Back to Journals » Research Reports in Clinical Cardiology » Volume 14
Utilization and Optimization of Beta-Blockers on Heart Failure Patients with Reduced Ejection Fraction (HFrEF) at Cardiac Clinic of Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia: A Cross-Sectional Study
Authors Demissie Z , Mekonnen D
Received 19 February 2023
Accepted for publication 29 April 2023
Published 3 May 2023 Volume 2023:14 Pages 21—33
DOI https://doi.org/10.2147/RRCC.S408047
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Kones
Zekewos Demissie,1 Desalew Mekonnen2
1Department of Internal Medicine, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia; 2Department of Internal Medicine, Division of Cardiology, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Zekewos Demissie, Email [email protected]
Introduction: Optimal use of guideline-directed medical therapy (GDMT) is an important strategy in preventing cardiovascular morbidity and mortality from heart failure (HF). The use of beta blockers (BBs) is an important pillar of GDMT. The utilization and optimization of BBs are understudied in Ethiopia.
Patients and Methods: A hospital-based cross-sectional study was conducted at the cardiology clinic of Tikur Anbessa Specialized Hospital on the utilization and optimization of BBs in the management of HFrEF patients from August 2022 to October 2022 G.C. Patients with a baseline HFrEF diagnosis and who had follow up for at least six months were included in the study. Patients were recruited during their routine clinic visit using systematic random sampling and consent was obtained from all participants. A total of 271 participants were included in this study. The optimal dose of BBs was predefined according to the 2021 European Society of Cardiology HF guideline. Data were collected through patients interview and reviewing the patient’s electronic medical records. The Collected Data was analysed using SPSS version 26.0.
Results: The study found BB utilization in 214 (79%) patients. Among the patients taking BBs only 102 (37.6%) participants were on guideline-recommended BBs. The other 41% of patients taking BBs were on Metoprolol tartrate (34.7%) and Atenolol (6.6%), which are not guideline-recommended BBs and these findings signify underutilization of guideline-recommended BBs. Duration of treatment follow-up for more than 1 year, absence of peripheral edema, presence of atrial fibrillation and diuretic use were positively associated with BB utilization.
Conclusion: Even though guideline-recommended BB at optimal doses exerts a clinical benefit, this study points out guideline-recommended BBs are underutilized and more importantly used at suboptimal doses among the study participants. To achieve the desired clinical benefit, physicians should attempt to provide guideline-recommended BBs at the optimum or maximum tolerable dose.
Keywords: heart failure, beta blocker, Tikur Anbessa Specialized Hospital, optimal therapy
Introduction
According to the 2021 universal definition of heart failure, HF is a clinical syndrome with cardinal symptoms and/or signs caused by a structural and/or functional cardiac abnormality and corroborated by elevated biomarkers (natriuretic peptide levels) and/or objective evidence of pulmonary or systemic congestion.1 Heart failure is accompanied by progressive left ventricular dilatation and adverse cardiac remodelling. This process is the culmination of a complex series of transcriptional, signalling, structural, electrophysiological, and functional events occurring within the cardiac myocyte.2,3 The diagnosis of HFrEF requires the fulfilment of the 2021 universal definition of HF and a reduced ejection fraction (LVEF ≤ 40%) that is most usually obtained by echocardiography.4
Globally, HF affects more than 26 million people, albeit the epidemiology varies by region.5 Sub-Saharan Africa lacks population-based incidence and prevalence. According to the published hospital prevalence studies, HF accounts for between 9.4% and 42.5% of all medical admissions and between 25.6% and 30.0% of admissions to cardiac units.6 There are no studies describing the prevalence or incidence of heart failure in Ethiopia’s adult population.7 After pneumonia and tuberculosis, HF was the third most common reason for hospitalization, accounting for 16% of all admissions, according to a retrospective research on the pattern of admission and prognosis at Saint Paul’s hospital.8
Significant morbidity and death, poor functional ability and quality of life, and high expenses are all characteristics of HF. According to the Framingham Heart Study, the death rate in USA after receiving a diagnosis of HF was approximately 10% after 30 days, 20–30% at 1 year, and 45–60% throughout the course of 5 years of follow-up.9 In Ethiopia, Non-communicable diseases (NCDs) account for 39% of all-cause of mortality of which, cardiovascular disease accounts for 16%.10 In a single-centre study in Ethiopia, the in-hospital mortality rate among patients admitted with acute heart failure was 21.29%.11 A prospective observational study done at a tertiary care hospital in Ethiopia on treatment outcomes of acute heart failure showed in-hospital mortality of 17.2%.12
The goals of heart failure treatment are to improve their clinical condition, functional capacity, and quality of life, and to prevent the events of hospital readmissions and mortality. According to many guidelines for the treatment of heart failure, an important step in reducing morbidity and mortality from heart failure is to implement optimal guideline-directed medical therapy with the right drug selection, dosage, and target dose to be achieved in HF patients. Hence, unless contraindicated or not tolerated, individuals with HFrEF should be treated with suitable neurohormonal blockers at maximum tolerated doses.13–15
Angiotensin receptor-neprilysin inhibitors (ARNIs), angiotensin receptor blockers (ARBs), angiotensin convertase enzyme inhibitors (ACEIs), beta-blockers, mineral corticoid receptor antagonists, and sodium-glucose co-transporter 2 inhibitors are the four drug classes that makeup GDMT for HFrEF.16 The beta-adrenergic receptors are G-protein coupled receptors that have a variable distribution. There are three known types of beta receptors, known as beta1 (β1), beta2 (β2), and beta3 (β3). These receptors are activated by norepinephrine and epinephrine. The beta-adrenergic receptors are found in the myocardium and their activation has detrimental effects in patients with chronic HF.17,18 BBs reduce morbidity and improve cardiac function. Carvedilol, Metoprolol succinate, and Bisoprolol have been shown to improve survival in multiple randomized trials.19–23 The mechanisms of β-blocker effects in heart failure are cardiac protection from β1-adrenoceptor overstimulation, antiarrhythmic effects, reduction in heart rate, and positive energetic effects or a combination thereof.24
The use of BB is the cornerstone in the management of HFrEF and one of the three BBs (Carvedilol, Metoprolol succinate, and Bisoprolol) should be initiated in all patients with HFrEFunless contraindicated or not tolerated.16 In HFrEF beta-blockers should be initiated at a low dose, and titration should be made to achieve the target doses of the beta-blockers. Titration can be made as frequently as 1 to 2 weeks. Achieving the target doses has shown to be effective and tolerable in several clinical trials.16,25 Recommended target doses in clinical trials include 200 mg of Metoprolol Succinate, 10 mg of Bisoprolol, and 50 mg of Carvedilol.13,19–23 If a target dose is not tolerated, then the highest tolerated dose should be maintained.26
The optimal use of beta blockers has significant mortality and morbidity benefits as shown in several clinical trials.19–23 On the other hand, suboptimal use of beta-blockers has been associated with poor treatment outcomes in HF patients.27 Despite having significant morbidity and mortality benefits, beta-blockers are often not seen being utilized in Ethiopia and there is a paucity of data on the standard and optimal use of guideline-recommended beta-blockers in our country. This study looked at the practice of optimal use of beta blockers among patients with HFrEF and remedies the gaps identified.
Materials and Methods
Study Design and Study Setting
We conducted a cross-sectional study at the cardiac referral clinic in Tikur Anbessa Specialized Hospital (TASH) among patients with heart failure with reduced ejection fraction on follow-up from August 2022 to October 2022 G.C. TASH is a university teaching hospital affiliated with Addis Ababa University. It is the largest referral hospital in the country. It provides specialized clinical services through its several departments.
Study Participants
Inclusion Criteria
- Age >/=18 years.
- Diagnosis of HF with a baseline left ventricular ejection fraction < 40%.
- On Follow-up for >/=06 months.
Exclusion Criteria
● Patients with precautions and contraindications to the use of beta-blockers including:
✓ Hypotension (<90/60mmg).
✓ Bradycardia (<60 beats per minute).
✓ 2nd and 3rd degree AV-block.
✓ Asthma/COPD.
✓ Decompensated heart failure:
● Patients with incomplete medical records.
● Critically ill patients.
● Who cannot provide written consent.
A sample of 294 was calculated by using single population proportion formula based on the results of a study conducted at Jimma University Hospital which revealed 34.2% beta-blocker utilization among patients with HF,28 95% confidence level, 5% margin of error, and 10% of contingency for nonresponse rate. From a total of 294 participants approached, 23 patients were excluded from the study due to precaution/contraindication to beta-blockers (15), declined consent (3) and incomplete medical record (5).
Data Collection Procedure
Patients were recruited into the study during their routine clinic visit using systematic random sampling. After explaining the purpose of the study, written informed consent was obtained from all study participants. Data were collected through both patient interviews and a review of patient’s medical records. Face-to-face interviews were used to gather socio-demographic information and clinical and treatment-related information was gathered from electronic medical record.
Operational Definitions
Guideline-recommended beta-blocker therapy is defined as when HFrEF patients are prescribed Carvedilol, Metoprolol Succinate, or Bisoprolol. Non-guideline recommended beta-blocker therapy is defined as when patients are prescribed beta-blockers other than Carvedilol, Metoprolol Succinate, or Bisoprolol. Underutilization of beta-blocker is defined as when guideline-recommended beta blockers are not used in the absence of contraindication. Suboptimal use is defined as when the optimal dose of guideline-recommended beta blocker is not used in the absence of contraindication. The optimal use of beta-blocker is defined as when the optimal dose of guideline-recommended beta-blocker is used. Guideline recommended daily target doses were defined as 200mg for Metoprolol Succinate, 50mg for Carvedilol and 10mg for Bisoprolol 10mg/day. This is based on 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure guideline.
Data Analysis
The collected data was verified, cleaned, and checked for quality before the analysis. The IBM SPSS Statistics software package version 26.0 was used for the entry of statistical data and analysis. Descriptive statistics was used as a statistical data analysis method and was expressed as frequencies and numbers (percentages %) for categorical variables. The results were summarized by using tables, and figures. Continuous variables were represented as means, standard deviations, and minimum and maximum values, and categorical variables as frequencies. Multicollinearity was assessed among independent variables using the variance inflation factor (VIF) and none was collinear. Univariable logistic regression analysis was performed to determine the association of each independent variable with the utilization of beta-blockers. Furthermore, a multivariable binary logistic regression model was done to identify predictors of beta-blockers utilization. P-values less than 0.05 are considered to determine the statistical significance of the association and an odds ratio with a 95% confidence interval is used to determine the presence, strength, and direction of association between covariates and the outcome variable.
Ethics Approval and Informed Consent
The ethical clearance of the present study was obtained from the institutional review board department of internal medicine, AAU, CHS. And permission was also obtained from the cardiology unit. Participants including data collectors were also asked for their willingness to participate in the study after explaining the objectives of the study. We fully explained the purpose and protocol of the study to all participants included in the study and written informed consent was obtained from each participant. All the information obtained was held with confidentiality and used only for the intended purpose. Participants’ identities will remain confidential. The obtained data was documented and analyzed anonymously. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki.
Results
Sociodemographic Profiles
The mean [± standard deviation] age of the participants was 54.1 ± (13.5) with minimum and maximum ages of 18 and 84, respectively. One-third of patients were above the age of 60. Two-thirds participants were male. The majority (83%) of the participants were urban dwellers and 73.8% resided in Addis Ababa.
The Sociodemographic data are shown in Table 1 and Figure 1.
 |
Table 1 Sociodemographic Characteristics of Study Participants at Cardiac Clinic of Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia, August 2022 – October 2022 |
 |
Figure 1 Region distribution of study participants at cardiac clinic of Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia, August 2022 – October 2022. |
Clinical Characteristics
The majority of participants were having New York Heart Association (NYHA) functional class II (39.1%) and class III (34.7%). About a third, (36%) of patients had had a history of hospitalization and 48 (18%) patients had 2 or more admissions in the past year.
The most common rhythm disturbance was atrial fibrillation, which was found in 33 (12.2%) participants. Three participants had atrial flutter. Ventricular tachycardia (VT) and supraventricular tachycardia (SVT), each identified in 2 participants and 6 participants had documented premature ventricular complexes (PVCs).
Ischemic heart disease (IHD) was the commonest etiology identified in 204 (75.3%) followed by dilated cardiomyopathy (DCM) in 64 (23.6%) participants. Peripartal cardiomyopathy was identified in 3 participants. Documented left ventricular apical thrombus was found in 6 patients among IHD patients during the study period. Degenerative valvular heart disease was found in 13 (5%) and Rheumatic heart disease was found in a single participant.
One-third 73 (27%) of the participants do not have identified comorbidities and about one-quarter of participants have more than one comorbidity. Hypertension was the commonest comorbidity identified in 120 (44.3%) patients followed by diabetes in 87 (32%). Fifty-two (19%) patients have both diabetes and hypertension. CKD and cancer of any type were found in 18 and 15 participants, respectively.
The mean [± standard deviation] Ejection fraction (EF) of the participants was 28.6% ± 7.3 and the leading proportion (69.4%) of participants were having EF below 30%. Only 31 (11.4%) participants had a pulse rate of above 100. The mean [± standard deviation] Mean arterial pressure (MAP) of the participants was 89.9 ± 11.6. Edema was found in 51 (18.8%) patients.
The clinical characteristics are shown in Table 2.
 |
Table 2 Clinical Characteristics of Study Participants at Cardiac Clinic of Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia, August 2022 – October 2022 |
Treatment-Related Characteristics
The mean (± SD) duration of follow-up for the management of HF was 4.36 (± 4.6) years and about two-thirds of patients were on follow-up for more than one year. One hundred and sixty-three (60.1%) patients were taking 5 or more drugs. RAAS-blockers are the most commonly used drugs in 233 (86%) patients followed by Beta-blockers in 214 (79%) and MRA in 159 (58.7%). Among RAAS-blockers Enalapril was a commonly used drug in 212 patients and only 3 participants were on ARNI. SGLT2i was used in 14 (5%) cases. More than three-quarters of the patients were receiving antiplatelet and/or anticoagulants. About three-quarters of participants were on a statin and 155 (57.2%) were taking diuretics. About two-thirds (61.6%) of the patients were taking Aspirin and 21 (7.7%) patients were taking DAPT.
The treatment-related characteristics are shown in. Table 3 and Figures 2 and 3
 |
Table 3 Treatment-Related Characteristics of Study Participants at Cardiac Clinic of Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia, August 2022 – October 2022 |
 |
Figure 2 Distribution of duration of follow up in year among patients with HFrEF at cardiac clinic of Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia, August 2022 - October 2022. |
 |
Figure 3 Distribution of utilization guideline medical therapy in patients with HFrEF at cardiac clinic of Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia, August 2022 – October 2022. |
Utilization of Beta-Blocker Among Patients with HFrEF
Among 214 (79%) participants taking beta-blocker, only 102 (37.6%) were on guideline-recommended beta-blocker. Among guideline-recommended beta-blockers, Metoprolol succinate was the most commonly used beta blocker in 79 (29.2%). Carvedilol and Bisoprolol were used by 13 and 10 participants, respectively. Ninety-four (34.7%) participants were getting Metoprolol tartrate and 18 (6.6%) were on Atenolol. Among patients receiving guideline-recommended beta-blockers (102), 16 (15.7%) were taking the optimal dose. Among patients who took the optimal dose of guideline-recommended beta-blocker 12 patients were taking Metoprolol succinate, 1 was on Carvedilol, and 3 were getting Bisoprolol. The mean daily doses of Metoprolol succinate, Carvedilol, and Bisoprolol were 72.18 mg, 18.75 mg, and 5.75 mg, respectively.
Utilization of beta blockers among patients with HFrEFis is shown. in Table 4 and Figure 4
 |
Table 4 Beta-Blocker Type and Dosage of Study Participants at Cardiac Clinic of Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia, August 2022 – October 2022 |
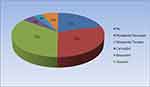 |
Figure 4 Types of beta-blockers used in patients with HFrEF at cardiac clinic of Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia, August 2022 – October 2022. |
Factors Associated with Beta-Blocker Utilization
Table 5 shows the logistic regression analysis of factors associated with beta-blocker utilization and odds ratio and a 95% confidence interval was used to measure the degree of association between the independent variables and beta-blocker utilization. Multicollinearity was assessed among independent variables using the variance inflation factor (VIF), and none was collinear. Initially, bivariate binary logistic regression was performed on selected sociodemographic, clinical, and treatment-related characteristics to identify variables candidate for multivariate binary logistic regression and a total of 8 variables were found to be candidates at a P-value of ≤0.2. These include age greater than 60 (Crude odd ratio (COR) 1.590, 95% confidence interval (CI) 0.817–3.095), residence in Addis Ababa (COR 1.558, 95% CI: 0.827–2.934), duration of treatment follow-up for more than 01 year (COR 3.152, 95% CI: 1.728–5.750), presence of atrial fibrillation (COR 4.658, 95% CI: 1.080–20.086), diuretic use (COR 4.272, 95% CI: 2.269–8.044), ejection fraction </=15% (COR 6.435, 95% CI: 0.811–51.045) and absence of edema (COR 5.037, 95% CI: 2.596–9.773). However, in the final multivariate regression model, only 4 variables were found to have a statistically significant association with beta blocker utilization at a P-value of ≤0.05. These included having duration of treatment follow-up for more than 01 year, presence of atrial fibrillation, diuretic use, and absence of edema.
Beta-blocker use among participants who had a follow-up for more than 1 year was more than 3 times as compared to patients who had to follow up for less than 1 year (Adjusted odds ratio (AOR 3.601), 95% CI: 1.707–7.597, p=0.001). The presence of atrial fibrillation increases the chance of beta-blocker utilization more than 5 times as compared to its absence (AOR 5.629, 95% CI: 1.063–29.817, p=0.042). Patients taking diuretics had increased the use of beta-blockers by more than 4 times as compared to patients not taking diuretics (AOR 4.498, 95% CI: 2.030–9.970, p=0.0002). Beta-blocker utilization among participants without edema was more than 9 times as compared to the presence of edema (AOR 9.588, 95% CI: 3.948–23.286, p<0.0001).
Discussion
Heart failure is a progressive disease associated with significant morbidity and mortality and disease-modifying agents are important in halting this process. Guideline-directed medical therapy is endorsed by most international guidelines and beta blockers are one of the four pillars of GDMT. The use of guideline-recommended beta-blockers (Metoprolol succinate, Bisoprolol, and Carvedilol) is associated with decreased adverse cardiovascular events, cardiovascular mortality, and all-cause mortality.19–23 Although these benefits have been demonstrated by many randomized controlled trials, different studies showed underutilization of these important components of GDMT.28–30
Our study found beta-blocker utilization in 214 (79%) patients, a figure that is comparable with the result of two France studies that showed a beta-blocker utilization rate of 65% and 70%.29,30 This result is higher as compared to a previous study (67%) done in Jimma Hospital.28 The variation in beta-blocker utilization rates among the various studies done could be due to differences in sample characteristics. The higher rate of beta-blocker utilization in our study could be due to increased healthcare access and drug availability since most of the study participants reside in the urban area and the study was conducted in the country’s highest hospital. In this study, RAAS blockers are the most commonly used drug in 86% of patients, which is in line with two studies conducted in France and one from Jimma, which showed RAAS-blocker use in 80%, 92%, and 82.6%, respectively. More than one-third (69.7%) of the participants were taking both RAAS-blocker and beta-blocker, which was 61% and 58% in France and Jimma studies, respectively.28–30
Despite the proven benefit of β-blockers in HFrEF, they are often underutilized in current clinical practice and this has been shown by different studies.28–31 It is also demonstrated by this study, 21% of the study participants were not taking beta-blockers. Among the patients taking beta-blockers only 102 (37.6%) participants were on guideline-recommended beta-blockers. The other 41% of patients taking beta-blockers were on Metoprolol tartrate (34.7%) and Atenolol (6.6%), which are not guideline-recommended beta blockers. The possible reasons for the higher prescription rate of Metoprolol tartrate and Atenolol might be due to the high local cost of guideline-directed beta-blockers (Metoprolol succinate, Carvedilol, and Bisoprolol), lack of patient awareness in differentiating between succinate and tartrate preparations of Metoprolol, lack of knowledge on the different effects of succinate and tartrate preparations of Metoprolol by pharmacists (drug dispensers), and incomplete prescriptions without specifying the type of Metoprolol by physicians.
In this study, four variables were found to be independent predictors of beta-blocker utilization. Duration of treatment follow-up more than 1 year, the absence of peripheral edema, the presence of atrial fibrillation and diuretic use were positively associated with beta-blocker utilization were positively associated with beta-blocker utilization.
Beta-blocker utilization was 3 times higher in participants who had a treatment follow-up for more than 1 year as compared to patients who had a treatment follow-up for more than 1 year. A possible justification might be a shorter duration of follow-up prevents the patients to attend multiple clinic visits and the physicians to initiate and escalate the dose of beta-blockers. This finding is consistent with IMPROVE HF registry.32 Beta-blocker utilization was 5 times lower in patients who had peripheral edema as compared to patients without edema. This could be due to lower physician inertia in prescribing beta-blockers in these patients. The presence of atrial fibrillation was significantly associated with beta-blocker utilization as compared to its absence. This could be due to the increased use of beta-blockers for rate control in these patients. The use of diuretics was significantly associated with beta-blocker utilization as compared to those patients not taking diuretics. This could be due to the use of diuretics decreasing congestion, which is one of the reasons not to prescribe beta-blockers or to escalate their doses. This finding is consistent with the Jimma study.28
None of the socio-demographic characteristics, underlying etiology, and comorbidities such as ischemic heart disease and hypertension were found to be significant predictors of beta-blocker utilization.
The impact of treatment with beta-blockers in HFrEF, in terms of overall mortality, cardiovascular mortality, and HF hospitalization is selectively seen with Carvedilol, Bisoprolol, and Metoprolol Succinate when titrated up to the maximum tolerated dose and these agents are mostly tolerable upon titration.19–23,33 In this study, among patients receiving guideline-recommended beta-blockers, 15.7% were taking the optimal doses and 49% reached more than or equal to 50% of the target dose. Although this figure is higher than the Jimma study in which only 10.5% of patients were at the optimal dose, it is quite comparable with the FUTURE survey, 56%.28,34
Even though the median dose achieved in major clinical trials for Metoprolol succinate, Carvedilol, and Bisoprolol was 159mg, 7.5mg, and 37mg, in this study the mean daily doses of Metoprolol succinate, Carvedilol, and Bisoprolol in this study are 72.18 mg, 18.75 mg, and 5.75 mg, respectively.21,22,30 The possible reason for sub-optimal dosing could be due to low physician inertia in up-titrating to the target dose, low awareness of dose titration among physicians, longer duration of clinic appointments, and patient refusal because of cost issues.
Conclusion
Even though the guideline-recommended beta-blocker at optimal doses exerts a clinical benefit, this study points out that beta-blockers are underutilized and more importantly used at suboptimal doses. Duration of treatment follow-up more than 1 year, the absence of peripheral edema, the presence of atrial fibrillation and diuretic use were positively associated with beta-blocker utilization and were positively associated with beta-blocker utilization. A significant association was not found for socio-demographic characteristics, underlying etiology, and comorbidities. To achieve the desired clinical benefit from beta-blockers physicians should attempt to provide guideline-recommended beta-blockers and should escalate to the optimum or maximum tolerable dose. Furthermore, factors associated with underutilization and suboptimal dosing should be identified and modified by further studies.
Recommendation
This is a hospital-based study with a small sample size, thus a large-scale and prospective study with a longer duration of follow-up is needed for the determination of factors associated with underutilization and suboptimal dosing of guideline-recommended beta-blockers in HFrEF patients. Clinicians’ knowledge and inertia in utilizing and optimizing beta-blockers should be determined in future studies.
Strengths and Limitations of the Study
The strength of this study is as it is a cross-sectional study, and data were collected over specified time duration and which enabled us to execute an accurate assessment of the descriptive and analytic analysis. This study is one of a few studies conducted both in TASH and Ethiopia. We believe this study will add more information regarding the current status of beta-blocker utilization, types, and dosages in the country’s largest hospital and motivate the cardiology unit and department of internal medicine to assess factors associated with underutilization and suboptimal dosing and remedy the gap.
Although the pattern of beta-blockers used and factors associated with beta-blocker utilization are assessed, this study did not assess patient-related factors including financial and educational status and physician knowledge and inertia in initiating and titrating beta-blockers; therefore further study is required. Even though the finding of this study is similar to many studies, it cannot be generalized to the general population because this is a single-center study.
Abbreviations
AAU, Addis Ababa University; AHA, American Heart Association; ACC, American College of Cardiology; ACE-I, Angiotensin Converting Enzyme Inhibitor; ASA, Aspirin; ARBs, Angiotensin Receptor Blockers; ARNI, Angiotensin receptor-Neprilysin Inhibitor; BP, Blood Pressure; BB, Beta Blockers; β-blockers, Beta Blockers; BPM, Beats Per-Minute; CHS, College of Health Sciences; CKD, Chronic Kidney Disease; CVD, Cardiovascular Disease; DAPT, Double Anti-Platelet Therapy; DBP, Diastolic Blood Pressure; DCM, Dilated Cardiomyopathy; DM, Diabetes Mellitus; EF, Ejection Fraction; ESC, European Society of Cardiology; GDMT, Guideline Directed Medical Therapy; HF, Heart Failure; HFrEF, Heart failure with reduced ejection fraction; HFpEF, Heart failure with preserved ejection fraction; IHD, Ischemic Heart Disease; LVEF, Left Ventricular Ejection Fraction; MRA, Mineralocorticoid receptor antagonist; NCD, Non-Communicable Diseases; PPCM, Peripartal Cardiomyopathy; PR, Pulse Rate; PVCs, Premature Ventricular Contractions; RAAS, Renin-angiotensin-aldosterone System; RCTs, Randomized controlled Trials; SBP, Systolic Blood Pressure; TASH, Tikur Anbessa Specialized Hospital; VHD, Valvular Heart Disease.
Acknowledgments
Foremost, I would like to express my heartfelt gratitude to my advisor; Dr. DesalewMekonnen, for his noble guidance through each stage of the process. I found his guidance to be instrumental in defining the path of my research. My sincere thanks also go to the members of the cardiology clinic staff for providing me with the necessary materials for the study. I would like to also give my thanks to the Department of Internal Medicine, Addis Ababa University, College of health sciences for giving me this golden opportunity to conduct my research including providing the necessary funding.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bozkurt B, Coats AJ, Tsutsui H, et al. Universal definition and classification of heart failure: a report of the heart failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing committee of the universal definition of heart failure. J Card Fail. 2021;27:S1071–S9164.
2. Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. doi:10.1056/NEJMra072139
3. Hill JA, Olson EN. Muscle: Fundamental Biology and Mechanisms of Disease. New York, NY: Acadamicpressl; 2012.
4. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contributio; 2021.
5. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017;3(1):7–11. doi:10.15420/cfr.2016:25:2
6. Ogah OS, Adebiyi A, Sliwa K. Heart failure in Sub-Saharan Africa. In: Rescigno G, Firstenberg MS, editors. Topics in Heart Failure Management [Internet]. London: IntechOpen; 2019.
7. Tsega TA, Demissei BG. A systematic review of epidemiology, treatment and prognosis of heart failure in adult in Ethiopia. J Cardiovasc Med. 2018;19(3):91–97.
8. Shewaye A, Bayisa T, Adamu F, Abdissa S. Medical admissions and outcomes at Saint Paul’s Hospital, Addis Ababa, Ethiopia: a retrospective study. Ethiop J Health Dev. 2017;30:50–56.
9. Levy D, Kenchaiah S, Larson MG, et al. Long term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. doi:10.1056/NEJMoa020265
10. World Health Organization. Noncommunicable diseases country profiles; 2018.
11. Meshesha MD, HussenKabthymer R, MechaAbafogi M. Mortality and its associated factors among hospitalized heart failure patients: the case of South West Ethiopia. Cardiol Res Pract. 2021;2021:8. doi:10.1155/2021/5951040
12. Mulubirhan T, Nedi T, Mekonnen D, Berha AB. Treatment outcome and its predictors among patients of acute heart failure at a tertiary care hospital in Ethiopia: a prospective observational study. BMC Cardiovasc Disord. 2020;20:16. doi:10.1186/s12872-019-01318-x
13. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2013;62(16):e147–e239. doi:10.1016/j.jacc.2013.05.019
14. Yancy CW, Jessup M, Bozkurt B, et al. ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. doi:10.1161/CIR.0000000000000509
15. Ponikowsk P, Voors AA, Anker SD, et al. ECS guidelines for heart failure treatment. Eur Heart J. 2016;37(27):2129–2200. doi:10.1093/eurheartj/ehw128
16. Heidenreich PA, Bozkurt B, Aguilar D, et al. AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;17:e263–e421.
17. Wallukat G. The beta-adrenergic receptors. Herz. 2002;27(7):683–690. doi:10.1007/s00059-002-2434-z
18. Florea VG, Cohn JN. The autonomic nervous system and heart failure. Circ Res. 2014;114:1815–1826. doi:10.1161/CIRCRESAHA.114.302589
19. Packer M, Coats AJS, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–1658. doi:10.1056/NEJM200105313442201
20. Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure.US Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–1355. doi:10.1056/NEJM199605233342101
21. The MERIT-HF Investigators. Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF). Lancet. 1999;353:2001–2007. doi:10.1016/S0140-6736(99)04440-2
22. The CIBIS-II Investigators. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13. doi:10.1016/S0140-6736(98)11181-9
23. Flather MD, Shibata MC, Coats AJS, et al; SENIORS Investigators. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J. 2005;26:215–225. doi:10.1093/eurheartj/ehi115
24. Böhm M, Maack C. Treatment of heart failure with beta-blockers. Mechanisms and results. Basic Res Cardiol. 2000;95:I15–I24. doi:10.1007/s003950070004
25. Basile JN. Titration of beta-blockers in heart failure. How to maximize benefit while minimizing adverse events. Postgrad Med. 2003;113:63–70. doi:10.3810/pgm.2003.03.1389
26. Wikstrand J, Hjalmarson A, Waagstein F, et al. Dose of metoprolol CR/XL and clinical outcomes in patients with heart failure: analysis of the experience in metoprolol CR/XL randomized intervention trial in chronic heart failure (MERIT-HF). J Am Coll Cardiol. 2002;40:491–498. doi:10.1016/S0735-1097(02)01970-8
27. Franke J, Wolter JS, Meme L, et al. Optimization of pharmacotherapy in chronic heart failure: is heart rate adequately addressed? Clin Res Cardiol. 2013;102:23–31. doi:10.1007/s00392-012-0489-2
28. Legesse Y, Asgedom S, Demoz G, Gidey K. Treatment optimization of beta-blockers in chronic heart failure therapy. Sci Rep. 2020;10:15903. doi:10.1038/s41598-020-72836-4
29. Berthelot E, Eicher J, Salvat M, Seronde M, de Groote P. Medical inertia in the optimization of heart failure treatment after discharge and its relationship to outcome. Health Care Curr Rev. 2018;2:12.
30. de Groote P, Isnard R, Assyag P, et al. Is the gap between guidelines and clinical practice in heart failure treatment being filled? Insights from the IMPACT RECO survey. Eur J Heart Fail. 2007;9:1205–1211. doi:10.1016/j.ejheart.2007.09.008
31. Loop MS, Van Dyke MK, Chen L, et al. Low utilization of beta-blockers among medicare beneficiaries hospitalized for heart failure with reduced ejection fraction. J Card Fail. 2018;16:31102–31107.
32. Gheorghiade M, Albert NM, Curtis AB, et al. Medication dosing in outpatients with heart failure after implementation of a practice-based performance improvement intervention: findings from IMPROVE HF. Congest Heart Fail. 2012;18(1):9–17. doi:10.1111/j.1751-7133.2011.00250.x
33. Bhatt AS, DeVore AD, DeWald TA, Swedberg K, Mentz RJ. Achieving a maximally tolerated β-blocker dose in heart failure patients: is there room for improvement? J Am Coll Cardiol. 2017;69(20):2542–2550. doi:10.1016/j.jacc.2017.03.563
34. Solal AC, Leurs I, Assyag P, et al. Optimization of heart failure medical treatment after hospital discharge according to left ventricUlaR Ejection fraction: the FUTURE survey. Arch Cardiovasc Dis. 2012;105:355–365. doi:10.1016/j.acvd.2012.04.003
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.