological failure in HIV-1 patients on highly active antiretroviral therapy from different healthcare settings in Mozambique. PLoS One. 2013;8(12):e82718. doi:10.1371/journal.pone.0082718
33. Gross R, Yip B, Re VL, et al. A simple, dynamic measure of antiretroviral therapy adherence predicts failure to maintain HIV-1 suppression. J Infect Dis. 2006;194(8):1108–1114. doi:10.1086/507680
34. Jorge AR, Jorge PB, Elsa GL, et al. Risk factors associated with virologic failure in HIV-infected patients receiving antiretroviral therapy at a public hospital in Peru. Revista Chilena de Infectologia. 2013;30(1):42. doi:10.4067/S0716-10182013000100006
35. Musa BM, Coker M, Bussell S, et al. Long-term outcomes of antiretroviral therapy in an adult HIV program: a 10-year retrospective cohort study in Kano, Nigeria. Ann Saudi Med. 2015;35(4):303–311. doi:10.5144/0256-4947.2015.303
36. Estill J, Kerr CC, Blaser N, et al. The Effect of Monitoring Viral Load and Tracing Patients Lost to Follow-Up on the Course of the HIV Epidemic in Malawi: A Mathematical Model. Open Forum Infectious Diseases. Oxford University Press US; 2018.
37. Asgedom SW, Maru M, Berihun B, Gidey K, Niriayo YL, Atey TM. Immunologic and clinical failure of antiretroviral therapy in people living with human immunodeficiency virus within two years of treatment. Biomed Res Int. 2020;2020:1–8. doi:10.1155/2020/5474103
38. Bayou B, Sisay A, Kumie A. Assessment of the magnitude and associated factors of immunological failure among adult and adolescent HIV-infected patients in St. Luke and Tulubolo Hospital, Oromia Region. Ethiop Pan Afri Med J. 2015;21(1):291.
39. Yirdaw KD, Hattingh S, Paraskevis D. Prevalence and predictors of immunological failure among HIV patients on HAART in Southern Ethiopia. PLoS One. 2015;10(5):e0125826. doi:10.1371/journal.pone.0125826
40. Ferreyra C, Yun O, Eisenberg N, et al. Evaluation of clinical and immunological markers for predicting virological failure in a HIV/AIDS treatment cohort in Busia, Kenya. PLoS One. 2012;7(11):e49834. doi:10.1371/journal.pone.0049834
41. Bulage L, Ssewanyana I, Nankabirwa V, et al. Factors associated with virological non-suppression among HIV-positive patients on antiretroviral therapy in Uganda, August 2014–July 2015. BMC Infect Dis. 2017;17(1):1–11. doi:10.1186/s12879-017-2428-3
42. Tran DA, Wilson DP, Shakeshaft A, Ngo AD, Doran C, Zhang L. Determinants of virological failure after 1 year’s antiretroviral therapy in Vietnamese people with HIV: findings from a retrospective cohort of 13 outpatient clinics in six provinces. Sex Transm Infect. 2014;90(7):538–544. doi:10.1136/sextrans-2013-051353
43. Ayalew MB, Kumilachew D, Belay A, et al. First-line antiretroviral treatment failure and associated factors in HIV patients at the University of Gondar Teaching Hospital, Gondar, Northwest Ethiopia. HIV/AIDS. 2016;8:141.
44. Ahmed M, Merga H, Jarso H. Predictors of virological treatment failure among adult HIV patients on first-line antiretroviral therapy in Woldia and Dessie hospitals, Northeast Ethiopia: a case-control study. BMC Infect Dis. 2019;19(1):1–7. doi:10.1186/s12879-019-3924-4
45. Deeks SG, Overbaugh J, Phillips A, Buchbinder S. HIV infection. Nat Rev Dis Primers. 2015;1:15035. doi:10.1038/nrdp.2015.35
46. Chesney MA, Morin M, Sherr L. Adherence to HIV combination therapy. Soc Sci Med. 2000;50(11):1599–1605. doi:10.1016/S0277-9536(99)00468-2
47. Getawa S, Fentahun A, Adane T, Melku M. Antiretroviral treatment failure and associated factors among HIV-infected children on antiretroviral therapy: a retrospective study. HIV/AIDS. 2021;13:229.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



