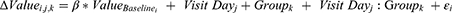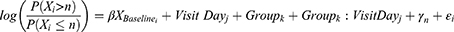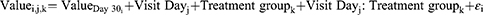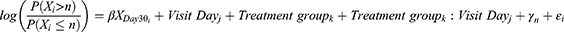Back to Journals » Clinical Ophthalmology » Volume 16
Use of Artificial Tears in Patients Undergoing Treatment with Anti-VEGF Intravitreal Injections
Authors Pastor-Pascual F, Pastor-Pascual R, Gálvez-Perez P, Dolz-Marco R, Gallego-Pinazo R
Received 24 September 2022
Accepted for publication 2 November 2022
Published 30 November 2022 Volume 2022:16 Pages 3959—3972
DOI https://doi.org/10.2147/OPTH.S391082
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Francisco Pastor-Pascual,1 Rafael Pastor-Pascual,1 Patricia Gálvez-Perez,1 Rosa Dolz-Marco,2 Roberto Gallego-Pinazo2
1Anterior Segment Department, Valencia, Spain; 2Posterior Segment Department, Valencia, Spain
Correspondence: Francisco Pastor-Pascual, Clínica Oftalvist, Valencia, Spain, Tel +34 963 51 33 04, Email [email protected]
Purpose: To analyze the use of artificial tears in patients undergoing treatment with anti-vascular endothelial growth (anti-VEGF) intravitreal injections.
Methods: Thirty-four eyes undergoing anti-VEGF treatment were analyzed. Each patient underwent a subjective and objective evaluation of the ocular surface, using the Ocular Surface Disease Index (OSDI), Dry Eye Questionnaire (DEQ)-5, tear meniscus height (TMH), first and average non-invasive Keratograph Break-Up Time (NIKBUT), bulbar conjunctival redness, meibography and the Vision Break-Up Time (VBUT). Patients attended 5 visits (days 0, 7, 30, 37, and 60). All patients continued with their intravitreal injection treatment during the study (days 0, 30, and 60). Patients did not receive any artificial tear treatment during the first month of the study, and at the baseline visit they were randomly assigned to one of two study groups to receive either the Systane Hydration or the Viscofresh 10mg/mL formulation. Patients were instructed to instill one drop of the assigned study treatment 3 times a day for 30 days during the second month of the study.
Results: According to the Mixed Models for Repeated Measures analysis, there is not enough statistical evidence for any of the parameters examined to determine significant differences between being treated with artificial tears and not being treated (p > 0.05). There is, however, a tendency toward improved outcomes in some parameters when artificial tears were used. OSDI, DEQ-5, TMH, and meibography were not affected by either the type of artificial tear used or by the time (from day 30 to day 60; p > 0.05), but the NIKBUT and VBUT values increased over time during this period regardless of which treatment the patient was receiving.
Conclusion: The use of artificial tears may help to keep the tear film stable Future studies with larger samples should be conducted to elucidate whether the tendency reported in our study becomes significant.
Keywords: ocular discomfort, artificial tears, intravitreal injection, anti-VEGF agents, dry eye
Introduction
Intravitreal injection of anti-vascular endothelial growth factor (anti-VEGF) agents is a commonly used treatment for various ophthalmic conditions. One of these includes age-related macular degeneration. These injections are used to prevent vision loss and this therapy requires patients to receive monthly injections for up to 2 years.1–5 It should be taken into consideration that identifying the specific role that intravitreal injections play in the development of dry eye syndrome is difficult, as the patient’s age and other age-related diseases may also contribute. Despite this, it is important to be aware that these preexisting conditions make patients more vulnerable to developing dry eyes when having intravitreal injections.6 It is likely, then, that this treatment is a factor in the development of dry eye syndrome.
Preliminary results suggest that intravitreal injections of anti-VEGF agents significantly impact ocular surface health.7 In fact, there are reports of an acute increase in corneal epitheliopathy and symptoms of ocular surface discomfort after intravitreal injections with povidone-iodine prophylaxis.8 Considering that this antiseptic is toxic to the corneal epithelium, clinicians should pay attention to the reaction caused on the ocular surface of patients undergoing treatment. In addition, patients often receive intravitreal injections on a monthly basis and over long periods of time (chronic basis) and this may affect ocular surface health and comfort. Common dry eye syndrome symptoms include pain, irritation, dryness, foreign bodies, sensation or light sensitivity among others.9 It has been indicated that optimizing ocular surface health between injection visits and saline irrigation immediately post-injection could help.8 Current post-intravitreal injection care guidelines do not include any kind of artificial tears or drops, even though it has been reported that artificial tears may be a safe and effective way of treating dry eye syndrome.10 Therefore, as the first‐line treatment for dry eyes is typically over the counter artificial tears,10 it seems interesting to assess whether using these in patients receiving intravitreal injections improves ocular comfort.
The main purpose of this study is, then, to assess the use of artificial tears in patients submitted to intravitreal injections of anti-VEGF agents using both subjective and objective metrics.
Methods
This randomized prospective comparative study included 40 eyes from 40 patients (only one eye per patient was considered). The present study was approved by the Ethics Committee of the Hospital Clínico San Carlos (Madrid, Spain) and was conducted in accordance with the tenets of the Declaration of Helsinki. Written informed consent was obtained from all patients prior to their enrollment in this study. This study was registered at https://www.isrctn.com/ISRCTN77853517 as ISRCTN 77853517.
Patients and Procedure
All the patients underwent a complete ophthalmological examination that included subjective refraction, visual acuity measurements, slit-lamp biomicroscopy, Schirmer I test (to measure tear production for 5 minutes), and posterior segment optical coherence tomography. The inclusion criteria were patients being treated with Lucentis 10 mg/mL (ranibizumab, Novartis) and aged between 51 and 90 years. The exclusion criteria were dry eye, glaucoma, previous refractive surgery, or recent cataract surgery (less than 6 months).
The patients attended five visits over the course of this study, day 0, day 7, day 30, day 37, and day 60. All the patients continued their intravitreal injection treatment during the study (1 per month, days 0, 30, and 60); they did not receive any artificial tear treatment in the first month of the study (visits on days 0, 7, and 30). At the baseline visit, all patients were randomly assigned (a 1:1 allocation ratio) to one of two study groups, by means of a computer randomization program, to receive either Systane Hydration (Alcon Laboratories, Fort Worth, TX, USA) or the Viscofresh 10mg/mL (Allergan Inc., Irvine, CA, USA) formulation. The Systane Hydration (Unitdose - Preservative Free) is a sterile solution containing sodium hyaluronate, polyethylene glycol 400, propylene glycol, hydroxypropyl guar, sorbitol, aminomethyl propanol, boric acid, sodium borate, potassium chloride, sodium chloride, hydrochloric acid and/or sodium hydroxide to adjust pH. And the Viscofresh ophthalmic drops contains carboxymethylcellulose sodium 5 mg, and also contains calcium chloride, magnesium chloride, potassium chloride, sodium chloride, sodium lactate and purified water.
The patients were instructed to instill one drop of the assigned treatment three times a day for 30 days during the second month of the study. Several subjective and objective metrics, described below, were measured over the two-month study. As indicated, in the first month, no patients received any artificial tear treatment and hence, the values obtained for the metrics on days 0, 7, and 30, indicated normal performance. From the visit on day 30, 20 patients were assigned to the Systane Hydration group and 20 to the Viscofresh group, according to the randomization scheme. The values obtained in the second month of the study served to assess the effect on these patients of using artificial tears. The differences between the two groups were also analyzed.
The subjective and objective metrics evaluated in this study were the Ocular Surface Disease Index (OSDI), Dry Eye Questionnaire (DEQ)-5, tear meniscus height (TMH), non-invasive keratograph break-up time (NIKBUT), bulbar conjunctival redness, meibography, and the vision break-up time (VBUT). The OSDI is a 12-item questionnaire designed to quickly assess ocular irritation symptoms consistent with dry eye disease and their impact on vision-related functioning. This index has been reported as a valid and reliable questionnaire for measuring the severity of dry eye disease.11 The DEQ-5 is a 1-page subset of 5 questions that is able to distinguish between patients with and without dry eye and facilitates the identification of even mild to moderate dry eye in patients.12 The Oculus Keratograph 5M (Oculus GmbH, Wetzlar, Germany) is a non-invasive method for analyzing the tear film. This device allowed us to obtain several parameters, including the TMH, the NIKBUT (NIKBUT-first, the time the first break-up of the tear film occurs; and NIKBUT-average, the average time of all break-up incidents), the bulbar conjunctival redness (on a 0 to 4-point scale in 0.1 steps), and the meibography of the upper right eyelid of each patient. This instrument has been found to be valid for objectively measuring these parameters.13–17 The HD Analyzer system (Visiometrics SL, Terrasa, Spain) is a double-pass system that evaluates the optical quality of the human eye. This system measures the tear film quality during the interblink period recording double-pass images every 0.5 seconds until it has completed a 10 (default) or 20-second capture. The program records 20 or 40 double-pass images, reflecting the evolution of the optical quality over those 10 or 20 seconds. The VBUT is defined as the time elapsed in seconds from 0 seconds to the time at which the patient’s vision quality index drops below a defined threshold. When the patient vision quality index exceeds the VBUT threshold, the result shows the time in seconds from blinking until the VBUT threshold is exceeded. This device provides an objective measurement of visual quality in patients with dry eye disease.18
Statistical Analysis
Mean ± standard deviation (SD) and range values were determined for all the parameters. The data was entered into a Microsoft Excel spreadsheet (Microsoft Corp, Redmond, WA, USA) and statistically analyzed using R software (The R Foundation). The required sample size was determined considering the OSDI parameter as the efficacy variable. There are no preliminary studies investigating the effect of Systane Hydration in patients with intravitreal injections in terms of the OSDI parameter. However, Deinema et al19 found an improvement in the OSDI parameter of −10±3.3 points when applying a placebo to patients with dry eye symptoms, and Jacobi et al20 also found an improvement of −19 when applying a treatment of Systane UD. Taking a SD for the Systane Hydration group of ±11.9 points, obtained from a similar study,21 and considering all the previous parameters (as well as a type I error of 5%, a power of 80% and a two-tailed hypothesis), the minimum sample size required for this study was 16 subjects per group.
Pre-Treatment versus Treatment
Mixed Models for Repeated Measures (MMRM) were used to compare differences between the values obtained in the period during which no artificial tears were applied (pre-treatment), and the values in the period using artificial tears (treatment) when the dependent variables were continuous quantitative variables. The treatment effect on each dependent variable was evaluated at a significance level of 0.05 (two-sided). For each variable (OSDI, DEQ-5, TMH, NIKBUT A, NIKBUT B, redness, and VBUT), the difference from the baseline at each time point (day 7 and day 30) was fitted using the baseline value as an adjusting covariate, whereas visit day (day 37 and day 60), group (control and treatment), and the interaction between these were treated as fixed factors in the MMRM. An autoregressive heterogeneous variance-covariance structure was employed to fit the MMRM. The model statement was:
where value i,j,k is the dependent variable value of subject i, on visit day j, in group k; εi ~ N (0, σ2) represents the residual variance component; and 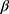 is the coefficient that multiplies the baseline value. The function used for the analysis was the gls() function from the {nlme} package from the R statistical software.
is the coefficient that multiplies the baseline value. The function used for the analysis was the gls() function from the {nlme} package from the R statistical software.
On the other hand, since meibography is an ordinal variable, the displacement of the ordinal scale end point was analyzed using the proportional odds model. The common odds ratio can be interpreted as the average change in the total ordinal result caused by the study treatment. The fitted model considered the meibography ordinal grade (0, 1, 2, or 3) on each visit day as the dependent variable, the baseline value was fitted as an adjusting covariate, and the visit day (day 7 and day 30), group (control/treatment), and the interaction between these two were treated as fixed factors. The proportional odds model statement was:
where Xi is the meibography ordinal grade of subject i, n is the nth level/category of the ordinal scale, on visit day j, in group k; 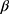 is the coefficient that multiplies the baseline value; γk is the intercept coefficient for the nth level of the ordinal scale; and ε is the residual error. By taking exponents, we get the odds ratio for each component in the formula. The treatment effect on the ordinal scale displacement was evaluated using a two-sided significance level of 0.05. The function used for the analysis was the polr() function from the {MASS} package in the R statistical software.
is the coefficient that multiplies the baseline value; γk is the intercept coefficient for the nth level of the ordinal scale; and ε is the residual error. By taking exponents, we get the odds ratio for each component in the formula. The treatment effect on the ordinal scale displacement was evaluated using a two-sided significance level of 0.05. The function used for the analysis was the polr() function from the {MASS} package in the R statistical software.
Treatment Differences Between Artificial Tears
Viscofresh was compared to Systane Hydration using MMRM when the dependent variables were continuous quantitative variables. The treatment effect on each (continuous quantitative) variable was evaluated at a significance level of 0.05 (two-sided). The values of the response variables (OSDI, DEQ-5, TMH, NIKBUT first, NIKBUT average, redness, and VBUT) at each visit were fitted using the baseline value (day 30) as an adjusting covariate, whereas visit day (day 37 and day 60), treatment group, and the interaction between these two were treated as fixed factors in the MMRM. An unstructured covariance matrix was employed to fit the MMRM. The model statement was:
Where Value I,j,k is the dependent variable value of subject i, on visit day j, in treatment group k; and εi ~ N (0, σ2) represents the residual variance component. The function used for the analysis was the gls() function from the {nlme} package in the R statistical software.
On the other hand, since meibography is an ordinal variable, the displacement of the ordinal scale end point was analyzed using the proportional odds model as previously indicated. The common odds ratio can be interpreted as the average change in the total ordinal result caused by the study treatment. The fitted model considered the meibography ordinal grade (0, 1, 2, or 3) on each visit day as the dependent variable, the baseline value (day 30) was fitted as an adjusting covariate, and visit day (day 37 and day 60), treatment group and the interaction between these two were treated as fixed factors. The proportional odds model statement was:
where Xi is the meibography ordinal grade of subject i, n is the nth level/category of the ordinal scale, on visit day j, in treatment group k; 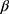 is the coefficient that multiplies the baseline value; γk is the intercept coefficient for the nth level of the ordinal scale; and ε is the residual error. By taking exponents, we get the odds ratio for each component in the formula. The treatment effect on the ordinal scale displacement was evaluated using a two-sided significance level of 0.05. The function used for the analysis was the polr() function from the {MASS} package in the R statistical software.
is the coefficient that multiplies the baseline value; γk is the intercept coefficient for the nth level of the ordinal scale; and ε is the residual error. By taking exponents, we get the odds ratio for each component in the formula. The treatment effect on the ordinal scale displacement was evaluated using a two-sided significance level of 0.05. The function used for the analysis was the polr() function from the {MASS} package in the R statistical software.
Results
Ultimately, the study considered a total of 34 eyes from 34 patients (13 females and 21 males), as 6 patients did not complete the follow-up. The mean age of the patients was 76.56±8.23 years (ranging from 51 to 88 years). Only one eye per patient was evaluated and no adverse events were encountered during the study. The data was registered in the analysis database, including the values that were obtained at each visit. Table 1 shows the mean values and SD for the different parameters obtained at the baseline (day 0).
 |
Table 1 Baseline patient demographics and clinical characteristics of the study groups |
Figures 1 and 2 show changes to the OSDI and DEQ-5 scores, respectively, over the course of the study for the two groups, before and after artificial tear treatment. Figure 3 depicts the mean TMH using the Keratograph 5M for the two groups at each visit. Figure 4 shows the first (top) and average (bottom) NIKBUT values obtained throughout the study for the two groups. Figures 5 and 6 show the mean bulbar conjunctival redness and mean meibography scores measured using the Keratograph 5M from the baseline up to the final visit. Figure 7 plots the mean VBUT measured using the HD Analyzer at the different visits for each group.
 |
Figure 1 Mean OSDI (ocular surface disease index) score from the baseline (day 0) on days 7, 30, 37, and 60 for each of the study groups. The values are expressed as the mean± standard deviation. |
 |
Figure 2 Mean DEQ-5 (dry eye questionnaire) score from the baseline (day 0) on days 7, 30, 37, and 60 for each of the study groups. The values are expressed as the mean± standard deviation. |
Comparison Analysis Between Periods “Without Using” (Pre-Treatment) and “Using” Artificial Tears (Treatment)
According to the MMRM results for assessing differences between the pre-treatment and treatment periods, there is not enough statistical evidence for any of the parameters examined to determine significant differences between being treated with artificial tears and not being treated (Table 2). However, it should be pointed out that there was a tendency toward better outcomes in some parameters when artificial tears were used. In addition, it should be considered that in all cases the baseline value is significant, in other words, the starting value influences the subsequent results.
 |
Table 2 Mixed Models for Repeated Measures regression results for the different parameters analyzed to compare periods without using (pre-treatment) and using artificial tears (treatment) |
Comparison Analysis Between Systane Hydration and Viscofresh in the Period Using Artificial Tears
According to the MMRM results for assessing possible differences between the treatments after 30 days of application (Table 3), the OSDI, DEQ-5, TMH, and meibography values were not affected by either the type of treatment (Systane Hydration or Viscofresh) or by the time (from day 30 to day 60); the values depend only on the day-30 starting value. There is no statistically significant evidence to allow us to determine differences between the treatments or a temporal evolution in the parameters (p > 0.05); nevertheless, the values of NIKBUT first and VBUT increased over time, irrespective of either their 30-day value (the day the treatment started) or the treatment they received. In contrast, the values of NIKBUT average and redness depended on the value of this parameter at the starting point (day 30) and also increased over time regardless of the treatment they received.
 |
Table 3 Mixed Models for Repeated Measures regression results for the different parameters analyzed during the period of using artificial tears comparing Systane Hydration versus Viscofresh |
Discussion
This randomized investigator-masked prospective comparative study was aimed at assessing the use of artificial tears in patients submitted to intravitreal injections of anti-VEGF agents. We used several objective and subjective metrics to properly analyze possible changes brought about by the use of artificial tears in these patients.
Our results revealed that there were no statistically significant changes for any of the parameters studied between the period of non-use and the period during which artificial tears were applied. Table 2 shows all the p values obtained in the statistical analysis and Figures 1–7 graphically represent the variations on different study days. Despite this, some parameters, such as the OSDI (Figure 1) and DEQ-5 (Figure 2), did show a trend toward lower values (reduced symptoms) over the course of the study. The OSDI score ranged from 0 to 100 and, based on their OSDI value, the patients can be categorized as having a normal ocular surface (0–12 points), or mild (13–22 points), moderate (23–32 points), or severe (33–100 points) ocular surface disease.22 The patients in this study can be categorized as having a normal ocular surface since the mean values for both groups, on any study day, were less than 12 points. However, as can be seen from the error bars in Figure 1, in some patients this value was actually exceeded, and they can be categorized as having mild or moderate ocular surface disease. Miller et al23 assessed the minimal clinically important difference for the OSDI in 310 patients (normal, 18, mild, 54, moderate, 98, and severe, 140). The level of symptomatic improvement substantially exceeds the OSDI minimal clinically important difference ranging from 4.5 to 7.3 units for mild or moderate dry eye disease and from 7.3 to 13.4 for severe disease. In our sample, the mean difference between the OSDI value obtained on day 60 (after treatment) and the mean of the values obtained on days 0, 7, and 30 (before treatment) were −1.36 and 1.30 for the Systane Hydration and Viscofresh groups, respectively. It should be pointed out that a negative value indicates a reduction in the mean OSDI value. In relation to the other questionnaire, Chalmers et al12 reported that a DEQ-5 score of ≥6 indicated that the person had symptoms similar to patients with a clinical diagnosis of non-Sjogren's Syndrome keratonjunctivitis sicca, and a DEQ-5 score of ≥12 matched the scores from most patients with a clinical diagnosis of Sjogren's Syndrome. The diagnostic criterion for dry eye disease, aqueous deficient dry eye disease, and evaporative dry eye disease is a DEQ-5 score of ≥6.24 Our mean values for the two groups were less than 5 on any study day (see Figure 2). Comparing pre- (mean for days 0, 7, and 30) and post-treatment (day 60) values, both groups showed a similar improvement, −0.10 and −0.17 change for Systane Hydration and Viscofresh, respectively.
In relation to the parameters obtained with the Keratograph 5M our results revealed that there were no significant changes in the TMH, NIKBUT (first and average), redness, and meibography during the treatment (Figures 3–6). However, despite there being no statistical significance, there was an improvement in tear film stability measured with the NIKBUT, mainly for the first but also for the average values (Figure 4, top and bottom, respectively). It should be borne in mind that tear film instability is a key pathogenic factor in dry eye disease and improving this is a basic strategy for managing the disease.25 NIKBUT first values improved in the Systane Hydration group from 9.42 seconds prior to treatment (mean of values obtained on days 0, 7, and 60) to 12.76 seconds on day 60 (1 month with treatment). These values changed to 8.21 and 8.16 seconds, respectively, in the Viscofresh group. For the NIKBUT average, the values were 14.70 and 16.47 seconds, and 12.91 and 13.40 seconds, for the Systane Hydration and Viscofresh groups, respectively. In other words, a patient who on day 30 (baseline) has a higher NIKBUT than another patient will see their NIKBUT increase further over time (on days 37 and 60). For the HD Analyzer outcomes, we obtained no significant variation in the mean VBUT values between the two periods or for the two groups during the treatment period (see Figure 7, days 37 and 60). However, the analysis of the two types of artificial tears applied revealed that the values of NIKBUT first and VBUT increase over time, irrespective of the value they present on day 30 (the day the treatment started) and regardless of which treatment they receive.
In a preliminary prospective study, presented in 2014 at the Association for Research in Vision and Ophthalmology annual meeting, Srinagesh et al7 assessed 12 patients receiving intravitreal injections of Avastin, Lucentis and/or Eyelea 2–4 weeks after the injections. They measured the OSDI symptoms of dry eye, tear break-up-time towa (TBUT), fluorescein staining, lissamine green staining, and the Schirmer test in eyes that received injections and compared the results with eyes that received no injections within the allotted time frame. These patients were evaluated 4–6 months after the cessation of intravitreal injections. The average OSDI for all eyes which had received an intravitreal injection within 2 weeks to 1 month was 21.91. In patients who received unilateral injections, the average OSDI for the injected eyes was 25.36, which was significantly higher than the average OSDI of 4.00 for non-injected eyes (p = 0.002). Fluorescein staining of the cornea was noted more frequently in the injected than the non-injected eye, specifically in the temporal quadrant (p = 0.017). The inferotemporal area in 50% of injected eyes and 25% of non-injected eyes was mildly stained by lissamine green. The mean TBUTs were 9.8 and 9.5 seconds for the injected and non-injected eyes, respectively. The mean Schirmer values were 14.6 mm for injected eyes and 15.1 mm for non-injected eyes. They concluded that these preliminary results could indicate that intravitreal injections of anti-VEGF agents significantly impact ocular surface health. They pointed out that, following these injections, clinical signs and symptoms of dry eye should be monitored and treated in order to optimize patient comfort and visual potential.
In another study published in 2019, Dohlman et al8 evaluated the signs and symptoms of ocular surface disease before and after intravitreal injection in 20 patients receiving unilateral intravitreal injections with povidone-iodine prophylaxis. These authors reported a significant post-injection increase in corneal epitheliopathy in treated but not untreated eyes (increase in corneal fluorescein staining scores in treated eyes were 9.9±3.5, compared with 2.05±2.4 in untreated eyes; p < 0.005), as well a significant increase in ocular discomfort using the symptom assessment in dry eye (SANDE) questionnaire (increase in SANDE severity score after injection, 23±23 pre-injection versus 54.4±26 post-injection; p < 0.005). They confirmed the effects of the intravitreal injections on the corneal epithelium and an increase in ocular surface discomfort after the injections, which may be attributable to povidone-iodine use as well as topical anesthetic.26 These authors also indicated that there was no clear correlation between the signs and symptoms and the injection history. Finally, they considered that copious saline irrigation immediately following injection may help to improve patient experience in terms of this type of treatment. We agree with them, taking into account the tendency we observed in some parameters after the use of artificial tears. Furthermore, our results suggest that the use of artificial tears may confer additional benefits (the values of NIKBUT first and VBUT will increase over time) but no differences between the two artificial tears used were found for any parameter during the period of their application (see Table 3).
Conclusion
In conclusion, we consider that the use of intravitreal injections might affect the ocular surface, and we suggest that the use of artificial tears could help to keep the tear film stable, and therefore, lead to an improved quality of life for those patients receiving intravitreal injections. Future studies should be conducted with large samples, long follow-up periods, and considering more types of artificial tears available on the market to elucidate whether the tendency reported in our study becomes significant.
Data Sharing Statement
Data are not available for sharing
Funding
This study was supported by an investigator-initiated study grant from Alcon Laboratories, Inc. receiving monetary funding and product support (IIT#63239827).
Disclosure
Dr Rosa Dolz-Marco reports grants from Roche, grants from Heidelberg Engineering, grants from IvericBio, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Talks J, Daien V, Finger RP, et al. The use of real-world evidence for evaluating anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration. Surv Ophthalmol. 2019;64(5):707–719. doi:10.1016/j.survophthal.2019.02.008
2. Li E, Donati S, Lindsley KB, et al. Treatment regimens for administration of anti-vascular endothelial growth factor agents for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2020;5(5):CD012208. doi:10.1002/14651858.CD012208.pub2
3. Holz FG, Figueroa MS, Bandello F, et al. Ranibizumab treatment in treatment-naive neovascular age-related macular degeneration, results from LUMINOUS, a global real-world study. Retina. 2020;40(9):1673–1685. doi:10.1097/IAE.0000000000002670
4. Carrasco J, Pietsch GA, Nicolas MP, et al. Real-world effectiveness and real-world cost-effectiveness of intravitreal aflibercept and intravitreal ranibizumab in neovascular age-related macular degeneration, systematic review and meta-analysis of real-world studies. Adv Ther. 2020;37(1):300–315. doi:10.1007/s12325-019-01147-6
5. Monés J, Singh RP, Bandello F, Souied E, Liu X, Gale R. Undertreatment of neovascular age-related macular degeneration after 10 years of anti-vascular endothelial growth factor therapy in the real world, the need for a change of mindset. Ophthalmologica. 2020;243(1):1–8. doi:10.1159/000502747
6. Labetoulle M, Schmickler S, Galarreta D, et al. Efficacy and safety of dual-polymer hydroxypropyl guar- and hyaluronic acid-containing lubricant eyedrops for the management of dry-eye disease, a randomized double-masked clinical study. Clin Ophthalmol. 2018;12:2499–2508. doi:10.2147/OPTH.S177176
7. Srinagesh V, Ellengerg D, Scharper PH, Etter J. Intravitreal dry eye study. Invest Ophthalmol Vis Sci. 2014;55(13):3696.
8. Dohlman T, Lertsuwanroj B, D’Amico DJ, Crialsky JB, Kiss S. Evaluation of signs and symptoms of ocular surface disease after intravitreal injection. Acta Ophthalmol. 2019;97:e1154–e1156. doi:10.1111/aos.14146
9. Ehlers JP, Shah CP. The Wills Eye Manual, Office and Emergency Room Diagnosis and Treatment of Eye Disease.
10. Pucker AD, Ng SM, Nichols JJ. Over the counter (OTC) artificial tear drops for dry eye syndrome. Cochrane Database Syst Rev. 2016;2:CD009729. doi:10.1002/14651858.CD009729.pub2
11. Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118(5):615–621. doi:10.1001/archopht.118.5.615
12. Chalmers RL, Begley CG, Caffery B. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5), discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Cont Lens Anterior Eye. 2010;33(2):55–60. doi:10.1016/j.clae.2009.12.010
13. Abdelfattah NS, Dastiridou A, Sadda SR, Lee OL. Noninvasive imaging of tear film dynamics in eyes with ocular surface disease. Cornea. 2015;34(Suppl 10):S48–52. doi:10.1097/ICO.0000000000000570
14. Tian L, Qu JH, Zhang XY, Sun XG. Repeatability and reproducibility of noninvasive keratograph 5M measurements in patients with dry eye disease. J Ophthalmol. 2016;2016:8013621. doi:10.1155/2016/8013621
15. Wu S, Hong J, Tian L, et al. Assessment of bulbar redness with a newly developed keratograph. Optom Vis Sci. 2015;92:892–899. doi:10.1097/OPX.0000000000000643
16. Downie LE, Keller PR, Vingrys AJ. Assessing ocular bulbar redness, a comparison of methods. Ophthalmic Physiol Opt. 2016;36:132–139. doi:10.1111/opo.12245
17. Schulze MM, Ng A, Yang M, et al. Bulbar redness and dry eye disease, comparison of a validated subjective grading scale and an objective automated method. Optom Vis Sci. 2021;98(2):113–120. doi:10.1097/OPX.0000000000001638
18. Gouvea L, Waring GO, Brundrett A, Crouse M, Rocha KM. Objective assessment of optical quality in dry eye disease using a double-pass imaging system. Clin Ophthalmol. 2019;13:1991–1996. doi:10.2147/OPTH.S211584
19. Deinema LA, Vingrys AJ, Wong CY, Jackson DC, Chinnery HR, Downie LE. A randomized, double-masked, placebo-controlled clinical trial of two forms of omega-3 supplements for treating dry eye disease. Ophthalmology. 2017;124(1):43–52. doi:10.1016/j.ophtha.2016.09.023
20. Jacobi C, Kruse FE, Cursiefen C. Prospective, randomized, controlled comparison of SYSTANE UD eye drops versus VISINE INTENSIV 1% EDO eye drops for the treatment of moderate dry eye. J Ocul Pharmacol Ther. 2012;28(6):598–603. doi:10.1089/jop.2012.0066
21. Pinto-Bonilla JC, Del Olmo-Jimeno A, Llovet-Osuna F, Hernández- Galilea E. A randomized crossover study comparing trehalose/hyaluronate eyedrops and standard treatment, patient satisfaction in the treatment of dry eye syndrome. Ther Clin Risk Manag. 2015;11:595–603. doi:10.2147/TCRM.S77091
22. Walt J. Ocular Surface Disease Index (OSDI) Administration and Scoring Manual. Irvine, CA, Allergan, Inc; 2004.
23. Miller KL, Walt JG, Mink DR, et al. Minimal clinically important difference for the ocular surface disease index. Arch Ophthalmol. 2010;128(1):94–101. doi:10.1001/archophthalmol.2009.356
24. Wolffsohn JS, Wang MTM, Vidal-Rohr M, et al. Demographic and lifestyle risk factors of dry eye disease subtypes, a cross-sectional study. Ocul Surf. 2021;21:58–63. doi:10.1016/j.jtos.2021.05.001
25. Lemp MA, Foulks GN. The definition and classification of dry eye disease, report of the definition and classification subcommittee of the international dry eye workShop (2007). Ocul Surf. 2007;5(2):75–92.
26. Saedon H, Nosek J, Phillips J, Narendran N, Yang YC. Ocular surface effects of repeated application of povidone iodine in patients receiving frequent intravitreal injections. Cutan Ocul Toxicol. 2017;36:343–346. doi:10.1080/15569527.2017.1291665
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.