Back to Journals » Clinical Ophthalmology » Volume 9
Virological and epidemiological analysis of coxsackievirus A24 variant epidemic of acute hemorrhagic conjunctivitis in Okinawa, Japan, in 2011
Authors Harada K, Fujimoto T, Asato Y, Uchio E
Received 22 January 2015
Accepted for publication 11 March 2015
Published 15 June 2015 Volume 2015:9 Pages 1085—1092
DOI https://doi.org/10.2147/OPTH.S81386
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Kazuhiro Harada,1 Tsuguto Fujimoto,2 Yoshimori Asato,3 Eiichi Uchio1
1Department of Ophthalmology, Fukuoka University School of Medicine, Fukuoka, 2Infectious Disease Surveillance Center, National Institute of Infectious Disease, Tokyo, 3Asato Eye Clinic, Itoman, Japan
Background: Acute hemorrhagic conjunctivitis (AHC) is a highly contagious enterovirus infection of the conjunctiva and cornea. Coxsackievirus A24 variant (CA24v) is one of its etiological agents. We report a clinical, epidemiological, and virological analysis of a large epidemic of AHC that occurred from May to September, 2011, in Okinawa, Japan.
Methods: Clinical and epidemic aspects were evaluated for 435 AHC patients (348 bilateral and 87 unilateral, 783 eyes). Virological studies were carried out on nine isolates from ten patients. Virus isolation and direct detection of the enterovirus genome by the reverse transcription polymerase chain reaction method and complete nucleotide sequencing of the VP1 gene and phylogeny-based classification using the VP4 sequences were carried out.
Results: The 11–15-year age group comprised the highest (62.0%) proportion of cases among all age groups. Conjunctival hyperemia was present in all patients, and subconjunctival hemorrhage, superficial punctate keratitis, and preauricular lymphadenopathy were present in 25.4%, 10.3%, and 7.8% of eyes, respectively. CA24v was isolated from the epidemic strain, and phylogenetic analysis based on a fragment of the VP1 gene showed 96%–97% identity between the current strain and the recent China/GD01/2010 strain.
Conclusion: These findings demonstrate that the clinical and epidemiological features of AHC observed in this study were similar to those of the past epidemic in the same region. It should be noted that sequential outbreaks of AHC due to CA24v might occur in the same location after a considerable period of time, and public health precautions are necessary to control this explosive epidemic.
Keywords: enterovirus, coxsackievirus, acute hemorrhagic conjunctivitis, polymerase chain reaction, epidemiology
Introduction
Acute hemorrhagic conjunctivitis (AHC), similar to epidemic keratoconjunctivitis, is a highly infectious conjunctivitis caused by a virus. Enterovirus (EV)70 and coxsackievirus A24 variant (CA24v) are known to be etiological agents. Epidemic AHC first occurred during June 1969 in a suburb of Accra, Ghana.1 Since then, AHC has spread to many parts of the world, affecting millions of people, including in Japan,2 and has gained recognition as a major international public health problem.3 CA24v first emerged in Singapore in 1970,4 followed by repeated epidemics in Asian countries.5 In the summer of 2011, a large epidemic of AHC occurred in Okinawa, Japan. Okinawa is located near the recent CA24v epidemics, including those in the People’s Republic of China,6 Taiwan,7 and Korea.8 In Okinawa, Japan, we experienced an unusually extensive AHC epidemic in the summer of 1985 that recurred in 1986.9 Many strains of CA24v were isolated from the patients, and wide dissemination of the virus was confirmed seroepidemiologically. The prefectural surveillance center was notified of 9,952 cases in 1985 and 6,096 cases in 1986 in Okinawa, Japan.10 The second epidemic of AHC was observed in 1993, and we reported that EV70 was the causative agent.11 There was an 8-year interval between the two AHC epidemics in Okinawa, Japan. Since these large outbreaks, no large outbreak of AHC has occurred in Okinawa. It was confined to Okinawa Prefecture and the virus did not spread to the mainland of Japan.9
Here we describe the virological and epidemiological features and clinical findings for the 2011 AHC epidemic in Okinawa, Japan, in which the etiological agent was identified as CA24v by reverse transcription polymerase chain reaction (RT-PCR).
Materials and methods
Location of Okinawa and sample collection
Okinawa Prefecture comprises 48 islands and constitutes the southern half of the Nansei Islands located between Kyushu, Japan, and Taiwan (Figure 1). It is located at latitude 24°–28° north and longitude 122°–133° east. Climatologically, the area is subtropical, with an average annual rainfall of 2,100 mm; the lowest temperature is 16.0°C in January and the highest 28.1°C in July. The prefecture covers an area of 2,250 km2 and the population numbered 1.40 million in 2011. Ninety percent of people live on Okinawa Honto Island. Several US military bases are located on the island, and there is official and military traffic to and from the US mainland, Southeast Asia, and the Pacific Islands. There are also busy international, domestic, and interisland flights and maritime traffic. AHC cases were diagnosed clinically according to characteristic findings, such as acute follicular conjunctivitis with conjunctival hemorrhage, bilateral conjunctivitis, and a short incubation period of up to several days. Clinical and epidemic aspects were evaluated in 783 eyes of 435 patients who were clinically diagnosed with AHC at Asato Eye Clinic, Itoman, Japan, from May 29, 2011 to September 4, 2011. A virological study was carried out in the following cases during this epidemic. After obtaining informed consent, ten eyes of ten patients with clinically diagnosed AHC at Asato Eye Clinic, from July 2, 2011 to July 7, 2011, were enrolled in this study for virological analysis.
  | Figure 1 Location of Okinawa Honto Island, Japan. Okinawa Honto Island is the main part of Okinawa Prefecture, which is located in the southwest rim of Japan. |
Virus isolation and detection
HEp-2, Vero, and RD-18 cells were used for isolation of enteroviruses from conjunctival swab samples collected from patients with AHC. Confluent cell cultures were seeded in microplate wells and inoculated with 100 μL of maintenance medium and 50 μL of conjunctival swab samples. The cell cultures were then incubated at 34°C in 5% CO2-95% air and observed for 7 days to check for cytopathic effects. A blind passage was performed once if no cytopathic effect was observed by the end of the observation period. Virus isolates were identified by a neutralization test using anti-CV-A24 polyclonal antibodies provided from the National Institute of Infectious Diseases in Japan. The isolates were stored at −80°C.
Direct detection of enterovirus genome by RT-PCR
To identify the pathogen, viral nucleic acid was extracted from 140 μL of ten culture supernatant fluids harvested for virus isolation using a QIAamp viral RNA mini kit (Qiagen NV, Venlo, the Netherlands), and suspended in DNase-free or RNase-free water, respectively. To detect enteroviruses, complementary DNA was synthesized using a Prime-Script RT reagent kit (Takara, Shiga, Japan) after RNA extraction, and RT-PCR and cycle sequencing reactions were performed according to the method by Tavares et al with modification.12
PCR and sequence determination of VP4 gene for classification
The methods used for molecular diagnosis of enteroviruses by nested RT-PCR and phylogeny-based classification using the VP4 sequences were carried out according to the report by Hosoya et al to confirm the results of virus isolation and RT-PCR.13 Briefly, the entire VP4 nucleotide sequences of the enrolled samples were determined and used for phylogeny-based analysis, along with those of 64 prototype enterovirus strains. We estimated the evolutionary distances using the Kimura two-parameter method14 and constructed unrooted phylogenetic trees with the neighbor-joining method.15 Bootstrap analysis was performed by resampling the data sets 1,000 times. Bootstrap values greater than 70% were considered to be statistically significant for the grouping.16 The VP4 sequences of the 54 representative coxsackievirus (CV)-A16 strains isolated in this study were also compared with those of 20 strains isolated in other areas of Japan, 29 strains in the People’s Republic of China, and one strain in the UK taken from international databases (GenBank), using phylogenetic analysis.
Complete nucleotide sequencing of VP1 gene
Complete nucleotide sequences of the VP1 gene were analyzed according to the report by Tavares et al.12 Products of 1,096 and 673 base pairs were obtained from PCR reactions using primer pairs that flank the VP1 gene. The products were then purified and analyzed using an ABI Prism® 310 genetic analyzer (Life Technologies Japan Ltd, Tokyo, Japan) as described above. The nucleotide sequences for VP1 were aligned using the Clustal W program included in the BioEdit version 7.0.9.0 software17 and hand-edited where necessary. The alignment was used to identify respective divergence and to infer the phylogenetic relationships among clinical isolates and sequences available in GenBank from outbreaks that had occurred in other parts of the world. Sequence analyses were performed using MEGA software version 4.0,18 and phylogenetic trees were constructed by the neighbor-joining method. The evolutionary distances were computed using the Kimura two-parameter model of nucleotide substitution. The robustness of each node was assessed by a bootstrap test with 1,000 replicates.
Results
Epidemic characteristics
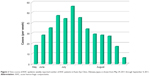
The weekly incidence of AHC diagnosed at Asato Eye Clinic in Okinawa from June to August, 2011 is presented in Figure 2. The epidemic started in the last week of May, 2011, and spread explosively, with a peak incidence in the middle of July, and then subsided toward the 1st week of September, 2011. The cumulated number of AHC cases was 435. The age distribution of the cases is presented in Figure 3. There were 246 male (56.6%) and 189 female (43.4%) patients. The age of the patients ranged from 3 to 79 (mean 17.3) years. The age group comprising the largest percentage of patients was junior high school children (11–15 years, 62.0%), followed by high school children (16–20 years, 16.8%).
  | Figure 3 Age distribution of patients with acute hemorrhagic conjunctivitis. The largest percentage of cases was observed in junior high school children (11–15 years, 62.0%). |
Clinical features of the epidemic
The eyes were infected bilaterally in 348 (80%) cases and unilaterally in 87 (20%) of the total cases. The clinical signs are shown in Table 1. Of the 783 eyes, conjunctival hyperemia was present in all patients, and 199 eyes (25.4%), 81 eyes (10.3%), and 61 eyes (7.8%) had subconjunctival hemorrhage, superficial punctate keratitis, and preauricular lymphadenopathy, respectively. The total number of patients reported through national surveillance in Japan was 4,094 in Okinawa in 2011 (data not shown). Considering the number of sentinel eye clinics in Okinawa in the national surveillance system, approximately 8% of total clinics, the number of infected patients with AHC in this epidemic was estimated to be 49,000.
  | Table 1 Clinical features of 435 cases of acute hemorrhagic conjunctivitis in Okinawa, 2011 |
Virus isolation, detection, and direct RNA detection in clinical specimens
Ten conjunctival swab samples were collected from AHC patients in Okinawa in 2011, and CA24v were isolated from nine cases. The remaining negative case did not show a positive finding for adenovirus on immunochromatography. The nine patients also showed PCR products of approximately 350 base pairs. Nucleotide sequences of the PCR products were compared with those available in GenBank using BLAST software3 and showed the presence of CA24v in the samples.
Phylogenetic analysis for classification of enteroviruses
When VP4 nucleotide sequences of the nine positive isolates in this study were compared with those of the 66 prototype strains, all isolates in this study showed an identical nucleotide sequence. Its homology rate to the prototype strain of CA24v (EH24/70) was 95.2%. All of them were identified as enterovirus Cluster C as reported by Ishiko et al.19 Among Cluster C enterovirus, CA24v is the sole type inducing keratoconjunctivitis.19
Phylogenetic analysis of VP1 gene
Sequences from the CA24v isolates in Okinawa were compared with those available in GenBank. In Table 2, two examples of tested case sample sequences of the VP1 gene are displayed. Compared with the human coxsackievirus A24 strain China/GD01/2010, registered in National Center for Biotechnology Information, those samples showed 96%–97% identity to human coxsackievirus A24 strain China/GD01/2010. This high homology rate indicates the possibility that the epidemiological origin of our cases might be from geographically neighbor strains, such as as strain China/GD01/2010, in this epidemic.
Discussion
Several large epidemics of AHC have occurred only in Okinawa Prefecture, Japan. CA24v caused the first outbreak in 1985 and 1986,9 and EV70 was the causative agent of the second epidemic in 1994, which has been reported by our group.11 There was a 25-year interval between the present and the last CA24v AHC epidemic in Okinawa, and 17 years have passed since the last AHC epidemic in the same region. It is reported that there is no cross reaction of neutralizing antibodies between EV70 and CA24v.20 Moreover, in spite of the repeated outbreaks caused by CA24v and EV70, antibody to both picornaviruses was not demonstrated in acute sera.20 It has been shown that neutralizing antibody to both CA24v and EV70 does not appear to last long, despite exposure to the viruses from recurring epidemics.21,22 There is a possibility that another AHC epidemic will occur after a considerable period in the near future. Therefore, the decreasing number of people having the antibody, such as school children, may have been a possible resource for this re-emergence. However, it was not explained why CA24v spread so suddenly and widely only on Okinawa Islands without a further epidemic on the Japan mainland. It is conceivable that the climate of the Japan mainland may be unfavorable for dissemination of enterovirus.9
Regarding this epidemic, Nidaira et al have recently reported molecular virological features regarding CA24v isolates from 26 samples on different occasions during the epidemic;23 however, epidemiological and clinical aspects of this epidemic were not described. In this epidemic, teenagers accounted for more than a half of patients. The large proportion of AHC patients comprising junior high school students suggests that AHC spread rapidly owing to the unhygienic habits of students (Figure 3). Person-to-person transmission among school-aged children and their family members seemed to occur simultaneously and accounted for the widespread epidemic on Okinawa Honto Island. Similar epidemic characteristics were reported by our group for the previous AHC epidemic in 1994 due to EV70.24 In this large outbreak, the 11–15-year age group comprised the highest (62.0%) proportion of cases among all the age groups.24 In the former AHC epidemic due to CA24v in Okinawa in 1985–1986, the age-specific attack rate was also highest in the 10–14-year group (20%) and lowest in adults (6%–8%).9 Although clinical and epidemiological features of CA24v AHC epidemics were reported in a few studies, Khan et al reported that the mean age of the patients was 24 years in the Pakistani epidemic,25 and Wu et al reported that 23.9% of cases were students, followed by factory workers (22.8%) and children in kindergartens (16.8%), from a Chinese epidemic.26 These epidemic features suggest a similar tendency of this disease in younger age. Past reports indicate that exclusion from school may actually have curtailed an epidemic,24,27,28 and school exclusion was carried out in some schools in the 2011 Okinawa epidemic. Thus, school exclusion might be effective in the control of AHC epidemics. Because no effective treatment is established for this infectious disease, it is reassuring to note the epidemiological aspects of AHC in order to prevent or control future outbreaks. These aspects include the association of AHC transmission with intrafamily personal hygienic factors.29,30 Climatologically, summer and autumn seem to have favored this AHC epidemic. The most recent epidemic occurred from summer to autumn (Figure 2).
Regarding the clinical findings in AHC, the presence of subconjunctival hemorrhage and superficial punctate keratitis was noted in 10%–70% and 0%–53%, respectively, of patients in past reports.1,2,20,21,24 Preauricular lymphadenopathy has been reported to be found in 4%–40% of AHC cases.1,2,20,21,24 Subconjunctival hemorrhage, superficial punctate keratitis, preauricular lymphadenopathy, and gross chemosis were observed in 25.4%, 10.3%, 7.8%, and 1.8%, respectively, in the most recent epidemic, versus 24.0%, 11.7%, 9.3%, and 2.2%, respectively, in earlier AHC epidemic due to EV70 in 1994.24 During the short period of follow-up in our patients, we did not see any neurological manifestations. Taken together with the consistent absence of neurological complications, the clinical findings for AHC have shown little change over several decades, in contrast with the continuing molecular evolution.
Several epidemics of AHC caused by CA24v have been reported in the last decade in Asia6–8,25,26,31–34 and other continents.12,35 Many of the studies in Asia were carried out in the People’s Republic of China6,26,32,34 and the East Asian countries7,8 neighboring Japan. From the virological analysis by Nidaira et al of the same epidemic, it was noted that the strains from this epidemic in Okinawa in 2011 could have divided in about 2010 from the same lineage detected in other countries, such as the People’s Republic of China,26,34 India,33 and Pakistan.25 These findings suggest that the present CA24v strains causing AHC in these regions are genetically related to each other, and AHC strains emerged around 2010. The high homology rate between our strains and the CA24v Chinese strain (Table 1) supports this hypothesis. From the social and epidemiological standpoints, numbers of immigrants and or overseas travelers from foreign countries outside Japan have been increasing in Japan in recent years. Therefore, it is deemed necessary to take some measures against the spread of imported AHC in Japan, especially in Okinawa. The reason for this re-emergence of CA24v AHC in Okinawa needs further investigation, such as a serological survey of the total population, and a molecular-based investigation is underway.
Acknowledgments
This work was supported by a Grant-in-Aid for Encouragement of Scientists (21592269) from the Ministry of Education, Science, Sports, and Culture of Japan. We thank W Gray for editing this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Chatterjee S, Quarcoopome CO, Apenteng A. Unusual type of epidemic conjunctivitis in Ghana. Br J Ophthalmol. 1970;54(9):628–630. | ||
Mirkovic RR, Kono R, Yin-Murphy M, Sohier R, Schmidt NJ, Melnick JL. Enterovirus type 70: the etiologic agent of pandemic acute haemorrhagic conjunctivitis. Bull World Health Organ. 1973;49(4):341–346. | ||
Arnow AP, Hierholzer JC, Higbee J, Harris DH. Acute hemorrhagic conjunctivitis: a mixed virus outbreak among Vietnamese refugees on Guam. Am J Epidemiol. 1977;105(1):68–74. | ||
Lim KH, Yin-Murphy M. An epidemic of conjunctivitis in Singapore in 1970. Singapore Med J. 1971;4(5):119–127. | ||
Higgins PG, Chapman TE. Coxsackievirus A24 and acute hemorrhagic conjunctivitis in Sri Lanka. Lancet. 1977;1(8007):361. | ||
Yan D, Zhu S, Zhang Y, Zhang J, Zhou Y, Xu W. Outbreak of acute hemorrhagic conjunctivitis in Yunnan, People’s Republic of China, 2007. Virol J. 2010;7:138. | ||
Kuo PC, Lin JY, Chen LC, et al. Molecular and immunocytochemical identification of coxsackievirus A-24 variant from the acute haemorrhagic conjunctivitis outbreak in Taiwan in 2007. Eye (Lond). 2010;24(1):131–136. | ||
Park K, Lee K, Lee J, et al. Acute hemorrhagic conjunctivitis epidemic caused by coxsackievirus A24 variants in Korea during 2002–2003. J Med Virol. 2006;78(1):91–97. | ||
Miyamura K, Yamashita K, Takeda N, et al. The first epidemic of acute haemorrhagic conjunctivitis due to a coxsackievirus A24 variant in Okinawa, Japan, in 1985–1986. Jpn J Med Sci Biol. 1988;41(4):159–174. | ||
Miyamura K. Epidemiological surveillance of acute haemorrhagic conjunctivitis in Japan, 1981–1986. In: Ishii K, Uchida Y, Miyamura K, Yamazaki S, editors. Acute Haemorrhagic Conjunctivitis. Tokyo, Japan: University of Tokyo Press; 1989. | ||
Uchio E, Yamazaki K, Aoki K, Ohno S. Detection of enterovirus 70 by polymerase chain reaction in acute haemorrhagic conjunctivitis. Am J Ophthalmol. 1996;122(2):273–275. | ||
Tavares FN, Campos Rde M, Burlandy FM, et al. Molecular characterization and phylogenetic study of coxsackievirus A24v causing outbreaks of acute hemorrhagic conjunctivitis (AHC) in Brazil. PLoS One. 2011;6(8):e23206. | ||
Hosoya M, Kawasaki Y, Sato M, et al. Genetic diversity of coxsackievirus A16 associated with hand, foot, and mouth disease epidemics in Japan from 1983 to 2003. J Clin Microbiol. 2007;45(1):112–120. | ||
Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16(2):111–120. | ||
Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4):406–425. | ||
Felsenstein J. Estimation of hominoid phylogeny from a DNA hybridization data set. J Mol Evol. 1987;26(1–2):123–131. | ||
Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Symposium on RNA Biology. III. RNA, Tool and Target. Research Triangle Park, NC, USA, October 15–17, 1999. Nucleic Acids Symp Ser. 1999;41:95–98. | ||
Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24(8):1596–1599. | ||
Ishiko H, Shimada Y, Yonaha M, et al. Molecular diagnosis of human enteroviruses by phylogeny-based classification by use of the VP4 sequence. J Infect Dis. 2002;185(6):744–754. | ||
Goh KT, Doraisingham S, Yin-Murphy M. An epidemic of acute conjunctivitis caused by enterovirus-70 in Singapore in 1980. Southeast Asian J Trop Med Public Health. 1981;12(4):473–480. | ||
Yin-Murphy M, Lim KH, Ho YM. A coxsackievirus A24 epidemic of acute conjunctivitis. Southeast Asian J Trop Med Public Health. 1976;7(1):1–5. | ||
Aoki K, Sawada H. Long-term observation of neutralization antibody after enterovirus 70 infection. Jpn J Ophthalmol. 1992;36(4):465–468. | ||
Nidaira M, Kuba Y, Saitoh M, et al. Molecular evolution of VP3, VP1, 3C(pro) and 3D(pol) coding regions in coxsackievirus group A type 24 variant isolates from acute hemorrhagic conjunctivitis in 2011 in Okinawa, Japan. Microbiol Immunol. 2014;58(4):227–238. | ||
Uchio E, Yamazaki K, Ishikawa H, et al. An epidemic of acute haemorrhagic conjunctivitis caused by enterovirus 70 in Okinawa, Japan, in 1994. Graefes Arch Clin Exp Ophthalmol. 1999;237(7):568–572. | ||
Khan A, Sharif S, Shaukat S, Khan S, Zaidi S. An outbreak of acute hemorrhagic conjunctivitis (AHC) caused by coxsackievirus A24 variant in Pakistan. Virus Res. 2008;137(1):150–152. | ||
Wu B, Qi X, Xu K, et al. Genetic characteristics of the coxsackievirus A24 variant causing outbreaks of acute hemorrhagic conjunctivitis in Jiangsu, China, 2010. PLoS One. 2014;9(1):e86883. | ||
Malison MD, Gunn RA, Hatch MH, Bernard KW, White MC. Acute haemorrhagic conjunctivitis, Key West, Florida. Am J Epidemiol. 1984;120(5):717–726. | ||
Onorato IM, Morens DM, Schonberger LB, Hatch MH, Kaminski RM, Turner JP. Acute haemorrhagic conjunctivitis caused by enterovirus 70: an epidemic in American Samoa. Am J Trop Med Hyg. 1985;34(5):984–991. | ||
Patriarca PA, Onorato IM, Sklar VE, et al. Acute haemorrhagic conjunctivitis: investigation of a large-scale community outbreak in Dade County, Florida. JAMA. 1983;249(10):1283–1289. | ||
Waterman SH, Casas-Benabe R, Hatch MH, et al. Acute haemorrhagic conjunctivitis in Puerto Rico, 1981–1982. Am J Epidemiol. 1984;120(3):395–403. | ||
Gopalkrishna V, Patil PR, Kolhapure RM, Bilaiya H, Fulmali PV, Deolankar RP. Outbreak of acute hemorrhagic conjunctivitis in Maharashtra and Gujarat states of India, caused by Coxsackie virus A-24 variant. J Med Virol. 2007;79(6):748–753. | ||
Wu D, Ke CW, Mo YL, et al. Multiple outbreaks of acute hemorrhagic conjunctivitis due to a variant of coxsackievirus A24: Guangdong, China, 2007. J Med Virol. 2008;80(10):1762–1768. | ||
Shukla D, Kumar A, Srivastava S, Dhole TN. Molecular identification and phylogenetic study of coxsackievirus A24 variant isolated from an outbreak of acute hemorrhagic conjunctivitis in India in 2010. Arch Virol. 2013;158(3):679–684. | ||
De W, Huanying Z, Hui L, et al. Phylogenetic and molecular characterization of coxsackievirus A24 variant isolates from a 2010 acute hemorrhagic conjunctivitis outbreak in Guangdong, China. Virol J. 2012;9:41. | ||
Ayoub EA, Shafik CF, Gaynor AM, et al. A molecular investigative approach to an outbreak of acute hemorrhagic conjunctivitis in Egypt, October 2010. Virol J. 2013;10:96. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.