Back to Journals » Cancer Management and Research » Volume 12
Usefulness of Inflammation-Based Prognostic Scores for Predicting the Risk of Complications After Radical Resection of Colorectal Carcinoma
Authors Man W , Lin H , Liu Z, Jin L , Wang J, Zhang J, Bai Z , Yao H, Zhang Z, Deng W
Received 15 October 2019
Accepted for publication 22 January 2020
Published 11 February 2020 Volume 2020:12 Pages 1029—1038
DOI https://doi.org/10.2147/CMAR.S234448
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmet Emre Eşkazan
Wenhao Man,* Huajun Lin,* Zhao Liu, Lei Jin, Jin Wang, Jun Zhang, Zhigang Bai, Hongwei Yao, Zhongtao Zhang, Wei Deng
Department of General Surgery, Beijing Friendship Hospital, Capital Medical University and National Clinical Research Center for Digestive Diseases, Beijing 100050, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wei Deng
Department of General Surgery, Beijing Friendship Hospital, Capital Medical University, No. 95 Yongan Road, Xicheng District, Beijing 100050, People’s Republic of China
Tel +86 134 2613 6152
Email [email protected]
Purpose: We aimed to investigate the value of inflammation-based prognostic scores for predicting early complications after radical surgery for colorectal carcinoma.
Methods: We retrospectively analyzed data of 154 patients who underwent elective resection of colorectal carcinoma between January 2017 and December 2018 at Beijing Friendship Hospital. Univariate, multivariate, and receiver operating characteristic curve analyses were conducted. As inflammation indices, we evaluated the preoperative modified Glasgow Prognostic Score (GPS), as well as the C-reactive protein/albumin ratio (CAR), postoperative GPS, and C-reactive protein levels on postoperative day 3 (POD3).
Results: Within 30 days postoperatively, complications occurred in 80 patients (51.9%). And high levels of preoperative mGPS (P=0.002), preoperative CAR (P=0.019), POD3 CAR (P< 0.001) and POD3 poGPS (P< 0.001) can significantly affect postoperative complications after surgery for colorectal cancer, with CRP on POD3 (odds ratio, 1.015; 95% confidence interval, 1.006– 1.024; P=0.001) as independent risk factors. Among all inflammation-based indicators, POD3 CAR had the highest area under the curve (0.711) and positive predictive value (83.2%). Higher CAR (≥ 2.6) on POD3 was associated with a higher rate of complications (92.9% vs 36.6%, P< 0.001), especially of infectious nature (54.8% vs 16.1%, P< 0.001).
Conclusion: CAR≥ 2.6 on POD3 reflects sustained systemic inflammation and represents a useful predictor of complications after surgery for colorectal carcinoma, facilitating early detection, timely intervention, and enhanced recovery.
Keywords: C-reactive protein, albumin, Glasgow Prognostic Score, colorectal carcinoma, surgery, postoperative complications, risk prediction
Introduction
Colorectal carcinoma is the third most common malignant tumor worldwide, with surgical resection being the only effective treatment currently available.1,2 Despite important advances in surgical techniques, the overall rate of complications remains high, at around 30%.3 Postoperative complications are associated with increased treatment costs, prolonged hospital stay, delayed adjuvant chemotherapy, increased risk of recurrence, and a detrimental impact on survival.4–7 Therefore, timely and precise detection and treatment of any complication are critical to improving prognosis after colorectal carcinoma surgery.
In recent years, inflammatory response-based prognostic scoring systems have been developed as tools for clinical evaluation to aid in decision-making. The most common predictors include the C-reactive protein/albumin ratio (CAR), modified Glasgow Prognostic Score (mGPS), postoperative Glasgow Prognostic Score (poGPS), and neutrophil-to-lymphocyte ratio. Most studies in patients with colorectal carcinoma focused primarily on the oncologic prognosis and rarely reported on the performance of predictors for postoperative complications. Tissue trauma caused by surgery affects the body’s metabolic, neuroendocrine, and immune response. An immune response is reflected as an increase in the expression of pro-inflammatory cytokines and the subsequent increase in the levels of C-reactive protein (CRP) and albumin in the acute phase protein.8 Warschkow et al conducted a meta-analysis of studies covering 1832 patients and found that increased CRP levels on postoperative days (PODs) 3 and 4 were good predictors of postoperative complications.9 Labgaa et al found that early postoperative reduction in albumin levels was a predictor of postoperative complications.10 Therefore, we hypothesized that indices that combine CRP and albumin information would be useful for patient stratification according to the risk of postoperative complications. In this study, we examined the value of CAR, mGPS, and poGPS as predictors of early postoperative complications in patients who underwent surgery for colorectal carcinoma.
Patients and Methods
Study Design and Selection of Participants
The retrospective study was approved by the Ethics Committee of Beijing Friendship Hospital. Between January 2017 to December 2018, 154 patients without preoperative infection of the respiratory, digestive, or urinary tract underwent radical surgery for colorectal cancer and were enrolled in the study. And the operations were performed by the same group of surgeons. All the patients have signed written informed consent forms for their data to be used in the study and the data were kept confidential. This study was conducted in accordance with the Declaration of Helsinki.
Data Collection
We retrospectively examined the records maintained in our hospital’s database and extracted data regarding age, sex, body mass index, presence of chronic diseases, tumor location, neoadjuvant therapy, and pathological stage. We also collect data regarding the surgery, including the operation time, operation method, and intraoperative blood loss. Laboratory data collected in this study included preoperative albumin levels, preoperative mGPS, CAR on POD3, and poGPS on POD3.
Preoperative and postoperative inflammatory responses, respectively, were evaluated based on the mGPS and poGPS (Table 1), which represent widely validated and independent systemic inflammation-based prognostic scores.11 Many studies have reported that poGPS on PODs 3 and 4 is a predictor of infectious complications.12
 |
Table 1 Overview of the Glasgow Prognostic Score Systems Based on Biomarkers of Systemic Inflammation |
Definition of Postoperative Complications
The severity of complications occurring within 30 days after surgery was graded based on the Clavien-Dindo classification.13 The main types of infectious complications included wound infection, urinary tract infection, pulmonary infection, abdominal pelvic infection, and sepsis. Surgical site infection was classified as superficial incision infection, deep incision infection, and organ infection, according to the guidelines issued by the Centers for Disease Control and Prevention in 2017.14
Data Analysis
Statistical analyses were conducted using SPSS version 22.0 (IBM Corp., Armonk, NY, USA) and MedCalc version 18.2 (MedCalc Software bvba, Ostend, Belgium). Continuous data were expressed as mean ± standard deviation and compared between groups using the independent sample t-test. Categorical data were compared using the chi-square test or Fisher’s exact probability test. To identify independent risk factors for postoperative complications, variables showing significant association (P<0.05) with the outcome on univariate analysis were entered into the multiple logistic regression analysis, and the results were expressed as odds ratios (ORs) with 95% confidence intervals (95% CIs). Receiver operating characteristic curve analysis was used to estimate the initial predictive value of POD3 CAR, preoperative mGPS, and POD3 poGPS. The area under the curve (AUC) was calculated, and the sensitivity, selectivity, positive predictive value (PPV), and negative predictive value (NPV) at the optimal cutoff value (corresponding to the maximum value of the Jordan index) were obtained for each predictor. Relationships with P<0.05 were considered statistically significant.
Results
Study Population and Baseline Characteristics
A total of 154 patients (95 male, 59 female) were included in the study, with an average age of 64.9±11.4 years. Within days postoperatively, complications occurred in 80 patients (51.9%), and two patients died.
Analysis of Possible Risk Factors for Postoperative Complications
Univariate analysis revealed that sex, preoperative albumin levels, preoperative CRP, use of neoadjuvant therapy, operative time, preoperative mGPS, preoperative CAR, POD3 CAR, POD3 poGPS, POD3 CRP and POD3 albumin were significantly associated with the 30-day incidence of postoperative complications (P<0.05) (Table 2). Both POD3 CAR and poGPS were derived from the same CRP and albumin in the same patients and highly interrelated, and the same for preoperative CAR and mGPS. We do not put them in the same multivariate analysis. On multivariate analysis, POD3 CRP (OR=1.015; 95% CI=1.006–1.024;P=0.001) and operative time (OR=1.006; 95% CI=1.001–1.010; P=0.018) were independent risk factors for postoperative complications (Table 3). While preoperative CRP appeared to have similar predictive value as that of POD3 CRP (OR=1.045; 95% CI=0.983–1.112), the association of preoperative CRP with the 30-day incidence of postoperative complications did not reach statistical significance (P=0.160). As POD3 CRP was an independent risk factor for postoperative complications, we speculated that the diagnostic value of POD3 CAR and poGPS was higher than that of preoperative indicators.
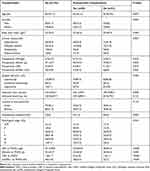 |
Table 2 Univariate Analysis of Risk Factors Associated with Postoperative Complications |
 |
Table 3 Multivariate Analysis of Factors Associated with Postoperative Complications |
Usefulness of CAR, mGPS, and poGPS as Predictors of Postoperative Complications
Because POD3 CRP was previously reported to serve as a predictor of postoperative complications,9 we conducted receiver operating characteristic curve analysis for all five indicators of inflammatory response (preoperative mGPS, preoperative CAR, as well as CRP, CAR, and poGPS on POD3). For POD3 CAR, the AUC was 0.711 and an optimal cutoff of 2.6 was identified (sensitivity, 51.3%; specificity, 87.8%; PPV, 83.2%; NPV, 61.3%). For preoperative mGPS (Figure 1, Table 4), the AUC was 0.613 and an optimal cutoff of 0.1 was identified (sensitivity, 66.3%; specificity, 50.0%; PPV, 58.9%; NPV, 57.8%). For preoperative mGPS, the AUC was 0.600, with the cutoff value at 0 (sensitivity, 73.8%; specificity, 6.9%; PPV, 46.1%; NPV, 19.2%), 1 (sensitivity, 18.8%; specificity, 93.2%; PPV, 75.0%; NPV, 51.5%) and 2 (sensitivity, 7.5%; specificity, 0.0%; PPV, 100.0%; NPV, 50.0%). For poGPS on POD3, the AUC was 0.599, with cutoff value at 0 (sensitivity, 77.5%; specificity, 2.7%; PPV, 46.3%; NPV, 10.0%), 1 (sensitivity, 21.3%; specificity, 97.3%; PPV, 89.5%; NPV, 53.3%) and 2 (sensitivity, 1.3%; specificity, 0.0%; PPV, 100.0%; NPV, 48.4%). For CRP on POD3, the AUC was 0.706 and an optimal cutoff of 65.5 mg/L was identified (sensitivity, 48.8%; specificity, 79.7%; PPV, 73.2%; NPV, 60.2%).
 |
Table 4 Predictive Power Analysis of Risk Factors Associated with Postoperative Complications |
The AUC was above 0.5 for all four variables analyzed, indicating good predictive value for postoperative complications (Figure 1, Table 4). From the perspective of clinical practice, PPV is the most impactful parameter because it reflects the likelihood that the screening will correctly identify patients with the outcome (high risk of postoperative complications). The present findings suggest that there is an 83.2% probability of correctly identifying the high risk of early complications among patients with CAR≥2.6 on POD3. Compared to preoperative CAR, preoperative mGPS, and POD3 poGPS, POD3 CAR provided significantly superior predictive value. Moreover, it reflected in the increased chance to correctly identify patients at risk. Therefore, we further examined the usefulness of POD3 CAR as an early predictor of specific postoperative complications.
CAR as a Predictor of Specific Postoperative Complications
For this analysis, the patients were stratified according to the CAR cutoff: high CAR (≥2.6) versus low CAR (<2.6). Complications occurred in 39 (92.9%) of the 42 patients with CAR≥2.6 and in 41 (36.6%) of the 112 patients with CAR<2.6 (Table 5). The difference between groups was statistically significant (P<0.001).
 |
Table 5 Incidence of Postoperative Complications According to Postoperative C-Reactive Protein/Albumin Ratio (CAR) |
The incidence of specific complications was examined in each group. Compared to patients with high CAR, those with low CAR on POD3 had a lower incidence of mild complications (grade I or II; 50.0% vs 26.8%, P<0.001), as well as a lower incidence of severe complications (grade III or higher; 42.9% vs 9.8%, P<0.001) (Table 5). In particular, infectious complications occur significantly more frequently in patients with high CAR on POD3 (54.8% vs 16.1%, P<0.001) (Table 5).
Usefulness of CAR as a Predictor of Surgical Site Infection, Infective Complications, and Anastomotic Leakage
For predicting surgical site infection (Figure 2), POD3 CAR exhibited an AUC of 0.760 (95% CI=0.685–0.825), the sensitivity of 75.00%, and specificity of 68.85%. For predicting infectious complications (Figure 3), POD3 CAR exhibited an AUC of 0.702 (95% CI=0.623–0.773), the sensitivity of 59.09%, and specificity of 74.55%. For predicting anastomotic leakage (Figure 4), POD3 CAR exhibited an AUC of 0.798 (95% CI=0.726–0.859), the sensitivity of 100%, and specificity of 58.11%.
Discussion
In this single-center retrospective analysis of 154 patients who underwent radical surgery for colorectal carcinoma, we found evidence suggesting that high levels of preoperative mGPS (P=0.002), preoperative CAR (P=0.019), POD3 CAR (P<0.001) and POD3 poGPS (P<0.001) can significantly affect postoperative complications after surgery for colorectal cancer. On receiver operating characteristic curve analysis, we found good predictive value for all three inflammation-based scores evaluated. However, CAR on POD3 provided the highest PPV, which is of particular importance in the clinical setting.
Surgery induces local tissue damage, physical barrier damage, and potential exposure to environmental and commensal microorganisms, all of which can lead to localized inflammation. Moreover, patients with surgery often undergo other invasive procedures such as venous catheterization, general anesthesia, endotracheal intubation, and catheterization. These procedures damaged the skin and epithelial defenses, causing inflammation at distant anatomical sites. Therefore, monitoring inflammation perioperatively is essential, as well as to understand the relationship between inflammation and the risk of postoperative complications. Indeed, an increasing number of studies are being designed to examine the role of the systemic inflammatory response as a contributing factor to postoperative complications.
Both preoperative and postoperative CRP levels have been reported as important predictors of postoperative survival in patients with colorectal cancer.9,15,16 CRP is an important marker of inflammation, and its elevation in cancer patients is mainly due to the inflammatory response to the tumor and surgery. Nason et al17 reported that CRP on POD3 is a useful predictor of infectious complications in patients who undergo colorectal surgery. However, in the clinical setting, there are several limitations to the use of postoperative CRP as a predictor of postoperative complications. The main disadvantages are low predictive accuracy and high time lag.18,19 CRP and white blood cell levels increase non-specifically in response to surgical stress.20 Moreover, as suggested by a recent study, postoperative changes in CRP levels are noted later than the changes in other inflammatory markers such as interleukin-6.19 Therefore, CRP alone is not a sufficiently sensitive descriptor of the inflammatory state of patients in the early stages after surgery.
In order to improve the accuracy of the prediction of postoperative complications, CAR considers CRP levels concomitantly with albumin levels. Hepatocytes synthesize albumin. And albumin serves as a multifunctional protein with antioxidant, immunomodulatory, and detoxifying action.21 Among patients with malignant tumors, albumin levels differ significantly between survivors and non-survivors.22 Some scholars have pointed out that decreased albumin levels reflect negative nitrogen balance and a decreased rate of toxic metabolite clearance. Abundant expression of inflammatory factors in the plasma causes damage to the endothelial cells of capillaries, which allows albumin to leak through the damaged capillary endothelium into the interstitial space, resulting in hypoproteinemia,21,23 which is an important prognostic index of surgical outcomes.24 In our study, the combined indices of CRP and albumin (CAR, mGPS, poGPS) provided superior prognostic efficacy compared to that noted for CRP. CAR on POD3 exhibited the highest probability to detect patients at risk (PPV=83.2%), and the overall predictive value of preoperative CAR was poor. Preoperative mGPS (PPV=100.0%) and poGPS on POD3 (PPV=100.0%) exhibited a higher probability to adequately identify patients with high risk when the cutoff value was 2.
Alazawi et al reported that most patients who underwent surgery had a systemic inflammatory response on POD3,25 which is consistent with our present findings. Specifically, we found high predictive value for all inflammation-based prognosis scores obtained on POD3, and the incidence of postoperative complications was significantly higher in patients with CAR≥2.6 than in those with CAR<2.6 on POD3 (overall incidence, as well as the incidence of severe complications and that of infectious complications).
In our study, subgroup analysis confirmed the good prognostic value of CAR on POD3 for predicting the 30-day incidence of surgical site infection (AUC=0.760; 95% CI=0.685–0.835; P=0.016), infectious complications (AUC=0.702; 95% CI=0.623–0.773), and anastomotic leakage (AUC=0.798; 95% CI=0.726–0.859). Surgical site infection is the most common complication after surgery, with a high incidence and strong impact on the duration of hospitalization.26 In a prospective study by Goulart et al.27 CAR was superior to white blood cell count and procalcitonin as an independent predictor of surgical site infection. On the other hand, CRP was reported as the best postoperative inflammatory response marker for predicting anastomotic leakage, providing high sensitivity.28 Our present findings confirm the usefulness of CRP as an inflammatory marker but highlight the benefit of considering albumin concomitantly with CRP (especially as CAR) to improve the accuracy of predicting the risk of complications after radical surgery for colorectal carcinoma.
In recent years, the concept of enhanced recovery after surgery has become increasingly adopted in the field of colorectal surgery. Within this framework, minimally invasive surgery is preferred, as it helps reduce surgical trauma and ensuing inflammatory response to surgical stress as much as possible. However, various complications may still occur. Therefore, further studies are warranted to elucidate the extent to which such principles can minimize inflammatory response to surgical stress.
Several limitations of our study should be acknowledged. First, this was a retrospective study with a relatively small sample. Second, because of the small sample size, our analysis did not differentiate between laparoscopic and open surgery. Future studies with prospective design and large sample size are warranted. In particular, it is desirable to develop a comprehensive score based on mGPS, CAR, and poGPS. And the score should be easy to evaluate in clinical practice. It would help clinicians detect early postoperative complications and intervene promptly, thereby reducing the duration and costs of hospitalization, and promoting rapid recovery.
Acknowledgments
This study was supported by the Natural Science Foundation of China (Grant No. 81702345).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors have no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
2. Zhang Y, Chen Z, Li J. The current status of treatment for colorectal cancer in China: a systematic review. Medicine. 2017;96(40):e8242. doi:10.1097/MD.0000000000008242
3. Nicholson A, Lowe MC, Parker J, Lewis SR, Alderson P, Smith AF. Systematic review and meta-analysis of enhanced recovery programmes in surgical patients. Br J Surg. 2014;101(3):172–188. doi:10.1002/bjs.2014.101.issue-3
4. Aoyama T, Oba K, Honda M, et al. Impact of postoperative complications on the colorectal cancer survival and recurrence: analyses of pooled individual patients’ data from three large Phase III randomized trials. Cancer Med. 2017;6(7):1573–1580. doi:10.1002/cam4.2017.6.issue-7
5. Brown SR, Mathew R, Keding A, Marshall HC, Brown JM, Jayne DG. The impact of postoperative complications on long-term quality of life after curative colorectal cancer surgery. Ann Surg. 2014;259(5):916–923. doi:10.1097/SLA.0000000000000407
6. Govaert JA, Fiocco M, van Dijk WA, et al. Costs of complications after colorectal cancer surgery in the Netherlands: building the business case for hospitals. Eur J Surg Oncol. 2015;41(8):1059–1067. doi:10.1016/j.ejso.2015.03.236
7. Hendren S, Birkmeyer JD, Yin H, Banerjee M, Sonnenday C, Morris AM. Surgical complications are associated with omission of chemotherapy for stage III colorectal cancer. Dis Colon Rectum. 2010;53(12):1587–1593. doi:10.1007/DCR.0b013e3181f2f202
8. Watt DG, McSorley ST, Horgan PG, McMillan DC. Enhanced recovery after surgery: which components, if any, impact on the systemic inflammatory response following colorectal surgery? A systematic review. Medicine. 2015;94(36):e1286. doi:10.1097/MD.0000000000001286
9. Warschkow R, Beutner U, Steffen T, et al. Safe and early discharge after colorectal surgery due to C-reactive protein: a diagnostic meta-analysis of 1832 patients. Ann Surg. 2012;256(2):245–250. doi:10.1097/SLA.0b013e31825b60f0
10. Labgaa I, Joliat GR, Kefleyesus A, et al. Is postoperative decrease of serum albumin an early predictor of complications after major abdominal surgery? A prospective cohort study in a European centre. BMJ Open. 2017;7(4):e013966. doi:10.1136/bmjopen-2016-013966
11. McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39(5):534–540. doi:10.1016/j.ctrv.2012.08.003
12. Watt DG, McSorley ST, Park JH, Horgan PG, McMillan DC. A postoperative systemic inflammation score predicts short- and long-term outcomes in patients undergoing surgery for colorectal cancer. Ann Surg Oncol. 2017;24(4):1100–1109. doi:10.1245/s10434-016-5659-4
13. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi:10.1097/01.sla.0000133083.54934.ae
14. Berrios-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017;152(8):784–791. doi:10.1001/jamasurg.2017.0904
15. Egenvall M, Morner M, Martling A, Gunnarsson U. Prediction of outcome after curative surgery for colorectal cancer: preoperative haemoglobin, C-reactive protein and albumin. Colorectal Dis. 2018;20(1):26–34. doi:10.1111/codi.13807
16. Li C, Xu Q, Chen L, Luo C, Ying J, Liu J. C-reactive protein (CRP) as a prognostic factor for colorectal cancer after surgical resection of pulmonary metastases. Bull Cancer. 2017;104(3):232–236. doi:10.1016/j.bulcan.2016.11.016
17. Nason GJ, Barry BD, Obinwa O, McMacken E, Rajaretnam NS, Neary PC. Early rise in C-reactive protein is a marker for infective complications in laparoscopic colorectal surgery. Surg Laparosc Endosc Percutan Tech. 2014;24(1):57–61. doi:10.1097/SLE.0b013e31828fa03e
18. Hubner M, Mantziari S, Demartines N, Pralong F, Coti-Bertrand P, Schafer M. Postoperative albumin drop is a marker for surgical stress and a predictor for clinical outcome: a pilot study. Gastroenterol Res Pract. 2016;2016:8743187. doi:10.1155/2016/8743187
19. Rettig TC, Verwijmeren L, Dijkstra IM, Boerma D, van de Garde EM, Noordzij PG. Postoperative interleukin-6 level and early detection of complications after elective major abdominal surgery. Ann Surg. 2016;263(6):1207–1212. doi:10.1097/SLA.0000000000001342
20. Cook EJ, Walsh SR, Farooq N, Alberts JC, Justin TA, Keeling NJ. Post-operative neutrophil-lymphocyte ratio predicts complications following colorectal surgery. Int J Surg. 2007;5(1):27–30. doi:10.1016/j.ijsu.2006.05.013
21. Garcia-Martinez R, Caraceni P, Bernardi M, Gines P, Arroyo V, Jalan R. Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology (Baltimore, Md.). 2013;58(5):1836–1846. doi:10.1002/hep.26338
22. Liu J, Wang F, Li S, Huang W, Jia Y, Wei C. The prognostic significance of preoperative serum albumin in urothelial carcinoma: a systematic review and meta-analysis. Biosci Rep. 2018;38(4). doi:10.1042/BSR20180214
23. Xu XL, Yu HQ, Hu W, Song Q, Mao WM. A novel inflammation-based prognostic score, the C-reactive protein/albumin ratio predicts the prognosis of patients with operable esophageal squamous cell carcinoma. PLoS One. 2015;10(9):e0138657. doi:10.1371/journal.pone.0138657
24. Moghadamyeghaneh Z, Hwang G, Hanna MH, et al. Even modest hypoalbuminemia affects outcomes of colorectal surgery patients. Am J Surg. 2015;210(2):276–284. doi:10.1016/j.amjsurg.2014.12.038
25. Alazawi W, Pirmadjid N, Lahiri R, Bhattacharya S. Inflammatory and immune responses to surgery and their clinical impact. Ann Surg. 2016;264(1):73–80. doi:10.1097/SLA.0000000000001691
26. Bhangu A, Ademuyiwa AO, Aguilera ML, et al. Surgical site infection after gastrointestinal surgery in high-income, middle-income, and low-income countries: a prospective, international, multicentre cohort study. Lancet Infect Dis. 2018;18(5):516–525. doi: 10.1016/S1473-3099(18)30101-4
27. Goulart A, Ferreira C, Estrada A, et al. Early inflammatory biomarkers as predictive factors for freedom from infection after colorectal cancer surgery: a prospective cohort study. Surg Infect (Larchmt). 2018;19(4):446–450. doi:10.1089/sur.2017.294
28. Smith SR, Pockney P, Holmes R, et al. Biomarkers and anastomotic leakage in colorectal surgery: C-reactive protein trajectory is the gold standard. ANZ J Surg. 2018;88(5):440–444. doi:10.1111/ans.2018.88.issue-5
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




