Back to Archived Journals » Journal of Vascular Diagnostics and Interventions » Volume 2
Vascular diagnostics for Raynaud's phenomenon
Authors Dinsdale G, Herrick A
Received 1 July 2014
Accepted for publication 5 August 2014
Published 3 October 2014 Volume 2014:2 Pages 127—139
DOI https://doi.org/10.2147/JVD.S52943
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Rahmi Oklu
Graham Dinsdale, Ariane L Herrick
Centre for Musculoskeletal Research, Institute of Inflammation and Repair, Salford Royal NHS Foundation Trust, University of Manchester, Manchester Academic Health Science Centre, Manchester, UK
Abstract: Raynaud's phenomenon (RP) is common, and in most patients is primary (idiopathic) when due to reversible vasospasm and does not progress to irreversible tissue injury. However, in those patients for whom RP is secondary to an underlying disease (eg, systemic sclerosis or atherosclerosis), progression to digital ulceration or critical ischemia can occur. Therefore, the key question for the clinician is “Why does this patient have RP?” Vascular diagnostics play a key role in answering this. In this review, we firstly discuss the different vascular investigations relevant to clinical practice: nail fold capillaroscopy (including the different methodologies for examining the nail fold capillaries, and the role of capillaroscopy in helping to differentiate between primary and systemic sclerosis-related RP), thermography (available in specialist centers), and evaluation of large vessel disease (for example, due to atherosclerosis). We then discuss research tools, mainly laser Doppler methods, including laser Doppler imaging and laser speckle contrast imaging. These are commercially available as complete imaging systems and are (relatively) easy to use. The main current goal in vascular imaging research is to validate these novel state-of-the-art techniques as outcome measures of digital vascular disease, and then apply them in early and later phase studies of new treatment approaches, thus facilitating drug development programs.
Keywords: Raynaud's phenomenon, systemic sclerosis, nail fold capillaroscopy, thermography, laser Doppler, angiography
Introduction
Raynaud’s phenomenon (RP), characterized by episodic color change of the fingers, usually as a result of cold exposure, is very common. The classic sequence of color changes is firstly white (ischemia), then blue (deoxygenation), and then red (reperfusion), although not all patients describe the complete triphasic change. Prevalence estimates vary widely, reflecting differences between studies in the definition of RP and in populations (eg, community-based or hospital-based). Some studies have reported a very high prevalence, with a UK study suggesting that as many as 21% of women and 16% of men attending general practice surgeries had RP,1 and a US community-based study reporting RP in 11% of women and 8% of men.2
RP is a symptom complex, and not a diagnosis. Therefore, when confronted by a patient who describes symptoms of RP, the first question the clinician must ask him/herself should be “What is the cause of this patient’s Raynaud’s?” The vast majority of patients with RP have primary (idiopathic) RP, which is a “benign” phenomenon in that it does not progress to tissue injury, eg, ulceration, digital pitting, or gangrene. In primary RP, the abnormality is thought to be reversible vasospasm (structural vascular changes do not occur). Most people with primary RP never seek medical advice.
In contrast, when RP occurs secondary to a number of different diseases/conditions, it can be very severe. The main causes of secondary RP are listed in Table 1. Many of these conditions are associated with structural vascular change and/or with intravascular (“circulating”) abnormalities which, singly or in combination, can further compromise blood flow. Systemic sclerosis, a multisystem connective tissue disease, is a good example of how the pathophysiology of RP can be complex and lead to very severe ischemia. In patients with systemic sclerosis, episodic vasospasm (functional change) is superimposed upon structural abnormalities of the digital arteries and microvasculature, and in addition, several intravascular factors (including increased platelet aggregation) may further compromise perfusion.3 Of all the causes of RP, systemic sclerosis-related RP has been the most researched.
  | Table 1 Main differential diagnosis of (and associations with) Raynaud’s phenomenon |
Vascular diagnostics play a key role in the diagnosis of RP by helping to separate primary from secondary RP and, in patients with secondary RP, identifying those with large vessel disease that might be amenable to surgical intervention. They can also play a role in the assessment of disease severity. In this review, we focus on the techniques of most interest to the practicing clinician: nail fold capillaroscopy, thermography, and assessment/imaging of large vessels by Doppler ultrasound and angiography. We also discuss laser Doppler methods, as these are being increasingly used in RP research studies, and briefly mention some other research methodologies. Circulating markers of vascular injury and measurement of tissue oxygenation are not discussed. In light of recently suggested diagnostic criteria for RP, it should be noted that although not all clinical centers will have access to all (or any) of these techniques, they are considered to be the cutting edge, particularly in relation to vascular research.4 Before describing the different methods, we describe the approach to the patient with RP, exemplified by three clinical scenarios to help put the problems into context.
Approach to the patient with RP
The clinician must fully assess the patient in order to diagnose the cause of the RP (Table 1) and assess its severity. This involves a careful history and examination (with particular attention given to the hands and feet), and a series of investigations usually including one or more vascular investigations.
Scenario 1
A 22-year-old woman was referred to the rheumatology clinic with a 5-year history of RP. Her feet were also affected. On examination there were no abnormalities. Specifically, there was no thickening of the skin of the fingers (sclerodactyly) or digital pitting, and all peripheral pulses were easily felt. Her full blood count and biochemical profile were normal and antinuclear antibody was negative. The rheumatologist arranged for nail fold capillaroscopy and this showed no abnormalities (Figure 1, top). Normal nail fold capillaries are one of the criteria for primary RP proposed by LeRoy and Medsger5 (others include episodic attacks of acral pallor or cyanosis, strong and symmetric peripheral pulses, no evidence of digital pitting, ulceration or gangrene, negative antinuclear antibody [titer <1/100], and normal erythrocyte sedimentation rate). Thermographic testing showed good rewarming after a cold challenge, albeit after a delay of 10 minutes. Additionally, although the fingers were cool at a room temperature of 23°C, at 30°C there were no persisting temperature gradients along any of the fingers (Figure 1, bottom). A functional abnormality was therefore confirmed, consistent with a diagnosis of primary RP.6
The mainstay of management for the patient in this scenario is to keep warm, for example, dressing warmly when out of doors. If, in future, troublesome symptoms continue, then vasodilator therapy could be considered. A diagnosis of primary RP usually allows a patient to be reassured and discharged from follow-up.
Scenario 2
A 50-year-old woman was referred to the rheumatology clinic with a 10-year history of RP that had become worse over the previous 12 months. Over the preceding winter, she had had two finger ulcers that had been slow to heal. On examination the fingers were puffy but there was no definite sclerodactyly. There was no digital pitting and all peripheral pulses were easily felt. Limited cutaneous systemic sclerosis was suspected and confirmed when investigation results included a positive anticentromere antibody and abnormal capillaroscopy, with several enlarged capillaries and areas of hemorrhage.
Abnormal capillaroscopy is a predictor of development of systemic sclerosis in the patient presenting with RP.7,8 However, this patient did not have “isolated” RP in that she had progressed to digital ulceration. With her RP, puffy fingers, digital ulcers, anticentromere antibody, and abnormal nail fold capillaries, she fulfilled the 2013 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) criteria for systemic sclerosis.9 Capillaroscopic abnormalities as demonstrated in Figure 2 are highly specific for an underlying systemic sclerosis spectrum disorder, and have been included in previous classification criteria for early systemic sclerosis.10–12 Therefore, unlike the patient described in scenario 1, this patient must be kept under review and screened for development of internal organ involvement related to systemic sclerosis.
Scenario 3
A 79-year-old man presented to his general practitioner with a 6-month history of a feeling of coldness in his left hand associated with tingling fingers that turned pale, then blue and mottled, and were aggravated by cold weather. His right hand was not affected. He had a history of hypertension and had stopped smoking 20 cigarettes a day 1 year previously.
On examination, there was no sclerodactyly or digital pitting, but the left radial pulse was weaker than the right. Full blood count and biochemical profile were normal and nail fold capillaroscopy was normal. Arterial Doppler ultrasound showed reduced brachial, ulnar, and radial pressures on the left side compared with the right. A referral to the vascular surgical team was made, and subsequent X-ray angiography showed a left subclavian stenosis that was treated with angioplasty.
In this situation, the RP is asymmetrical, meaning that large vessel disease (or external vascular compression) should be suspected. The key clinical finding is the asymmetrical nature of the pulses. Arterial Doppler ultrasound was supportive of the clinical suspicion of proximal large vessel disease and angiography was diagnostic. Angioplasty was performed at the same time, meaning that the procedure was both diagnostic and therapeutic.
Nail fold capillaroscopy
Rationale
At the nail fold, the capillaries run parallel rather than perpendicular to the skin surface. They can be well visualized by nail fold capillaroscopy, which therefore provides a window into the finger microcirculation and is part of the routine assessment of any patient presenting with RP. As such, capillaroscopy is the vascular diagnostic test most commonly used in the assessment of RP. Its availability and application are likely to increase over the next few years because of the recent publication of the 2013 ACR/EULAR criteria for systemic sclerosis,9 which include abnormal capillaroscopy. Essentially, these new criteria require the clinician to perform capillaroscopy on anyone in whom the diagnosis of systemic sclerosis is suspected, and in whom RP is often the first clinical feature (as in scenario 2).
Methods
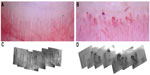
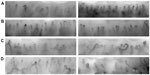
There are several different methods used to perform capillaroscopy (Figure 3). Ideally, for clinical purposes all the nail beds should be examined, because there can be considerable heterogeneity between nail folds (Figure 4), and diagnostic changes (for example, giant capillaries) might be missed if only (say) two fingers are examined. However, examining all nail folds can be time-consuming, depending on the exact technique used, and current research is ongoing to address the question as to how many nail folds must be examined to preserve sensitivity while at the same time recognizing the time constraints of busy clinical practice.
Widefield microscopy
This was the original technique used by Maricq and LeRoy in their seminal work on capillaroscopy dating from the 1970s.13,14 A drop of oil is applied to the nail bed to improve visualization; the oil fills in microscopic surface irregularities and consequently reduces specular reflection and decreases light scatter. The nail bed is then examined using a light microscope, usually at a magnification of around 12–14×. A standard digital camera is often coupled to the microscope to allow capture of nail fold images for later review or for comparison with previous time points. An advantage of the low magnification is that the whole of the nail fold can be seen (or imaged) “at a glance”, allowing the clinician to detect easily whether the capillary pattern is normal (classically with even “hairpin” loops) or whether there are one or more abnormalities (enlarged capillaries including “giant” capillaries, areas of avascularity, hemorrhage, or distortion of the normal capillary architecture). The widefield technique is used primarily for diagnosis: the low magnification limits accurate measurement of capillary density and dimensions which may be useful in research studies to document change over time.
Video capillaroscopy
This is currently considered the gold standard for imaging capillary architecture,15,16 but tends to be available only in specialist centers. It is used in both clinical practice and in research. Video capillaroscopy (Figure 3, bottom left and right) is an extension of the widefield technique but using much higher magnifications (in the order of 200–600×). The microscope is connected to a video camera. The high magnification allows measurement of capillary density and dimensions, which can be done using commercially available software. Images are captured digitally and can be stored for the reporting clinician, and also for the researcher who may wish to perform more complex quantitative assessment. There are different commercially available systems, some incorporating a hand-held probe, whereas others have a fixed microscope format (Figure 3, bottom left). A fixed microscope format can prove problematic when applied in patients with systemic sclerosis, many of whom have fixed flexion deformities of their fingers. As with the widefield technique, a drop of oil is used to improve visualization of capillaries. One disadvantage of most commercially available systems is that only a small area of the nail fold is visualized at any one time; abnormalities may therefore be missed unless the microscope is “panned” across the whole nail fold, since capillary morphology can be very heterogeneous, not only between but also within individual nail folds (Figure 4). For this reason, computer software has been developed that can build up a mosaic of single image frames covering the entire nail fold, thereby combining the advantages of high magnification with the ability to view the whole nail fold.17
Dermoscopy and other lower magnification techniques
The hand-held dermatoscope (Figure 3, top) allows nail fold capillaries to be examined at a magnification of around 10×, comparable with the widefield technique. As with other capillaroscopy techniques, a drop of oil on the nail fold prior to viewing will increase vessel visualization. A recent study suggested good reliability of dermoscopy among rheumatologists,18 and it is likely that interest in dermoscopy will increase, because this will allow practicing rheumatologists to perform diagnostic capillaroscopy without having to invest in relatively expensive equipment. Dermatoscopes can be coupled to a standard digital camera (or even a smartphone), allowing images to be recorded.
Some rheumatologists use the opthalmoscope,19 but this has the disadvantage of showing only a small field of view and does not give as good visualization as other techniques. It is possible that low-cost USB microscopes will be further used by rheumatologists. These cheap, plastic devices can provide images of a standard comparable with dermoscopy, and plug directly into a computer for image capture, making them potentially a good choice for clinical use.
Clinical applications in patients with RP
The main clinical application of capillaroscopy is for diagnosis of an underlying systemic sclerosis spectrum disorder in the patient presenting with RP. It has long been recognized that the “scleroderma pattern” of capillary abnormality (with dilated loops, areas of avascularity, and hemorrhage), is highly specific for systemic sclerosis. Further classification of this “scleroderma pattern” into “early”, “active”, and “late” subclasses has highlighted the association between the progression of capillary abnormality and overall disease duration.20 For comparison, the “normal” spectrum of capillary architecture has also been described,21 with the majority of subjects having around seven capillaries per mm of mostly a uniform U shape.
The most definitive study examining prediction of systemic sclerosis in patients with “isolated” RP was by Koenig et al,7 who followed 586 patients for 3,197 patient-years (median duration of follow-up, 4 years). Systemic sclerosis developed in 1.8% of those with neither abnormal capillaroscopy nor a systemic sclerosis-specific autoantibody, in 25.8% with abnormal capillaroscopy, in 35.4% with a specific autoantibody, and in 79.5% with both predictors.7 Patients with both abnormal capillaroscopy and a specific autoantibody were 60 times more likely to develop systemic sclerosis than those with neither. This study provides the evidence base for reassuring the patient described in scenario 1 that she is unlikely to develop systemic sclerosis.
Although it has been shown that the degree of capillaroscopic abnormality is predictive of the risk of digital ulceration in a patient with systemic sclerosis,22,23 this finding has not yet impacted on clinical practice. Also, although capillaroscopic abnormalities have been reported in other connective tissue diseases, eg, hemorrhages in antiphospholipid syndrome24 and non-specific changes in systemic lupus erythematosus,25 these findings have been relatively little studied. Capillaroscopic changes observed in just 35.8% of cases in systemic lupus erythematosus may be too non-specific to be of diagnostic significance,25 although if changes are observed in a patient with systemic lupus erythematosus the possibility of an overlap condition with (eg) systemic sclerosis should be considered.
Research applications in patients with RP
Measurement of disease progression and treatment response
High magnification video capillaroscopy brings the potential of quantifying the structural microvascular abnormality, and thereby providing a biomarker of microangiopathy related to systemic sclerosis. Several authors have included capillaroscopy in reports of treatment response.26,27 This is an exciting area of research. Challenges to overcome include ensuring that the same part of the nail bed is examined on each occasion over time28 (therefore ideally the whole nail bed should be examined) and how best to quantify abnormality.
As a predictor of digital ulceration and internal organ involvement
This is an area of current research.29 Different investigators have found associations between the severity of capillaroscopic abnormality and disease “complications”, eg, digital ulceration22,23 and pulmonary arterial hypertension.30 These findings could potentially help to identify “at risk” patients non-invasively and at an early stage, facilitating early intervention.
Measurement of red blood cell velocity
While velocity can be measured using commercially available software, this is a highly complex area of research. For example, flow rates vary with respiration and temperature, and often between adjacent capillaries. This is an area requiring further research, because accurate measurement of velocity would allow development of protocols of dynamic testing (eg, to a standard temperature challenge) which could pave the way for an outcome measure (sensitive to change over short time periods) in clinical trials of RP.
Fluorescence video microscopy
This technique assesses capillary permeability, which is increased in patients with systemic sclerosis.31 However, the technique is invasive (a fluorescent contrast agent must be injected) and therefore unlikely to be widely used.
Conclusion
Nail fold capillaroscopy is rapidly gaining ground as an essential diagnostic test for the clinician who assesses patients with RP. There are different methods, ranging from the easy-to-use, hand-held dermatoscope through to high magnification video capillaroscopy. Research is ongoing, including into trying to establish capillaroscopy as a biomarker of microvascular disease related to systemic sclerosis.
Infrared thermography
Rationale
Infrared thermography measures skin surface temperature and is therefore an indirect measure of blood flow in both small and large blood vessels. Like capillaroscopy, it is entirely non-invasive.
Methods
As a result of advances in methodology, thermography is now more accessible than previously to the clinician and clinical researcher; cameras are now more compact and more portable, and have high thermal (to 0.1°C) and spatial resolutions. Thermography equipment, as well as being relatively expensive, should optimally be housed in a temperature-controlled and humidity-controlled laboratory. Thus its use is generally confined to specialist centers. Most thermography protocols incorporate some form of dynamic challenge, usually a cold challenge involving immersion of the hands in cool water for a period of time.32–34 There is debate as to which parameter(s) derived from baseline images and from images following cold challenge are most useful to clinicians and researchers, but the degree of temperature gradient along the fingers has been advocated by several researchers as a useful measure.6,34–36 One problem is that different centers use different protocols for thermography, including for dynamic testing, and ideally these should be standardized across centers. A further problem is that (as for other vascular physiology tests) there are concerns about reproducibility.34
Clinical applications in the patient with RP
The main clinical application of thermography in the context of RP is to help differentiate between primary and secondary RP. Investigators have also used thermography to give objective evidence of an abnormal response to cold (ie, to differentiate patients with RP from healthy controls), because sometimes a patient’s history can be difficult to interpret.
Single images (thermograms) of the hands at a baseline temperature (for example, 23°C) show different patterns of temperature distribution in patients with RP compared with healthy controls: in patients with RP the fingertips tend to be cooler than the hand, whereas in healthy controls the reverse is true (Figure 5, top and middle). The pattern of rewarming after a cold challenge is helpful in separating between healthy controls, patients with primary RP, and patients with systemic sclerosis.6,32,33 In our center, we additionally measure response to a heat challenge (20 minutes of heating to 30°C) because we found that a temperature gradient along at least one finger of greater than 1°C (fingertip cooler; Figure 5, middle and bottom) was more specific for an underlying connective tissue disease than parameters derived after a cold challenge.6,34
Dynamic testing inevitably adds complexity (and time) to the investigation. It has been suggested that, in a population setting, a baseline image alone could help predict RP,37 and that a cold challenge test provided little additional information. This finding was supported by a recent study suggesting that “baseline” images are more discriminatory between primary and secondary RP than imaging after cold challenge testing.35
Most papers describing differentiation between primary and secondary RP refer primarily to RP related to systemic sclerosis, although thermographic imaging in response to cold has also been described in patients with hand-arm vibration syndrome.38
Research applications in the patient with RP
Thermography has been advocated as an objective (albeit indirect) measure of blood flow response in clinical trials of vasoactive treatments. When interpreting results, the reproducibility of whichever protocol is being used should be taken into account, and this is a further argument for standardization of protocols across centers.39 Thermography also gives insights into the nature and pathophysiology of RP. For example, it has shown that the thumb is less involved than other digits.40
Conclusion
Thermography helps to differentiate between patients with primary and secondary RP. At present, its use is restricted to specialist centers and to research, mainly due to the high cost of the equipment and facilities required. Most clinicians and researchers incorporate a dynamic challenge (usually a cold challenge) into their thermography protocols. Urgent standardization of these protocols across centers is necessary in order to encourage increased use of this technique.
Imaging of large vessels
Rationale
In the patient with RP, large vessel imaging is only required when there is concern about large vessel disease, including atherosclerotic disease (eg, causing a subclavian stenosis, as in scenario 3) and extrinsic vascular compression (eg, by a cervical rib). Key indicators that should make the clinician suspect large vessel disease are (in the history) asymmetric RP and (in the examination) absence or asymmetry of peripheral pulses.
Large vessel imaging is therefore only briefly described here, because it is very rarely indicated. However, those patients with RP with suspected large vessel disease are an important minority, because to miss this diagnosis may lead to critical ischemia and tissue loss, which could have been preventable with appropriate diagnosis and treatment.
Methods
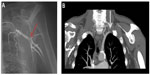
Arterial Doppler ultrasound studies (as in scenario 3) are performed as a first step. If these are abnormal (or even if normal, but the clinical features are of concern), then further vascular imaging is required, usually in discussion with an endovascular specialist. Conventional X-ray (usually digital subtraction) angiography is now less used than previously because of the increasing use of magnetic resonance angiography and computed tomography angiography (Figure 6), especially in evaluating proximal vessel disease. However, for visualization of the peripheries, X-ray angiography is generally still advocated, although has the disadvantages of requiring intra-arterial puncture and a relatively high contrast load.
Clinical and research applications in the patient with RP
Investigation of suspected large vessel (proximal) disease
As stated above, this is the main clinical indication for large vessel imaging in patients with RP (Figure 6).
RP related to systemic sclerosis
Patients with RP related to systemic sclerosis often have severe structural digital vasculopathy, and for reasons unknown, the ulnar artery is often involved.41,42 Some lesions, including ulnar artery stenosis, may be amenable to surgical intervention. Therefore, in the patient with recurrent ulcers in whom a revascularization procedure might be considered, angiography should be considered and discussed with the vascular or hand surgeon. At present, the best visualization of distal vessels is with X-ray angiography. However, advances in magnetic resonance angiography have led to its recent application (both contrast and non-contrast) in systemic sclerosis-related angiopathy.43–45 Demonstration of distal vessels with magnetic resonance angiography holds promise as a research tool, as well as a clinical tool, and, since no radiation is involved, repeat studies are possible.
Conclusion
Large vessel imaging should be considered if there is a possibility that RP is due to large vessel disease. Arterial Doppler ultrasound is useful in initial screening for large vessel disease, followed (if appropriate) by angiography. The type of angiography (X-ray, magnetic resonance imaging, computed tomography) will depend on the level (proximal or distal) and nature of the suspected pathology.
Research methodologies
Recent years have seen the development and refinement of a number of methods that are currently confined to research use but may, in the future, find clinical application. Of these, laser Doppler imaging is currently most commonly applied. Many research studies have applied techniques in combination, and this can be particularly useful when techniques examine different aspects of vascular structure and function, eg, studies combining capillaroscopy (microvascular structure) with laser Doppler (microvascular function).
Laser techniques
Laser Doppler methods have been used for many years in studies of RP, including studies of pathophysiology and of treatment response. There are a number of different laser Doppler methodologies,46 all of which measure microcirculatory blood flow. All methodologies rely on laser light incident on skin being Doppler-shifted (ie, subtly changed in wavelength/color) by interaction with moving objects (generally blood cells). The Doppler-shifted light is collected following reflection and the degree of color change is related to the velocity of the moving object.
Laser Doppler flowmetry
This is the method used in early laser Doppler studies of RP. Here blood flow is measured at a single point, using a single probe. Disadvantages are that there is a large site-to-site variability in blood flow, so even minute movements of the probe can result in marked changes in blood flow, and the direct contact of the probe with the skin may in itself influence blood flow. However, an advantage is that a continuous reading can be obtained, allowing changes in blood flow, for example, in response to vasodilator therapy to be monitored over short time periods.
Laser Doppler imaging
This technique measures blood flow over an area rather than at a single site.47 As for laser Doppler flowmetry, a single laser is used, but it is scanned across the area of interest, building up a perfusion map (Figure 7). In patients with RP, the area of interest normally includes the fingers and hands. The problem of site-to-site variability is resolved, since blood flow is averaged over an area. A second advantage is that the system is non-contact. However, a disadvantage is that scans take in the order of seconds to minutes to perform. For example, if perfusion is measured in response to a cold challenge, then a series of images (each of which takes time to acquire) is collected, and so the “rewarming curve” can be viewed as a number of snapshots of perfusion rather than a continuous recording. Different laser wavelengths, and use of dual wavelength systems, allow different levels of the microcirculation to be studied due to their different penetrations.47
Laser speckle contrast imaging
This method also measures blood flow over an area of skin, but relies on a slightly different physical process.46 The scattering of coherent light, such as that produced by a typical laser, results in an interference pattern known as speckle. If there are moving objects (eg, blood cells) in the illuminated field, this speckle pattern changes temporally, and this can be imaged and related to the velocity of the moving object. Laser speckle contrast imaging has the advantage of producing images much faster than laser Doppler imaging, since the whole area of interest is illuminated and imaged in one “snapshot”. Consequently, this can allow near-real-time video rate monitoring of blood flow, and therefore monitoring and capture of transient dynamic processes. The major disadvantage of this technique is that only superficial blood flow near to the skin surface is imaged, potentially missing processes occurring at deeper levels.
Research applications in the patient with RP
Studies of pathophysiology
The reasons for abnormal vascular reactivity in patients with different forms of RP are not fully understood. These are likely to differ between primary and secondary RP. Of the different forms of secondary RP, systemic sclerosis-related RP has been most studied, and researchers often compare blood flow responses between patients with primary RP, patients with systemic sclerosis, and healthy control subjects in order to understand the differences between these three groups. Many of these studies of pathophysiology have involved laser Doppler techniques.48 Examples of where laser Doppler techniques have been applied to elucidate pathophysiology include studies examining endothelial-dependent and endothelial-independent vasodilation in the fingers of patients with primary RP and systemic sclerosis-related RP; laser Doppler techniques have been used to measure blood flow response to iontophoresis of acetylcholine chloride (endothelial-dependent) and of sodium nitroprusside (endothelial-independent).49 It is now generally accepted that endothelial-dependent vasodilation is impaired in the fingers of patients with systemic sclerosis.
Studies examining interrelationships between microvascular structure and function
A recent study showed that in patients with systemic sclerosis, impaired blood flow (as measured by both laser speckle and laser Doppler flowmetry) was related to the degree of structural vascular abnormality on nail fold capillaroscopy,50 although other studies have not shown correlations between the degree of capillaroscopic abnormality and perfusion.51,52
Studies discriminating between primary and secondary RP
Many investigators have examined the ability of laser Doppler techniques to distinguish between primary and secondary (usually systemic sclerosis-related) RP, including recently in children.53 In the future, laser Doppler techniques may enter the clinical arena to aid the clinician in diagnosis.
Studies of treatment response
Different laser Doppler methods have been applied in studying treatment response. Many of the protocols used have included some form of dynamic challenge, primarily a cold challenge.54–56 Although laser Doppler methods have the potential of providing an objective outcome measure that is sensitive to change, for use in treatment studies including randomized controlled trials, the same challenges apply as in thermography, ie, the lack of standardization of protocols and concerns about reproducibility. As with thermography, reproducibility has to be set against the background of physiological variation within any one individual over time, although this can be minimized by precautions, such as ensuring that the subject is acclimatized to the room temperature and is “caffeine-free”.
Other research methods
Several other methods have been used to assess RP in research studies. These include finger systolic blood pressure measurement, which is a test of digital artery function. The rationale is that patients with RP have a more marked response to cooling than control subjects. Maricq et al reported that digital blood pressure responses to temperature change could help differentiate between patients with systemic sclerosis, patients with primary RP, and cold-sensitive individuals.57 However, this technique is now little used, probably in part because it is complex,58 involving two digital cuffs (one a blood pressure cuff around the proximal phalanx, the other [with water circulating at different temperatures] around the middle phalanx) and a method of recording the finger pulse (eg, a phototoelectric transducer or laser Doppler flowmetry).
Plethysmography
Several different plethysmography techniques have been applied to studying patients with RP. Venous occlusion plethysmography measures finger swelling after venous occlusion, reflecting blood flow into the finger.57 The technique of photoplethysmography46 assesses microcirculatory volume (volume of circulating erythrocytes) optically using a probe similar to those used for pulse oximetry, and has also been applied in studies of RP.59,60
Emerging techniques
Developing techniques are likely to find future applications in research studies of RP. These include optical Doppler tomography61 and correlation mapping optical coherence tomography62 for combined structural and functional imaging of the microvasculature and (for measurement of oxygenation) spectroscopy and multispectral imaging.
Conclusion
Vascular diagnostics play a key role in the assessment of RP. For the practicing clinician, nail fold capillaroscopy allows separation between primary and systemic sclerosis-related RP and (in consequence) allows early diagnosis and optimal management of an underlying systemic sclerosis. Evaluation of large vessel disease firstly with Doppler ultrasound and then (if indicated) with angiography is rarely necessary, but mandatory in that minority of patients in whom large vessel disease is suspected and which would be potentially amenable to angioplasty or surgical treatment. In specialist centers, thermography complements nail fold capillaroscopy in differentiating between primary and systemic sclerosis-related RP.
For the researcher with an interest in RP, this is an exciting time because many new and emerging techniques (and refinements of older techniques) hold promise for the non-invasive investigation of RP. An example of this is the recent development of new laser Doppler modalities, which are commercially available and (relatively) easy to use. The ultimate goal is to apply these techniques to early and later phase studies of new treatment approaches, thus facilitating drug development programs.
Acknowledgment
We are grateful to Tonia Moore and Joanne Manning, Salford, UK, for their help in acquiring the capillaroscopy, thermography, and laser Doppler images.
Disclosure
The authors have no conflicts of interest to report in this work.
References
Silman A, Holligan S, Brennan P, Maddison P. Prevalence of symptoms of Raynaud’s phenomenon in general practice. BMJ. 1990;301:590–592. | |
Suter LG, Murabito JM, Felson DT, Fraenkel L. The incidence and natural history of Raynaud’s phenomenon in the community. Arthritis Rheum. 2005;52:1259–1263. | |
Herrick AL. The pathogenesis, diagnosis and treatment of Raynaud phenomenon. Nat Rev Rheumatol. 2012;8:469–479. | |
Maverakis E, Patel F, Kronenberg DG, et al. International consensus criteria for the diagnosis of Raynaud’s phenomenon. J Autoimmun. 2014;48–49:60–65. | |
LeRoy EC, Medsger TA. Raynaud’s phenomenon: a proposal for classification. Clin Exp Rheumatol. 1992;10:485–488. | |
Anderson ME, Moore TL, Lunt M, Herrick AL. The ‘distal-dorsal difference’: a thermographic parameter by which to differentiate between primary and secondary Raynaud’s phenomenon. Rheumatology (Oxford). 2007;6:533–538. | |
Koenig M, Joyal F, Fritzler MJ, et al. Autoantibodies and microvascular damage are independent predictive factors for the progression of Raynaud’s phenomenon to systemic sclerosis: a twenty-year prospective study of 586 patients, with validation of proposed criteria for early systemic sclerosis. Arthritis Rheum. 2008;58:3902–3912. | |
Ingegnoli F, Boracchi P, Gualtierotti R, et al. Improving outcome prediction of systemic sclerosis from isolated Raynaud’s phenomenon: role of autoantibodies and nail-fold capillaroscopy. Rheumatology (Oxford). 2010;49:797–805. | |
Van den Hoogen F, Khanna D, Fransen J, Johnson S, Baron M, Tyndall A. Classification criteria for systemic sclerosis. Arthritis Rheum. 2013;65:2737–2747. | |
LeRoy EC, Medsger TA Jr. Criteria for the classification of early systemic sclerosis. J Rheumatol. 2001;28:1573–1576. | |
Matucci-Cerinic M, Allanore Y, Czirják L, et al. The challenge of early systemic sclerosis for the EULAR Scleroderma Trial and Research group (EUSTAR) community. It is time to cut the Gordian knot and develop a prevention or rescue strategy. Ann Rheum Dis. 2009;68:1377–1380. | |
Avouac J, Fransen J, Walker UA, et al. Preliminary criteria for the very early diagnosis of systemic sclerosis: results of a Delphi Consensus Study from EULAR Scleroderma Trials and Research Group. Ann Rheum Dis. 2011;70:476–481. | |
Maricq HR, LeRoy EC. Patterns of finger capillary abnormalities in connective tissue disease by ‘wide-field’ microscopy. Arthritis Rheum. 1973;16:619–628. | |
Maricq HR. Widefield capillary microscopy. Technique and rating scale for abnormalities seen in scleroderma and related disorders. Arthritis Rheum. 1981;24:1159–1165. | |
Herrick AL, Cutolo M. Clinical implications from capillaroscopic analysis in patients with Raynaud’s phenomenon and systemic sclerosis. Arthritis Rheum. 2010;62:2595–2604. | |
Cutolo M, Sulli A, Smith V. How to perform and interpret capillaroscopy. Best Pract Res Clin Rheumatol. 2013;27:237–248. | |
Anderson ME, Allen PD, Moore T, Hillier V, Taylor CJ, Herrick AL. Computerised nailfold video capillaroscopy – a new tool for the assessment of Raynaud’s phenomenon. J Rheumatol. 2005;32:841–848. | |
Moore TL, Roberts C, Murray AK, Helbling I, Herrick AL. Reliability of dermoscopy in the assessment of patients with Raynaud’s phenomenon. Rheumatology (Oxford). 2010;49:542–547. | |
Baron M, Bell M, Bookman A, et al. Office capillaroscopy in systemic sclerosis. Clin Rheumatol. 2007;26:1268–1274. | |
Cutolo M, Sulli A, Pizzorni C, Accardo S. Nailfold videocapillaroscopy assessment of microvascular damage in systemic sclerosis. J Rheumatol. 2000;27:155–160. | |
Ingegnoli F, Gualtierotti R, Lubatti C, et al. Nailfold capillary patterns in healthy subjects: a real issue in capillaroscopy. Microvasc Res. 2013;90:90–95. | |
Sebastiani M, Manfredi A, Colaci M, et al. Capillaroscopic skin ulcer risk index: a new prognostic tool for digital skin ulcer development in systemic sclerosis patients. Arthritis Rheum. 2009;61:688–694. | |
Smith V, De Keyser F, Pizzorni C, et al. Nailfold capillaroscopy for day-to-day clinical use: construction of a simple scoring modality as a clinical prognostic index for digital trophic lesions. Ann Rheum Dis. 2011;70:180–183. | |
Vaz JLP, Dancour MAA, Bottino DA, Bouskela E. Nailfold videocapillaroscopy in primary antiphospholipid syndrome (PAPS). Rhematology (Oxford). 2004;43:1025–1027. | |
Ingegnoli F, Zeni S, Meani L, Soldi A, Lurati A, Pantini F. Evaluation of nailfold videocapillaroscopic abnormalities in patients with systemic lupus erythematosus. J Clin Rheumatol. 2005;11:295–298. | |
Moore TL, Vail A, Herrick AL. Assessment of digital vascular structure and function in response to bosentan in patients with systemic sclerosis-related Raynaud’s phenomenon. Rheumatology (Oxford). 2007;46:363–364. | |
Cutolo M, Zampogna G, Vremis L, Smith V, Pizzorna C, Sulli A. Longterm effects of endothelin receptor antagonism on microvascular damage evaluated by nailfold capillaroscopic analysis in systemic sclerosis. J Rheumatol. 2013;40:40–45. | |
Murray AK, Vail A, Moore TL, Manning JB, Taylor CJ, Herrick AL. The influence of measurement location on reliability of quantitative nailfold videocapillaroscopy in patients with SSc. Rheumatology (Oxford). 2012;51:1323–1330. | |
Smith V, Riccieri V, Pizzorni C, et al. Nailfold capillaroscopy for prediction of novel future severe organ involvement in systemic sclerosis. J Rheumatol. 2013;40:2023–2028. | |
Hofstee HM, Vonk Noordegraaf A, Voskuyl AE, et al. Nailfold capillary density is associated with the presence and severity of pulmonary arterial hypertension in systemic sclerosis. Ann Rheum Dis. 2008;68:191–195. | |
Bollinger A, Jager K, Siegenthaler W. Microangiopathy of progressive systemic sclerosis. Evaluation by dynamic fluorescence videomicroscopy. Arch Intern Med. 1986;146:1541–1545. | |
Darton K, Black CM. Pyroelectric vidicon thermography and cold challenge quantify the severity of Raynaud’s phenomenon. Br J Rheumatol. 1991;30:190–195. | |
O’Reilly D, Taylor L, El-Hadidy K, Jayson MIV. Measurement of cold challenge responses in primary Raynaud’s phenomenon and Raynaud’s phenomenon associated with systemic sclerosis. Ann Rheum Dis. 1992;51:1193–1196. | |
Clark S, Hollis S, Campbell F, Moore T, Jayson M, Herrick A. The ‘distal-dorsal difference’ as a possible predictor of secondary Raynaud’s phenomenon. J Rheumatol. 1999;26:1125–1128. | |
Pauling JD, Flower V, Shipley JA, Harris ND, McHugh NJ. Influence of the cold challenge on the discriminatory capacity of the digital distal-dorsal difference in the thermographic assessment of Raynaud’s phenomenon. Microvasc Res. 2011;82:364–368. | |
Schuhfried O, Vacariu G, Lang T, Korpan M, Keiner HP, Fialka-Moser V. Thermographic parameters in the diagnosis of secondary Raynaud’s phenomenon. Arch Phys Med Rehabil. 2000;81:495–499. | |
Cherkas LF, Carter L, Spector TD, Howell KJ, Black CM, MacGregor AJ. Use of thermographic criteria to identify Raynaud’s phenomenon in a population setting. J Rheumatol. 2003;30:720–722. | |
Coughlin PA, Chetter IC, Kent PJ, Kester RC. The analysis of sensitivity, specificity, positive predictive value and negative predictive value of cold provocation thermography in the objective diagnosis of the hand-arm-vibration syndrome. Occup Med (Lond). 2001;51:75–80. | |
Pauling JD, Shipley JA, Harris ND, McHugh NJ. Use of infrared thermography in therapeutic trials of Raynaud’s phenomenon and systemic sclerosis. Clin Exp Rheumatol. 2012:30 Suppl 71:S103–S115. | |
Chikura B, Moore T, Manning J, Vail A, Herrick AL. Sparing of the thumb in Raynaud’s phenomenon. Rheumatology (Oxford). 2008;47:219–221. | |
Hasegawa M, Nagai A, Tamura A, Ishikawa O. Arteriographic evaluation of vascular changes of the extremities in patients with systemic sclerosis. Br J Dermatol. 2006;155:1159–1164. | |
Taylor MH, McFadden JA, Bolster MB, Silver RM. Ulnar artery involvement in systemic sclerosis (scleroderma). J Rheumatol. 2002;29:102–106. | |
Allanore Y, Seror R, Chevrot A, Kahan A, Drapé JL. Hand vascular involvement assessed by magnetic resonance angiography in systemic sclerosis. Arthritis Rheum. 2007;56:2747–2754. | |
Sheehan JJ, Fan Z, Davarpanah AH, et al. Nonenhanced MR angiography of the hand with flow-sensitive dephasing-prepared balanced SSFP sequence: initial experience with systemic sclerosis. Radiology. 2011;259:248–256. | |
Zhang W, Xu JR, Lu Q, Ye S, Liu XS. High-resolution magnetic resonance angiography or digital arteries in SSc patients on 3 Tesla: preliminary study. Rheumatology (Oxford). 2011;50:1712–1719. | |
Allen J, Howell K. Microvascular imaging: techniques and opportunities for clinical physiological measurements. Physiol Meas. 2014;35:R91–R141. | |
Murray AK, Herrick AL, King TA. Laser Doppler imaging: a developing technique for application in the rheumatic diseases. Rheumatology (Oxford). 2004;43:1210–1218. | |
Roustit M, Blaise S, Millet C, Cracowski J-L. Impaired transient vasodilation and increased vasoconstriction to digital local cooling in primary Raynaud’s phenomenon. Am J Physiol Heart Circ Physiol. 2011;301:H324–H330. | |
Gunawardena H, Harris ND, Carmichael C, McHugh NJ. Maximum blood flow and microvascular regulatory responses in systemic sclerosis. Rheumatology (Oxford). 2007;46:1079–1082. | |
Ruaro B, Sulli A, Alessandri E, Pizzorni C, Ferrari G, Cutolo M. Laser speckle contrast analysis: a new method to evaluate peripheral blood perfusion in systemic sclerosis patients. Ann Rheum Dis. 2014;73:1181–1185. | |
Murray AK, Moore TL, Manning JB, Taylor C, Griffiths CE, Herrick AL. Noninvasive imaging techniques in the assessment of scleroderma spectrum disorders. Arthritis Care Res. 2009;61:1103–1111. | |
Correa MJ, Andrade LE, Kayser C. Comparison of laser Doppler imaging, fingertip lacticemy test, and nailfold capillaroscopy for assessment of digital microcirculation in systemic sclerosis. Arthritis Res Ther. 2010;12:R157. | |
Piotto DG, Correa MJ, Miotto e Silva VB, Kayser C, Terreri MT. Laser Doppler imaging for assessment of microcirculation in juvenile systemic sclerosis. Rheumatology (Oxford). 2014;53:72–75. | |
Roustit M, Hellman M, Cracowski C, Blaise S, Cracowski JL. Sildenafil increases digital skin blood flow during all phases of local cooling in primary Raynaud’s phenomenon. Clin Pharmacol Ther. 2012;91:813–819. | |
Hummers LK, Dugowson CE, Dechow FJ, et al. A multi-centre, blinded, randomised, placebo-controlled, laboratory-based study of MQX-503, a novel topical gel formulation of nitroglycerine, in patients with Raynaud’s phenomenon. Ann Rheum Dis. 2013;72:1962–1967. | |
Herrick AL, Murray AK, Ruck A, et al. A double-blind, randomized, placebo-controlled crossover trial of the alpha2C-adrenoceptor antagonist ORM-12741 for prevention of cold-induced vasospasm in patients with systemic sclerosis. Rheumatology (Oxford). 2014;53:948–952. | |
Maricq HR, Weinrich MC, Valter I, Palesch YY, Maricq JG. Digital vascular responses to cooling in subjects with cold sensitivity, primary Raynaud’s phenomenon, or scleroderma. J Rheumatol. 1996;23:2068–2078. | |
Neilson SL. Raynaud phenomena and finger systolic pressure during cooling. Scand J Clin Lab Invest. 1978;38:765–770. | |
Suichies HE, Aarnoudse JG, Wouda AA, Jentink HW, de Mul FF, Greve J. Digital blood flow in cooled and contralateral finger in patients with Raynaud’s phenomenon. Comparative measurements between photoelectrical plethysmography and laser Doppler flowmetry. Angiology. 1992;43:134–141. | |
Rosato E, Molinaro I, Rossi C, Pisarri S, Salsano F. The combination of laser Doppler perfusion imaging and photoplethysmography is useful in the characterization of scleroderma and primary Raynaud’s phenomenon. Scand J Rheumatol. 2011;40;292–298. | |
Chen Z, Zhao Y, Srinivas SM, Nelson JS, Prakash N, Frostig RD. Optical Doppler tomography. IEEE J Sel Top Quant. 1999;5:1134–1142. | |
Jonathan E, Enfield J, Leahy MJ. Correlation mapping method for generating microcirculation morphology from optical coherence tomography (OCT) intensity images. J Biophotonics. 2011;4:583–587. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.