Back to Journals » Chronic Wound Care Management and Research » Volume 4
Utility of a three-dimensional wound measurement device in pressure ulcers
Authors Goto T , Nakagami G, Nakai A, Noyori S, Sasaki S, Hayashi C, Miyagaki T, Akamata K, Sanada H
Received 24 July 2017
Accepted for publication 12 September 2017
Published 24 October 2017 Volume 2017:4 Pages 129—133
DOI https://doi.org/10.2147/CWCMR.S147139
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Marco Romanelli
Taichi Goto,1–3 Gojiro Nakagami,1,4 Ayano Nakai,1 Shuhei Noyori,1,5 Sanae Sasaki,6 Chieko Hayashi,6 Tomomitsu Miyagaki,7 Kaname Akamata,7 Hiromi Sanada1,4
1Department of Gerontological Nursing/Wound Care Management, Graduate School of Medicine, 2Global Leadership Initiative for an Age-Friendly Society, The University of Tokyo, Bunkyo-ku, 3Japan Society for the Promotion of Science, Chiyoda-ku, Japan; 4Global Nursing Research Center, Graduate School of Medicine, The University of Tokyo, 5Graduate Program for Social ICT Global Creative Leaders, The University of Tokyo, 6Department of Nursing, 7Department of Dermatology, The University of Tokyo Hospital, Bunkyo-ku, Tokyo, Japan
Introduction: Depth assessment is important for severe pressure ulcers (PUs); however, a device for the metric measurement of wounds, including depth, is lacking in clinical settings. Recent technological advancements have enabled the evaluation of the depth of wounds, and three-dimensional measurements are now available. The aim of this study was to test the utility of a newly developed three-dimensional wound measurement device in the clinical setting.
Methods: We recruited three patients, each with a PU, who were being treated by a PU team at a university hospital. We measured the length, width, area, and maximal depth of the ulcers by using the device and with the conventional method. The ulcer volume was measured only with the device. The difference in measurement results of the device before and after debridement was compared in the first patient. The difference in measurement results between the conventional method and the device was compared in the second patient. Correlation coefficients between the conventional method and the device obtained from longitudinal data were calculated in the third patient.
Results: The changes in measurements between before and after debridement were easily detected by the device in the first patient. Although the maximal depth was different, the length, width, and area were consistent between the conventional method and the device in the second patient. The correlation coefficients of the length, width, and area between the conventional method and the device were 0.35, 0.48, and 0.59, respectively, in the third patient whose PU exhibited a vague wound edge.
Conclusion: Although two-dimensional measurements were comparable between the conventional method and the device, there were some challenges to performing three-dimensional measurement more precisely. A further study is needed to determine the specific characteristics of wounds that result in poor measurement accuracy.
Keywords: three-dimensional wound measurement device, wound depth, wound volume
Background
The prevalence of pressure ulcers (PUs) dramatically decreased within a decade following the recognition that PUs are a central cause of deterioration in elderly people.1,2 However, severe PUs are still a challenge in clinical settings,3 being a major cause of fatal infection. Although assessing the depth of such severe PUs is important for monitoring, only macroscopic observations, such as those obtained with the DESIGN-R® tool,4 are performed owing to the lack of a device for precisely measuring the depth in clinical settings.
Recently, a three-dimensional wound measurement device, inSightTM (eKare, Fairfax, VA, USA), has been developed. The device is attached to an Apple iPad miniTM for the measurement of wound dimension. A previous study that used an artificial wound model and an animal wound model demonstrated an improvement in measurement accuracy and a significant reduction in measurement time compared with a conventional method.5,6 However, in such wound models, wounds can be easily measured because of the readily detectable wound edge. Thus, it is still unknown whether the device can be applied to patients with PUs with various wound edge characteristics in the clinical setting. Recently, the PU team of our university hospital has begun using inSightTM during clinical rounds. In this study, we aimed to discuss the utility of this three-dimensional wound measurement device in the context of severe PUs.
Methods
Setting and participants
This case study was conducted at a university hospital in an urban area of Tokyo, Japan. We recruited three inpatients with a PU. The three patients provided written informed consent to participate in this research and to have the case details and images published. The procedure of the present study was approved by the ethics committee of the Graduate School of Medicine, The University of Tokyo.
Three-dimensional wound measurement
A three-dimensional wound measurement device (inSightTM) was used in this study. It consists of an Apple iPad miniTM, a Structure Sensor, and computer vision algorithms. The region of interest on the depth map was defined by using the wound edge, which is provided by the structure sensor. The region of interest on the depth map was then transformed into a three-dimensional space where the metric measurement was performed. Wound border segmentation was performed by using the interactive Graph Cuts algorithm developed by Boykov and Jolly.7 The wound area was distinguished from the non-wound area through simple finger swipes on the touch screen of the iPad miniTM. The segmentation result was displayed in real time, and the user had the flexibility to fine-tune the segmentation as needed.5,6
Wound measurement
During the clinical rounds of the PU team for patients included in this study, we photographed the PUs by using a digital camera and evaluated the wound healing status by using DESIGN-R®.4 DESIGN-R® consists of seven items: depth, exudate, size, inflammation/infection, granulation tissue, necrotic tissue, and undermining. A higher score indicates a worse condition of the PU. Then, we measured the length, width, area, and maximal depth of the wound by using an image analyzing software (ImageJ; National Institutes of Health, Bethesda, MD, USA), which served as reference measurements. The wound was also analyzed by using inSightTM during clinical rounds of the PU team. Wound characteristics, including the length, width, area, maximal depth, and volume, were calculated in accordance with the company’s product description.
Statistical analyses
To examine the utility of inSightTM, we performed comparisons 1) between the measurement results before and after debridement and 2) between the reference measurements and the device measurements. For repeatedly measured data, we calculated Pearson’s correlation coefficients.
Results
Comparison of the measurement results between before and after debridement
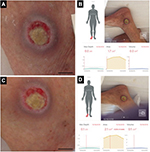
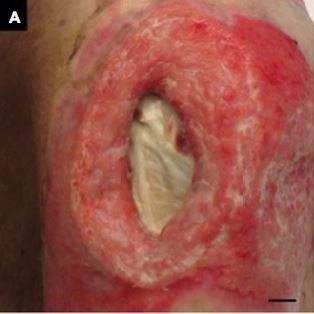
The first patient was a man in his 70s who exhibited decreased consciousness because of hepatic encephalopathy. A PU developed near his right lateral malleolus. Figure 1A and C shows the gross appearances of the wound before and after debridement, and Figure 1B and D shows the measurement results indicated on the touch screen of the iPad miniTM. The DESIGN-R® score at the first examination was D3-e1s3i0G5N3p0 (12 points). The inSightTM software-enabled measurements of the PU before and after debridement were as follows: area (1.7 vs. 2.1 cm2), maximal depth (0.0 vs. 0.1 cm), and volume (0.0 vs. 0.1 cm3; Table 1).
  | Table 1 Measurement results before and after debridement |
Comparison between the reference measurements and the device measurements
The second patient was a woman in her 60s who experienced septic shock. A PU developed near her left greater trochanter. Figure 2A shows the gross appearance of the wound, and Figure 2B shows the measurement results indicated on the touch screen of the iPad miniTM. The DESIGN-R® score at the first examination was D4-e3s8i1G5N3p0 (20 points). The measurements according to the reference and inSightTM software were as follows: length (4.5 vs. 4.5 cm), width (2.9 vs. 2.9 cm), area (9.8 vs. 9.2 cm2), and maximal depth (1.2 vs. 0.4 cm). Additionally, the volume measured by the device was 1.8 cm3 (Table 2).
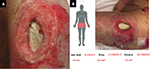  | Figure 2 Gross appearance and measurement results of the second patient. Note: (A) Gross appearance and (B) measurement results. The bar indicates 1.0 cm. Abbreviation: Max, maximum. |
  | Table 2 Measurement differences between the reference tool and inSightTM |
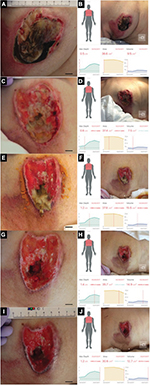
The third patient was a woman in her 80s who developed an acute extradural hematoma. We observed this patient longitudinally. A PU developed in her right breast. Figure 3 shows the gross appearance of her wound and its measurements. The DESIGN-R® score at the first examination was DU-e3s8i1G6N6p0 (24 points). Figure 4 shows the scatter plots of the reference measurements and the inSightTM software measurements. The correlation coefficients of the length, width, and area were 0.35, 0.48, and 0.59, respectively.
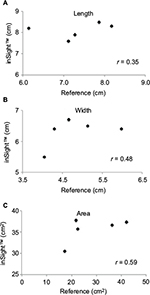  | Figure 4 Scatter plots and Pearson’s correlation coefficients of length, width, and area of the third patient. Note: (A) Length, (B) width, and (C) area. |
Discussion
The present study examined the utility of a newly developed three-dimensional wound measurement device, inSightTM. As a result, we found that this device has both advantages and disadvantages.
To assess whether the device can determine the wound area, maximal depth, and volume change after debridement, we compared the measurement results between before and after debridement in the first patient. We hypothesized that the measurement results after debridement would be larger than those before debridement because debridement is expected to increase the wound area, maximal depth, and volume. The results were consistent with our hypothesis, which suggests that inSightTM may be a valid tool for intra-patient measurement.
To examine the consistency between the reference measurements and the inSightTM measurements, the PUs of the second and third patients were measured. Whereas the length, width, and area were highly consistent between the two methods, the device-enabled maximal depth value was much smaller than the reference result. Thus, it is possible that the device-measured volume was not accurate. The device measures maximal depth by calculating the difference between the wound edge and the wound bed by using the reflection of infrared radiation. Therefore, the inaccurate measurement of the maximal depth may be owing to the inability of the device to precisely detect the wound edge because of the mortar-shaped wound of the second patient. Moreover, another possible reason was that the device failed to detect the deepest point of the PUs, which resulted in an underestimation of the maximal depth. According to the results of the third patient, the correlation coefficients were low to moderate. The vague wound edge of the third patient may have rendered the measurement results inaccurate. This may mean that there are certain wound characteristics that are unsuitable for measurement with this device.
This study has several limitations. First, the sample size was not sufficient to perform statistical analyses. However, we believe that the three patients included in this study each had individual PUs with variable measurements, thereby allowing this pilot study to inform a larger clinical study. Second, we could not evaluate the accuracy of the volume measurement because certain volume measurement methods could not be applied in this clinical setting.8
Conclusion
inSightTM can be applied to the two-dimensional measurement of wounds with obvious edges. While real-time measurements can inform patient care, there are some challenges to conducting accurate three-dimensional measurements. To use the device more efficiently, further studies that examine its reliability and validity need to be conducted, and specific characteristics of wounds for which the device is suitable should be identified.
Acknowledgment
inSightTM was offered for clinical use by Daewoong Pharmaceutical Co., Ltd. (Seoul, Korea).
Disclosure
The authors report no conflicts of interest in this work.
References
Sanada H, Miyachi Y, Ohura T, et al. The Japanese pressure ulcer surveillance study: a retrospective cohort study to determine prevalence of pressure ulcers in Japanese hospitals. Wounds. 2008;20(6):176–182. | ||
Gorecki C, Brown JM, Nelson EA, et al. Impact of pressure ulcers on quality of life in older patients: a systematic review. J Am Geriatr Soc. 2009;57(7):1175–1183. | ||
Coyer F, Miles S, Gosley S, et al. Pressure injury prevalence in intensive care versus non-intensive care patients: a state-wide comparison. Aust Crit Care. 2017;30(5):244–250. | ||
Matsui Y, Furue M, Sanada H, et al. Development of the DESIGN-R with an observational study: an absolute evaluation tool for monitoring pressure ulcer wound healing. Wound Repair Regen. 2011;19(3):309–315. | ||
Bills JD, Berriman SJ, Noble DL, Lavery LA, Davis KE. Pilot study to evaluate a novel three-dimensional wound measurement device. Int Wound J. 2016;13(6):1372–1377. | ||
Sheng J, Li H, Jin J, et al. Application of 3-dimensional wound analyzer in small area wound healing of animal. J Burn Care Res. Epub 2017 May 23. | ||
Boykov YY, Jolly M-P. Interactive graph cuts for optimal boundary & region segmentation of objects in N-D images. Proceedings of the Eighth IEEE International Conference on Computer Vision, ICCV. Vol. 1. IEEE; 2001:105–112. | ||
Little C, McDonald J, Jenkins MG, McCarron P. An overview of techniques used to measure wound area and volume. J Wound Care. 2009;18(6):250–253. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.