Back to Journals » Clinical Ophthalmology » Volume 16
Utility of Liquid-Based Cytology and Cell Block Procedure Obtained by Vitrectomy to Diagnose Ocular Sarcoidosis–The Significance of Epithelioid Granuloma and Epithelioid Cells
Authors Ohe R , Kaneko Y, Namba H, Nishi K , Goto JI, Futakuchi M , Nishitsuka K
Received 25 May 2022
Accepted for publication 28 September 2022
Published 10 October 2022 Volume 2022:16 Pages 3289—3296
DOI https://doi.org/10.2147/OPTH.S376141
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Rintaro Ohe,1 Yutaka Kaneko,2 Hiroyuki Namba,2 Katsuhiro Nishi,2 Jun-Ichi Goto,3 Mitsuru Futakuchi,1 Koichi Nishitsuka2
1Department of Pathology, Yamagata University Faculty of Medicine, Yamagata, Japan; 2Department of Ophthalmology and Visual Sciences, Yamagata University Faculty of Medicine, Yamagata, Japan; 3Department of Physiology, Yamagata University Faculty of Medicine, Yamagata, Japan
Correspondence: Koichi Nishitsuka, Department of Ophthalmology and Visual Sciences, Yamagata University Faculty of Medicine, 2-2-2 Iida-Nishi, Yamagata, 990-9585, Japan, Tel +81-23-628-5374, Fax +81-23-628-5377, Email [email protected]
Purpose: The eyes are one of the most frequently involved organs in sarcoidosis in Asia, including Japan. Sarcoid uveitis is the major complaint of ocular sarcoidosis. The detection of epithelioid granuloma (EG) requires histological biopsy of the uvea for the precise diagnosis of sarcoid uveitis, because it is challenging to diagnose sarcoid uveitis without a history of systemic sarcoidosis. To diagnose sarcoid uveitis, we have established novel methods.
Patients and Methods: In this study, we included 30 eyes of 21 patients with granulomatous uveitis diagnosed via slit-lamp examinations, gonioscopy, fundus photography, and fluorescein angiography. Vitrectomy was performed to remove the vitreous opacity with vision loss. To examine vitreous cell components, we used liquid-based cytology (LBC). To detect EG in an intraocular irrigating solution, we collected vitreous cell components, and then the cell pellets were embedded in the cell block procedure.
Results: Here, we demonstrated the usefulness of the histological detection of EG and epithelioid cells (ECs) in LBC from vitreous body specimens and in the cell block procedure from vitreous cell components in an intraocular irrigating solution. Our results showed that the detection rates of EG were 6.3% (1/16) in LBC and 9.1% (1/11) in the cell block procedure in the sarcoid uveitis-suspected group and 7.7% (1/13) in LBC and 28.6% (2/7) in the cell block procedure in the sarcoidosis group. We would discuss the specificity of the EG/EC detection of ocular sarcoidosis.
Conclusion: Our methods are helpful in the precise diagnosis of ocular sarcoidosis and the control of the development of systemic sarcoidosis.
Keywords: uveitis, sarcoidosis, vitrectomy, liquid-based cytology, cell block procedure
Introduction
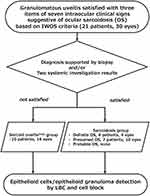
The eyes are well known to be one of the most frequently involved organs in sarcoidosis in Asia, including Japan.1 A major manifestation of ocular sarcoidosis is sarcoid uveitis, and the eyes may be the initial organs involved in systemic sarcoidosis.2 It is challenging to diagnose sarcoid uveitis without a history of systemic sarcoidosis.2 In a previous study, the patients in the sarcoidosis group were classified into definite ocular sarcoidosis, presumed ocular sarcoidosis, and probable ocular sarcoidosis based on the intraocular clinical signs, histological biopsy, and systemic investigation results of the International Workshop on Ocular Sarcoidosis (IWOS) criteria.3 In contrast, patients were classified into the sarcoid uveitis-suspected group (sarcoid uveitissusp group) when more than two items were gained out of seven intraocular clinical signs without a histological biopsy and systemic investigation results.3
The uvea and retina are the main tissues involved in sarcoid uveitis. For a precise diagnosis of sarcoid uveitis, the detection of epithelioid granuloma (EG) would be required by histological biopsy.4 However, a histological biopsy of the uvea would increase the risk of blindness. Therefore, the appropriate examination of vitreous body specimens is required to replace the histological detection of EG in the uvea for ophthalmologists and sarcoidosis specialists in patients with vision loss.5,6
Previously, we established liquid-based cytology (LBC) to examine vitreous cell components.7,8 This method required a volume of 0.4–1.0 mL of vitreous body specimens and would reflect the histological detection of EG in the uvea without the risk of blindness. However, our LBC method may not reflect the entire vitreous body because the volumes of vitreous body specimens used in our method do not correspond to the total volume of the vitreous body (4 mL).
To treat uveitis with vitreous opacity, vitrectomy is often performed at our university hospital. During vitrectomy at our hospital, the vitreous body is replaced by an intraocular irrigating solution (IS). As a result, approximately 100–250 mL of the intraocular IS mixes with the vitreous body. It has been difficult for us to detect EG in such a high volume of this intraocular IS because of the low cell density. Therefore, to detect EG in the intraocular IS, we collected vitreous cell components via centrifugation, and the cell pellet was embedded in the cell block procedure. In this paper, for the precise diagnosis of sarcoid uveitis, we demonstrated the usefulness of the histological detection of EG and epithelioid cells (ECs) in LBC from vitreous body specimens and in the cell block procedure from vitreous cell components in the intraocular IS.
Materials and Methods
Patients and Eyes
Thirty eyes of 21 patients with granulomatous uveitis diagnosed via slit-lamp examination, gonioscopy, fundus photography, and fluorescein angiography were included in this study (Figures 1 and 2A–D). Before vitrectomy, all patients used betamethasone eye drops and only one patient took prednisolone in the sarcoid uveitissusp group. Vitrectomy was performed to remove vitreous opacity with vision loss for the 16 eyes in the sarcoid uveitissusp group that did not meet the diagnostic criteria for sarcoidosis and the 14 eyes in the sarcoidosis group (Figure 1). Two patients with sarcoid uveitissusp who developed cardiac sarcoidosis in our hospital were excluded from this study because they did not have vision loss that required vitrectomy.
All patients were treated using a 25-gauge pars plana vitrectomy instrument with either the Constellation Vision System (Alcon Laboratories, Fort Worth, Texas, USA) or EVA vitrectomy system (DORC, Zuidland, The Netherlands) for the removal of vitreous opacity. Standard core vitrectomy was performed under infusion with BSS Plus™ (Alcon, Fort Worth, USA), and diluted vitreous fluid (0.4 mL) filling the vitrectomy cutter tube was obtained for LBC.7,8 After the vitrectomy, diluted vitreous cell components in the drainage bag (100–250 mL) were collected for the cell block procedure.
CD4/CD8 Ratio by Flow Cytometry
Navios (Beckman Coulter, Miami, FL) performed flow cytometry according to the standard protocol. To measure the CD4/CD8 ratio of the diluted vitreous body, we used the Dual Color Kit CD4/CD8 [CD4-FITC (clone 13B8.2), CD8a-PE (clone B9.11), Beckman Coulter] as was done in a previous study.9
LBC and Cell Block Procedure
We performed LBC on 30 samples from vitreous bodies to identify EG/ECs in the sarcoid uveitissusp group and sarcoidosis group (Tables 1 and 2, respectively). LBC specimens were performed using a Surepath® (BD Diagnostics, Tokyo, Japan) system as previously described.7 For Papanicolaou staining, these properly processed specimens were fixed in 95% ethanol for 10 min. Out of 30 samples, this method produced 29 LBCs.
 |
Table 1 Epithelioid Granuloma/Epithelioid Cell in Vitreous Body Sample in the Group of the Patients with Sarcoid Uveitis, Suspected |
 |
Table 2 Epithelioid Granuloma/Epithelioid Cell in Vitreous Body Sample in the Group of the Patients with Sarcoidosis |
We performed cell block procedure specimens on 19 samples from vitreous bodies to identify EG/ECs in sarcoid the uveitissusp group and the sarcoidosis group (Tables 1 and 2, respectively). After the supernatant liquid was decanted, the sediment was fixed in 10% neutral-buffered formalin (five times the volume of the sediment) for 24 hours. After centrifugation at 3000 rpm for 5 min (Model 5911, KUBOTA), the formalin was decanted. We added 0.1 mL of 1% sodium alginate (JUNSEI CHEMICAL, Tokyo, Japan) and 0.2 mL of calcium chloride (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) to the sediment to solidify it. The solid sediment was embedded in paraffin and used for hematoxylin–eosin staining. Out of 19 samples, this method produced 18 cell block procedures.
Cytological and pathological diagnoses were made at the Yamagata University Hospital between 2015 and 2021. All cases were reviewed by experienced pathologists (OR and FM). This study was approved by the Research Ethics Committee of the Yamagata University Faculty of Medicine (2019–314) and conducted in accordance with the principles of the Declaration of Helsinki. Because of the retrospective study design, the requirement for patients’ informed consent was waived by the institutional review board. All data were handled with confidentiality to ensure participants’ privacy.
Statistical Analysis
To compare the detection rates of EG/ECs, Fisher’s exact test was used.
Results
EG/ECs in Vitreous Body Samples from the Group of Patients with Suspected Sarcoidosis
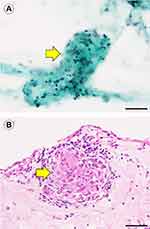
The findings of EG/ECs in vitreous body specimens in the sarcoid uveitissusp group are shown in Figure 3A and B and in Table 1. Pathologically, EG was defined as a granuloma consisting of more than 10 ECs. Pathologically, ECs were defined as cells with monocyte-like nuclei, broad cytoplasm, and adhesive properties. In LBC, the detection rate of EG was 6.3% (1/16), whereas that of ECs was 93.8% (15/16). In the cell block procedure, the detection rate of EG was 9.1% (1/11), whereas that of ECs was 81.8% (9/11). In summary, specific findings of sarcoid uveitis by LBC were observed in 15 of 16 eyes, and specific findings of sarcoid uveitis by the cell block procedure were observed in 9 of 11 eyes (Table 1).
EG/ECs in Vitreous Body Samples in the Group of Patients with Sarcoidosis
The findings of EG/ECs in vitreous body specimens in the sarcoidosis group are shown in Table 2. Similar to the sarcoid uveitissusp group, we identified EG/ECs in vitreous body specimens in the sarcoidosis group. In LBC, the detection rate of EG was 7.7% (1/13), whereas that of ECs was 100% (13/13). In the cell block procedure, the detection rate of EG was 28.6% (2/7), whereas that of ECs was 100% (7/7). In summary, the specific findings of sarcoid uveitis by both LBC and the cell block procedure were observed in all eyes that were examined (Table 2).
Comparison of the Detection Rates of Epithelioid Granuloma/Epithelioid Cells Between the Sarcoid Uveitissusp Group and the Sarcoidosis Group
Our results revealed that the detection rate of EG in the sarcoid uveitissusp group was similar to that in the sarcoidosis group (LBC, P = 1; cell block procedure, P = 0.528), and the detection rate of ECs in the sarcoid uveitissusp group was similar to that in the sarcoidosis group (LBC, P = 1; cell block procedure, P = 0.096).
Discussion
The uvea is adjacent to the retina, and the retina touches the vitreous body. These anatomical pieces of evidence indicate that inflammation in the uvea (uveitis) would spread to the retina, resulting in retinitis, and retinitis would spread to the vitreous body. Therefore, the inflammation of sarcoid uveitis spreads to the vitreous body through the retina, resulting in vitreous opacity and vision loss. In this study, we considered that the cell components in the vitreous body would reflect the inflammatory cells associated with sarcoid uveitis. Therefore, a vitreous biopsy LBC could assess sarcoid uveitis.
In non-granulomatous uveitis, neutrophils, lymphocytes, and macrophage would be detected in the vitreous body. In granulomatous uveitis, epithelioid cells would be detected in the vitreous body because these cells are specifically induced in granulomatous inflammation, such as tuberculosis and sarcoidosis. Therefore, we considered that the present methods would contribute to the diagnosis of sarcoid uveitis.
Systemic sarcoidosis is known to occur EG in several organs, including the lungs, lymph nodes, eyes, heart, and skin.10 Ocular sarcoidosis is one of the initial manifestations of systemic sarcoidosis, and sarcoid uveitis is a major manifestation of ocular sarcoidosis.2 For the treatment of patients with sarcoid uveitis, vitrectomy would be performed to remove vitreous opacity, and then vitreous body specimens and an intraocular IS were obtained. Thus, to detect EG, we used LBC for vitreous body specimens and the cell block procedure for vitreous cell components in the intraocular IS.
In this study, we examined 10 patients with sarcoid uveitissusp and 11 patients with sarcoidosis. Among the 11 patients with sarcoidosis, several lesions were observed in the eyes and other organs. Eleven patients with sarcoid uveitissusp gained more than two items out of seven intraocular clinical signs based on the IWOS criteria for ocular sarcoidosis.3 However, granulomatous lesions were not detected in other organs except the eyes. Up to 32.6–80% of patients with sarcoid uveitis develop systemic sarcoidosis.1,2 In our hospital, two patients with sarcoid uveitis developed cardiac sarcoidosis (data not shown). Thus, a prior diagnosis of ocular sarcoidosis predicts the subsequent development of systemic sarcoidosis and underscores the need for constant observation by the physician. If we found EG in other organs, we would start the initial treatment for systemic sarcoidosis without delay. The detection of EG via histological biopsy would be required for the precise diagnosis of systemic sarcoidosis.4 In this study, we identified EG via LBC of vitreous body specimens and via the cell block procedure in the intraocular IS by vitrectomy (Figure 3A and B). In the sarcoid uveitissusp group, the detection rates of EG were 6.3% (1/16) in LBC and 9.1% (1/11) in the cell block procedure. However, in the sarcoidosis group, the detection rates of EG were 7.7% (1/13) in LBC and 28.6% (2/7) in the cell block procedure. Our results revealed that the detection rate of EG in the sarcoid uveitissusp group was similar to that in the sarcoidosis group. We could recognize clear EG in both LBC and the cell block procedure in each group despite these low detection rates. Recognition of clear EG in LBC and/or cell block procedure would reflect histological detection of EG in the uvea. If we could recognize clear EG in LBC and cell block procedure from vitrectomy, sarcoid uveitissusp group should be considered as the sarcoidosis group.
In this study, the detection rates of EG in LBC and the cell block procedure were low because a few EGs were detected in vitreous cell components, whereas many ECs were detected in these components. In sarcoidosis lesions, ECs assemble and form EGs. However, the detection of EC was not included in the diagnostic criteria for systemic sarcoidosis. To include the detection of EC in the list of diagnostic criteria for systemic sarcoidosis, we should discuss the specificity of EC detection to ocular sarcoidosis. ECs in vitreous body specimens would be observed in patients with sarcoid uveitis, tuberculous uveitis, and Vogt–Koyanagi–Harada disease. Because ECs in the vitreous body could reflect choroidal granuloma in tuberculous uveitis11 and Vogt–Koyanagi–Harada disease,12 these two diseases should be ruled out for a precise diagnosis of sarcoid uveitis. A combination of a chest X-ray, purified protein derivative interferon-γ-release assay, and history of pulmonary tuberculosis would help diagnose tuberculous uveitis.13 The diagnosis of Vogt–Koyanagi–Harada disease is easy because of specific clinical examinations, such as the detection of the specific human leukocyte antigen type, fluorescein angiography, indocyanine angiography, and optical coherence tomography.12 Altogether, the specificity of EC detection for the diagnosis of ocular sarcoidosis would increase with the help of these clinical examinations. It is possible that the sarcoid uveitissusp group (sarcoid uveitis) is considered the pathologically compatible (sarcoid uveitispathol compati) group by the recognition of ECs in LBC and the cell block procedure from vitrectomy. Our methods may bring benefits to the patients in the sarcoid uveitispathol compati group by having them undergo the following examination to check for signs of progression to systemic sarcoidosis.
Because we used only 16 samples in the sarcoid uveitissusp group, further studies on more samples are required to draw conclusions with certainty.
Conclusion
In summary, we have established novel methods to diagnose sarcoid uveitis. We detected EG/ECs in LBC from vitreous body specimens and in the cell block procedure from vitreous cell components in the intraocular IS. It is possible that those in the sarcoid uveitissusp group would be considered patients with ocular sarcoidosis by the recognition of EG in LBC and the cell block procedure from vitrectomy. Our methods would be helpful in the precise diagnosis of ocular sarcoidosis.
Abbreviations
IWOS, International Workshop on Ocular Sarcoidosis; EG, epithelioid granuloma; LBC, liquid-based cytology; IS, irrigating solution; ECs, epithelioid cells.
Ethics Approval and Informed Consent
This study was approved by the Research Ethics Committee of the Yamagata University Faculty of Medicine (2019-314) and performed in accordance with the principles of the Declaration of Helsinki. Because of the retrospective study design, the requirement for participants’ informed consent was waived by the institutional review board. All data were handled with confidentiality to ensure participants’ privacy.
Acknowledgments
This work was supported by JSPS KAKENHI (Grant numbers JP21K06901 and JP20K18373). The authors are grateful to Toshinori Suzuki (Division of Clinical Laboratory, Yamagata University Hospital) for their valuable assistance during this study.
Funding
Japan Society for the Promotion of Science; Grant/Award Numbers JP21K06901 (Rintaro Ohe) and JP20K18373 (Katsuhiro Nishi).
Disclosure
Dr Jun-Ichi Goto reports grants from Yamagata University, during the conduct of the study. Mr Koichi Nishitsuka reports personal fees from Santen, RE MEDICAL, SENJU Pharmaceutical Co., Ltd., NOVARTIS, HOYA, Otsuka Pharmaceutical, CHUGAI PHARMACEUTICAL CO., LTD., and ALCON, outside the submitted work; the authors report no other conflicts of interest in this work.
References
1. Reid G, Williams M, Compton M, Silvestri G, McAvoy C. Ocular sarcoidosis prevalence and clinical features in the Northern Ireland population. Eye. 2021;36(10):1918–1923. doi:10.1038/s41433-021-01770-0
2. Ma SP, Rogers SL, Hall AJ, et al. Sarcoidosis–related uveitis: clinical presentation, disease course, and rates of systemic disease progression after uveitis diagnosis. Am J Ophthalmol. 2019;198:30–36. doi:10.1016/j.ajo.2018.09.013
3. Mochizuki M, Smith JR, Takase H, Kaburaki T, Acharya NR, Rao NA. Revised criteria of International Workshop on Ocular Sarcoidosis (IWOS) for the diagnosis of ocular sarcoidosis. Br J Ophthalmol. 2019;103:1418–1422. doi:10.1136/bjophthalmol-2018-313356
4. Crouser ED, Maier LA, Wilson KC, et al. Diagnosis and detection of sarcoidosis. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201:e26–e51. doi:10.1164/rccm.202002-0251ST
5. Matsuo T, Ichimura K. Immunocytochemical diagnosis as inflammation by vitrectomy cell blocks in patients with vitreous opacity. Ophthalmology. 2012;119:827–837. doi:10.1016/j.ophtha.2011.10.020
6. Kinoshita Y, Takasu K, Adachi Y, et al. Diagnostic utility of vitreous humor fluid cytology for intraocular sarcoidosis: a clinicopathologic study of 7 cases. Diagn Cytopathol. 2012;40:210–213. doi:10.1002/dc.21540
7. Narumi M, Nishitsuka K, Yamakawa M, Yamashita H. A survey of vitreous cell components performed using liquid-based cytology. Acta Ophthalmol. 2015;93:e386–e390. doi:10.1111/aos.12623
8. Nishituka K, Kaneko Y, Narumi M, Namba H, Ohe R, Yamashita H. Usefulness and safety of liquid-based cytology in vitreous pathology following vitrectomy. Indian J Ophthalmol Case Rep. 2021;1:818–819. doi:10.4103/ijo.IJO_3811_20
9. Kojima K, Maruyama K, Inaba T, et al. The CD4/CD8 ratio in vitreous fluid is of high diagnostic value in sarcoidosis. Ophthalmology. 2012;119:2386–2392. doi:10.1016/j.ophtha.2012.05.033
10. Tana C, Drent M, Nunes H, et al. Comorbidities of sarcoidosis. Ann Med. 2022;54:1014–1035. doi:10.1080/07853890.2022.2063375
11. Cunningham ET, Rathinam SR, Albini TA, Chee SP, Zierhut M. Tuberculous uveitis. Ocul Immunol Inflamm. 2015;23:2–6. doi:10.3109/09273948.2014.1004016
12. Knecht PB, Mantovani A, Herbort CP. Indocyanine green angiography-guided management of Vogt-Koyanagi-Harada disease: differentiation between choroidal scars and active lesions. Int Ophthalmol. 2013;33:571–577. doi:10.1007/s10792-012-9692-4
13. Agrawal R, Testi I, Mahajan S, et al. Collaborative ocular tuberculosis study consensus guidelines on the management of tubercular uveitis–report 1: guidelines for initiating antitubercular therapy in tubercular choroiditis. Ophthalmology. 2021;128:266–276. doi:10.1016/j.ophtha.2020.01.008
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.