Back to Journals » International Journal of General Medicine » Volume 13
Venovenous Extracorporeal Membrane Oxygenation Combined with Fiberoptic Bronchoscopy–Assisted CO2 Cryotherapy in the Treatment of Massive Hemoptysis in Pregnancy: A Case Report
Received 19 October 2020
Accepted for publication 11 November 2020
Published 27 November 2020 Volume 2020:13 Pages 1291—1296
DOI https://doi.org/10.2147/IJGM.S287666
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Ting Chen,1 Li Yao,1 Chunyan Zhu2
1Department of Critical Care Medicine, The Second People’s Hospital of Hefei, Hefei, Anhui 230009, People’s Republic of China; 2Department of Critical Care Medicine, The First Affiliated Hospital of USTC, Division of Life Science and Medicine, University of Science and Technology of China, Hefei, Anhui 230032, People’s Republic of China
Correspondence: Chunyan Zhu
Department of Critical Care Medicine, The First Affiliated Hospital of USTC, Division of Life Science and Medicine, University of Science and Technology of China, Hefei, Anhui 230032, People’s Republic of China
Tel +86-551-62282731
Fax +86-551-62283409
Email [email protected]
Background: Massive hemoptysis in pregnancy is very rare but can be life-threatening for both the pregnant woman and fetus. Extracorporeal membrane oxygenation (ECMO) is extremely rare in the treatment of severe hemoptysis in pregnancy. Here we describe the case of massive hemoptysis in the second trimester of pregnancy successfully treated with a combination of venovenous (VV)-ECMO, and bronchial artery embolization combined with fiberoptic bronchoscopy–assisted CO2 cryotherapy.
Case Presentation: A 34-year-old patient at 28 2/7 weeks gestation with a history of hemoptysis for 3 days was transferred to our care. Massive hemoptysis completely blocked the trachea and main bronchus, and a ventilator could not carry out ventilation. ECMO was performed immediately when oxygenation was not maintained. A right lower bronchial artery hemorrhage was found by bronchial arteriography under ECMO, and embolization with microcoils and gelatin sponge particles was then performed. An emergency bedside carbon dioxide cryo-thrombectomy was performed under fiberoptic bronchoscopy because of obstruction of the trachea and main bronchus. Endotracheal cryotherapy was repeated (for a total three times) until bronchoscopic evaluation confirmed no obstruction of the trachea and no active bleeding in the airway. On day 7, ECMO was successfully evacuated. On day 15, the patient was extubated. On day 17, the tracheotomy was closed and replaced by nasal oxygen inhalation. On day 20, the patient was discharged from hospital. The patient has had no recurrence of hemoptysis in 3-month follow-up.
Conclusion: VV-ECMO combined with carbon dioxide cryotherapy in the treatment of pregnancy complicated with massive hemoptysis is an effective treatment, when massive hemoptysis completely blocked the trachea.
Keywords: VV-ECMO, bronchial artery embolization, carbon dioxide freezing, pregnancy, massive hemoptysis
Introduction
Massive hemoptysis in pregnancy is very rare, and no specific incidence rate has been reported. The common causes of hemoptysis include bronchiectasis, pulmonary infection, lung cancer, and pulmonary embolism.1 In the case of bronchial artery hemorrhage, embolization is recognized as a safe and effective treatment method.2 Carbon dioxide cryotherapy is another therapeutic option. Carbon dioxide cryotherapy is easy to use and has few technical requirements, making it suitable for bedside procedures in the intensive care unit, and it has become an important method of treating airway obstruction caused by intratracheal tumor;3 however, there are few reports on its use for the removal of blood clots formed during pulmonary hemorrhage. Extracorporeal membrane oxygenation (ECMO) has been successfully used for various reversible and refractory cardiopulmonary conditions, such as acute respiratory distress syndrome, massive pulmonary embolism, and fulminant myocarditis, and the number of cases reported has been increasing.4 Improved understanding of the physiological effects of ECMO on the pregnant woman and fetus has also led to its increased use in treatment of such conditions as perinatal cardiomyopathy, myocarditis, acute respiratory distress syndrome, and amniotic fluid embolism.5 Nevertheless, there is little report of its use in maternal massive hemoptysis.
Herein, we report a case of massive hemoptysis in the second trimester of pregnancy successfully treated with the a combination of venovenous (VV) ECMO, and fiberoptic bronchoscopy–assisted CO2 cryotherapy.
Case Report
A 34-year-old patient weighing 60 kg and 162 cm in height at 28 2/7 weeks gestation with a history of hemoptysis for 3 days and dyspnea for 2 days was transferred to our care 10 hours after emergency tracheotomy at a local hospital. The patient reported a past history of hemoptysis 20 years earlier, which had resolved without treatment. Three days prior to admission, the patient had a spontaneous occurrence of hemoptysis, with loss of approximately 10–20 mL of bright-red blood. The following day (2 days prior to admission), the hemoptysis increased to 100 mL and was accompanied by cyanosis (blue lips), which prompted the patient to visit the local hospital. Computed tomography (CT) of the lungs at that time revealed an infiltrative shadow in the left upper lung. After initial hemostasis and phentolamine treatment, hemoptysis recurred 6 hours after admission and was accompanied by markedly decreased oxygenation –radial artery blood gas analysis revealed an oxygen partial pressure (PO2) of 42 mmHg. An emergency endotracheal intubation was performed, and negative pressure suction was applied to assist ventilation, with maintenance of arterial oxygen saturation (SaO2) at approximately 95%. Subsequently, a tracheotomy was performed, and the patient was transferred by ambulance to our hospital.
On admission, the patient was conscious and had a transcutaneous oxygen saturation (SpO2) of 95%. She was scheduled to undergo a CT pulmonary angiogram; however, the hemoptysis increased significantly, preventing the examination. A lung CT scan was done instead and showed large infiltrating shadows in both lungs. After admission to the intensive care unit, the patient coughed up a large amount of pink blood and demonstrated obvious symptoms of airway obstruction. The ventilator parameters were then set to a tidal volume 350 mL, respiratory rate 20 breaths/min, positive end-expiratory pressure (PEEP) 5 cmH2O, inspiratory time 1.0 s, oxygen concentration 100%, and the actual delivered tidal volume 125 mL. Fiberoptic bronchoscopy revealed the trachea and main bronchi to be completely blocked by blood clots (Figure 1). Blood gas analysis showed PO2 50 mmHg, partial pressure of carbon oxide (PCO2) 76 mmHg, and pH 7.102. At the same time, the fetal heart rate decreased, from 150 beats/min to 50–60 beats/min. Obstetrician consultation suggested urgent correction of hypoxia and fetal heart rate monitoring. Multidisciplinary consultation with Thoracic Surgery, Respiratory Medicine, and Interventional Radiology suggested management with bronchial artery embolization performed with VV-ECMO support.
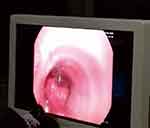 |
Figure 1 Blockage of trachea by blood clots. |
A 17 French ECMO catheter (Duraflo, Edwards Lifesciences; Irvine, CA, USA) was inserted via the right internal jugular vein, between the superior vena cava and right atrium; a 19 French catheter was inserted into the femoral vein and advanced under B-mode ultrasound guidance. Then, both catheters were connected to their respective ECMO sheath. The ECMO was set to a flow rate of 4.0 L/min, with a pump speed of 3000 r/min and return pressure of 120 mmHg. Following the establishment of VV-ECMO, radial artery blood gas showed pH 7.35, PO2 389 mmHg, PCO2 34 mmHg, and SaO2 100%, and the fetal heart rate increased to 120 beats/min.
Prior to transfer to the operating room for embolization, hemoptysis resumed. As the airway became completely obstructed, the tidal volume fell to 50 mL, and SpO2 decreased to 60%. Midazolam (0.15mg/kg) and continuous remifentanil (0.1 µg/kg/min) were administered for analgesia and sedation and vecuronium (0.1 mg/kg injected intermittently) for neuromuscular block. Following this, the PO2 rose to 88%, and the patient was immediately brought to the operating room for embolization. Embolization, with microcoils and gelatin sponge particles (350–560 µm; Hangzhou Alekang), was then performed (Figure 2), and the patient was transferred back to the intensive care unit, where a repeat fiberoptic bronchoscopy showed complete airway obstruction. Repeated irrigation and aspiration were performed, yielding only a small amount of clot. An emergency bedside carbon dioxide cryo-thrombectomy (Kooland 300 Flexible Cryoprobe, Beijing Kulan Medical Treatment Equipment Co., Ltd.; Beijing, China) was performed under fiberoptic bronchoscopy (Olympus bf-260; Germany). Endotracheal cryotherapy was repeated (for a total three times) until bronchoscopic evaluation confirmed no obstruction of the trachea and no active bleeding in the airway (Figure 3).
 |
Figure 2 Contrast agent spilling from the distal end of the right pulmonary bronchial artery. |
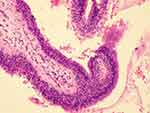 |
Figure 3 Histopathological examination of a clot specimen showed degenerative necrotic tissue but no epithelial cell component, and congestion and bleeding in the bronchial mucosa. |
At 24 hours on ECMO, heparin anticoagulation was confirmed with the finding of activated partial thromboplastin time (aPTT) 55–60 seconds. However, on day 4 of admission, the fetal heart sounds abruptly stopped, and fetal monitoring confirmed an absent heartbeat. The obstetrician diagnosed stillbirth and suggested cesarean section once the patient stabilized. On day 6, only a small exudative shadow was seen in the right lower lung on bedside chest X-ray. On day 7, oxygenation was stopped for 2 hours, and the heart rate, blood pressure, and SpO2 remained unchanged, and blood gas analysis showed pH 7.36, PO2 86 mmHg, and PCO2 38.2 mmHg. Routine lower extremity vascular ultrasound on this day revealed intramuscular venous thrombosis in both lower limbs, and this prompted weaning from ECMO. On day 9 of admission, the cesarean section was performed. On day 11, bronchoscopy confirmed no obstruction in the trachea or in either main bronchi; CT pulmonary angiogram showed little inflammation bilaterally and no obstruction of the pulmonary artery or its branches. On day 15, after a successful spontaneous breathing trial, the patient was extubated, and oxygen therapy via tracheotomy was commenced. On day 17, the tracheotomy was closed and replaced by nasal oxygen inhalation. Finally, on day 20, the patient was discharged from hospital. At 3-month follow-up, there was no hemoptysis, and a few cord-shaped shadows remained in the left upper lung on CT scan (Figure 4).
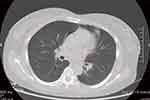 |
Figure 4 Computed tomography of the lung showed a few cords in the left upper lung. |
Discussion
While there is no consensus regarding definition, massive hemoptysis is understood as bleeding in the trachea, bronchus, and lung tissue in varying amounts exceeding 100 mL or up to 600 mL in 24 hours, which compromises lung function. Massive hemoptysis is commonly seen in chronic underlying diseases, such as tuberculosis and lung cancer, and may occur as an early symptom. The incidence of massive hemoptysis in pregnancy is low, but it is generally dangerous, posing serious threat to the safety of both the pregnant woman and the fetus.6 The cause of hemoptysis in our patient was confirmed to be bronchial artery rupture, which, in turn, may have been caused by increased blood volume and changes in hormone levels related to the pregnancy (the etiology remains unclear). The patient’s trachea was completely blocked by clotted blood, and an artificial airway was ineffective in enabling ventilation. With the support of a multidisciplinary team, we successfully used VV-ECMO to treat our pregnant patient with massive hemoptysis.
ECMO describes extracorporeal circulation equipment with a lung membrane and centrifugal pump as the core components, and can save time when treating reversible heart and lung diseases.4 With progressive developments in ECMO, it has been used in an increasingly wider range of clinical situations, from recovery after major cardiac surgery or the wait to lung transplantation, to use in various critical conditions, including cardiopulmonary resuscitation, acute myocardial infarction, severe fulminant myocarditis, and poisoning. Thus, ECMO has been playing an increasingly pivotal role in clinical practice. The use of ECMO during pregnancy has also been previously reported,7 in the context of H1N1 infection, amniotic fluid embolism, and perinatal cardiomyopathy. Despite this, ECMO is not commonly used in treatment of pregnant women, and VV-ECMO has rarely been used in the treatment of massive hemoptysis in pregnancy. One reason is that ECMO requires a skilled and specialized management team; more important, however, are concerns about the effects of ECMO anticoagulation and hemodynamics in pregnancy. Moore et al9 found the survival rate for pregnant women receiving ECMO treatment to be 77.8% and the fetal survival rate to be 65%. In the present case, massive hemoptysis completed obstructed the trachea and left and right main bronchi, and the patient was obviously cyanotic. ECMO was the only option and was prescribed.
Ramanathan et al10 summarized 280 cases of pregnant women placed on VV- or venoarterial (VA)-ECMO treatment from 1997 to 2017; among them, the indications for VV-ECMO in pregnancy included: severe reversible or irreversible respiratory failure; hypercapnia with severe respiratory acidosis despite optimal conventional mechanical ventilation at >35 breaths/min; and ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2)<100 at oxygen concentration ≥90% and PEEP ≥10 cm H2O, despite use of ventilation measures such as low tidal volume, appropriate PEEP, lung recruitment maneuvers, and prone position ventilation. The indications for VA-ECMO included: refractory left heart failure caused by perinatal cardiomyopathy, myocarditis, and myocardial infarction; amniotic fluid embolism; prolonged cardiopulmonary resuscitation following bupivacaine poisoning; and large pulmonary embolism. Our patient met the first two criteria for VV-ECMO, and, prior to ECMO, both left and right ventricular contractions were normal, left ventricular ejection fraction was 65%, and stroke volume was 65 mL, so we elected VV-ECMO assistance. VV-ECMO was started at pump speed 3,500 rpm, flow 4.0 L/min, and flow/cardiac output ≥0.6, in line with Levy et al,11 who reported ECMO management requirements of continuous hypoxemia and SaO2 >88%.
Complications, such as hemorrhage, thrombosis, and thrombocytopenia, are not uncommon with ECMO. The physiological changes of pregnancy, the increase of volume and cardiac output, especially the changes of coagulation system, increase the difficulty of anticoagulant management for ECMO treatment during pregnancy. One third of patients with ECMO during pregnancy have been reported to have bleeding.12,13 Notably, our patient experienced all the aforementioned complications. Bleeding occurred at the right femoral vein puncture site, leaving a hematoma (from the joint action of puncture injury and heparin anticoagulation) despite local compression; Significantly, the hemoptysis, which immediately stopped after bronchial artery embolization, remained controlled for the duration of heparin therapy. As per the standards for ECMO anticoagulation management described by the Extracorporeal Life Support Organization,14 unfractionated heparin therapy was initiated for anticoagulation 24 hours after ECMO was established, with a target aPTT of 55–60 seconds. Despite the clinical benefits of unfractionated heparin (among which is its inability to cross the placenta), its use requires special attention in monitoring for the occurrence of thrombocytopenia, which, according to Reese et al,15 occurs in as many as 8% of pregnancies. Thrombocytopenia is mainly an ECMO centrifugal pump, pipeline adsorption, and activated coagulation. If platelets fall below 20,000/mL, platelet transfusion is required. While our patient was thrombocytopenic, her platelets were maintained at ≥50,000/mL, and transfusion was unnecessary. Thrombosis is another common complication of ECMO, occurring mainly at the puncture site, and may lead to life-threatening pulmonary embolism. Indeed, before withdrawing ECMO, we found bilateral intermuscular venous thrombosis in our patient, which required anticoagulation therapy. Upon examination at 2 weeks post-discharge, thrombosis was resolved.
The effect of ECMO on the fetus is unclear at present.8 In the present case, the fetal heart rate suddenly disappeared at day 5 after ECMO initiation. Possible factors in the fetal demise include: hypoxia that occurred prior to the start of ECMO; stimulation of the uterus related to catheterization of the femoral jugular vein; and secondary effects of the imaging agent (iodine contrast), analgesic/sedative and muscle relaxant, and the antibiotics required during the ECMO intervention. It may be that preferential selection of a dual-lumen tube and catheterization through only the distant jugular vein will avoid trauma to the uterus and reduce the impact of drugs on the fetus. Despite the reports of successful use of ECMO to preserve the lives of pregnant women,16 the effects of ECMO on the fetus warrant further investigation.
In our patient, the massive hemoptysis led to the formation of clots that completely blocked the trachea and required removal. To perform the procedure, we elected to use bronchoscopy in combination with CO2 cryotherapy. Use of a rigid bronchoscope, while providing superior direct suctioning ability,17 has complex technical requirements, and, thus, we opted to use a fiberoptic bronchoscope. Previously, the combination of fiberoptic bronchoscopy and CO2 cryotherapy has mainly been used in minimally invasive removal of tracheal tumors; however, it has promise as a simple and effective method for removal of clots obstructing the trachea after massive hemoptysis. The use in our case provides further evidence of the feasibility, previously proposed by Schmidt et al,18 of using bedside fiberoptic bronchoscopy for blood clot removal in critically ill patients with pulmonary hemorrhage.
In conclusion, the incidence of massive hemoptysis in pregnancy is low but poses a serious threat to the health of both the pregnant woman and the fetus. ECMO technology is now simpler and safer because of improvements addressing biocompatibility and anticoagulation, and can be considered for respiratory function support in otherwise fatal hemoptysis of pregnancy. Further, fiberoptic bronchoscopy–assisted CO2 cryotherapy can provide a simple and effective method for bedside clearance of clots in critically ill patients with massive hemoptysis.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author, Chunyan Zhu.
Ethics and Consent Statement
Based on the regulations of The Second People’s Hospital of Hefei, institutional review board approval is not required for case reports.
Consent for Publication
Written informed consent has been provided by the patient and her guardian to have the case details and any accompanying images published.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no conflicts of interest to declare.
References
1. Lordan JL, Gascoigne A, Corris PA. The pulmonary physician in critical care illustrative case 7: assessment and management of massive haemoptysis. Thorax. 2003;58(9):814–819. doi:10.1136/thorax.58.9.814
2. Jean-Baptiste E. Clinical assessment and management of massive hemoptysis. Crit Care Med. 2000;28(5):1642–1647. doi:10.1097/00003246-200005000-00066
3. El-Helbawya R, Hussein SA, Tawab AA. Fiberoptic bronchoscopic cryotherapy for endobronchial lung cancer: outcomes and predictors of success. EJCT. 2019;68(3):394–403.
4. Eleuteri K, Koerner MM, Horstmanshof D, El Banayosy A. Temporary circulatory support and extracorporeal membrane oxygenation. Cardiol Clin. 2018;36(4):473–485. doi:10.1016/j.ccl.2018.06.002
5. Loyaga-Rendon RY, Pamboukian SV, Tallaj JA, et al.Outcomes of patients with peripartum cardiomyopathy who received mechanical circulatory support.Data from the interagency registry for mechanically assisted circulatory support. Circ Heart Fail. 2014;7(2):300–309. doi:10.1161/CIRCHEARTFAILURE.113.000721
6. Stiff A, Harrison R, Palatnik A. Case report of massive hemoptysis in pregnancy requiring veno-venous extracorporeal membrane oxygenation. J Obstet Gynaecol Res. 2019;45(12):2452–2455. doi:10.1111/jog.14110
7. Saad AF, Rahman M, Maybauer DM, et al. Extracorporeal membrane oxygenation in pregnant and postpartum women with H1N1-related acute respiratory distress syndrome: A systematic review and metaanalysis. Obstet Gynecol. 2016;127(2):241–247. doi:10.1097/AOG.0000000000001236
8. Pacheco LD, Saade GR, Hankins GDV. Extracorporeal membrane oxygenation (ECMO) during pregnancy and postpartum. Semin Perinatol. 2018;42(1):21–25. doi:10.1053/j.semperi.2017.11.005
9. Moore SA, Dietl CA, Coleman DM. Extracorporeal life support during pregnancy. J Thorac Cardiovasc Surg. 2016;151(4):1154–1160. doi:10.1016/j.jtcvs.2015.12.027
10. Ramanathan K, Tan CS, Rycus P, et al. Extracorporeal membrane oxygenation in pregnancy: an analysis of the extracorporeal life support organization registry. Crit Care Med. 2020;48(5):696–703. doi:10.1097/CCM.0000000000004269
11. Levy B, Taccone FS, Guarracino F. Recent developments in the management of persistent hypoxemia under veno-venous ECMO. Intensive Care Med. 2015;41(3):508–510. doi:10.1007/s00134-014-3579-y
12. Costantine MM. Physiologic and pharmacokinetic changes in pregnancy. Front Pharmacol. 2014;5:65. doi:10.3389/fphar.2014.00065
13. Agerstrand C, Abrams D, Biscotti M, et al. Extracorporeal membrane oxygenation for cardiopulmonary failure during pregnancy. Ann Thorac Surg. 2016;102:774–779. doi:10.1016/j.athoracsur.2016.03.005
14. Extracorporeal Life Support Organization ELSO guidelines 2009. Available from: http://www.elso.med.umich.edu/WordForms/ELSO%20Guidelines%20General%20All%20ECLS%20Version21.21.pdf.
15. Reese JA, Peck JD, Deschamps DR, et al. Platelet counts during pregnancy. New Engl J Med. 2018;379(1):32–43. doi:10.1056/NEJMoa1802897
16. Sharma NS, Wille KM, Bellot SC, Diaz-Guzman E. Modern use of extracorporeal life support in pregnancy and postpartum. ASAIO J. 2015;61(1):110–114. doi:10.1097/MAT.0000000000000154
17. Batra H, Yarmus L. Indications and complications of rigid bronchoscopy. Expert Rev Respir Med. 2018;12(6):509–520. doi:10.1080/17476348.2018.1473037
18. Schmidt LH, Schulze AB, Goerlich D, et al. Blood clot removal by cryoextraction in critically ill patients with pulmonary hemorrhage. J Thorac Dis. 2019;11(10):4319–4327. doi:10.21037/jtd.2019.09.46
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
