Back to Journals » Medical Devices: Evidence and Research » Volume 15
Use of a Portable Electronic Interface Improves Clinical Handoffs and Adherence to Lung Protective Ventilation
Authors Euliano NR , Stephan P, Michalopoulos K, Gentile MA , Layon AJ, Gabrielli A
Received 29 April 2022
Accepted for publication 5 July 2022
Published 5 August 2022 Volume 2022:15 Pages 263—275
DOI https://doi.org/10.2147/MDER.S372333
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Neil R Euliano,1 Paul Stephan,1 Konstantinos Michalopoulos,1 Michael A Gentile,2 A Joseph Layon,3 Andrea Gabrielli4
1Convergent Engineering, Inc, Gainesville, FL, USA; 2Department of Anesthesiology, Duke University, Durham, NC, USA; 3Department of Anesthesiology, College of Medicine, University of Central Florida, Orlando, FL, USA; 4Miller School of Medicine, University of Miami, Miami, FL, USA
Correspondence: Neil R Euliano, Convergent Engineering, Inc, 100 SW 75th Street, STE 106, Gainesville, FL, 32607, USA, Tel +1 352 378 4899 x 107, Fax +1 352 378 9202, Email [email protected]
Background: Mechanical ventilation (MV) is used to support patients with respiratory impairment. Evidence supports the use of lung-protective ventilation (LPV) during MV to improve outcomes. However, studies have demonstrated poor adherence to LPV guidelines. We hypothesized that an electronic platform adapted to a hand-held tablet receiving real-time ventilatory parameters could increase clinician awareness of key LPV parameters. Furthermore, we speculated that an electronic shift-change tool could improve the quality of clinician handoffs.
Methods: Using a specially designed Wi-Fi dongle to transmit data from three ventilators and a respiratory monitor, we implemented a system that displays data from all ventilators under the care of a Respiratory Care Practitioner (RCP) on an electronic tablet. In addition, the tablet created a handoff checklist to improve shift-change communication. In a simulated ICU environment, we monitored the performance of eight RCPs at baseline and while using the system.
Results: Using the system, the time above guideline Pplat decreased by 74% from control, and the time outside the VT range decreased by 60% from control, p = 0.007 and 0.015, respectively. The handoff scores improved quality significantly from 2.8 to 1.6 on a scale of 1 to 5 (1 being best), p = 0.03.
Conclusion: In a simulated environment, an electronic RT tool can significantly improve shift-change communication and increase the RCP’s level of LPV adherence.
Keywords: respiration, lung protective ventilation, handoff, respiratory therapy, clinical decision support software, health information technology
Introduction
Mechanical ventilation (MV) is an integral part of care for critically ill patients. Lung-protective mechanical ventilation (LPV) is a strategy aiming to reduce the incidence of ventilator-induced lung injury.1–3 Perioperative use of LPV has also shown benefits in patients without preexisting lung impairment, suggesting a role in reducing pulmonary complications and length of stay.4,5 LPV focuses on reducing the stress of lung opening, or barotrauma, and lung parenchymal strain, or volutrauma.1,6 The evidence that LPV is potentially life-saving was reported over 20 years ago by the NHLBI ARDS Net Group as they applied tidal volume (Vt) between 4 and 8 mL/kg predicted body weight (PBW) and Plateau pressure (Pplat) <30 cmH2O for patients receiving MV.1,7 While these findings have achieved the status of Best Practices, their implementation into routine clinical care has proven to be a complex task. Indeed, there are data indicating that clinicians still do not effectively implement these LPV strategies.8,9
Several studies have identified barriers to guideline adherence, including lack of awareness, lack of familiarity, lack of agreement, lack of outcome expectancy, and inertia of previous practice.10 More specifically, reported reasons for non-compliance with LPV standards include: (1) wrong calculation of predicted body weight (PBW, also termed Ideal Body Weight [IBW]); (2) underdiagnosed ARDS despite international standards of definition; (3) perceived patient discomfort from low Vt ventilation, and (4) knowledge gap in recognizing and treating patient-ventilator asynchrony.11–13 Many of these barriers may be overcome by enhancing communication between Respiratory Care Practitioners (RCPs) and other bedside personnel, increasing awareness of patient–ventilator interaction and consolidating key patient information in a user-friendly format, both in real time and during clinical handoffs. Several studies have demonstrated that simple communication tools can be used to improve compliance with LPV ventilation.14,15
While optimization of patient–ventilator interaction is multidisciplinary, RCPs have a central role in managing ventilator settings. Still, they face many challenges in implementing evidence-based medicine at the bedside in critically ill patients: maintaining compliance with best ventilator practices, patient situational awareness while facing high patient workload, and efficient communication during clinical handoffs. It has been demonstrated that the implementation of a standard handoff bundle could reduce preventable adverse events by 30%, without increasing transition of care time.16 Several key actions suggested by The Joint Commission to improve the quality of transition of care include standardized content between sender and receiver including standardizing tools and methods (eg checklists), face-to-face handoff in a quiet environment, use of electronic health record capabilities and other technologies (apps and patient portals) to enhance hand-offs, and process improvement through monitoring success of interventions.17. Electronic adjuncts are an alternative strategy to increase compliance with LPV by providing continuous physiological feedback, cognitive prompts, and behavioral nudges.18
We created such a system to increase awareness of LPV parameters and improve clinical handoffs conducted at shift change and then assessed the efficacy of this electronic platform. We hypothesized that an electronic platform adapted to a hand-held tablet receiving real-time ventilatory parameters such as Vt, frequency of mechanical breaths (f), flow/volume graphs, and alarms, and containing an efficient handoff checklist could assist RCPs in maintaining established LPV parameters (Pplat <30 cmH2O and Vt 4 to 8 mL/kg PBW) and improve hand-offs.
Materials and Methods
To collect and distribute data wirelessly, we designed Wi-Fi dongles for three mechanical ventilators (Puritan Bennett 840, Medtronic Inc., Boulder, CO; Drager V500, Drager Inc, Telford, PA; Hamilton G5, Hamilton Medical Inc, Reno, NV) plus a respiratory graphics monitor (NM3, Philips Healthcare Inc, Carlsbad, CA) (Figure 1). The dongles were programmed to read data from the serial ports of the four devices and transmit them wirelessly to a custom-designed server. The server collected the data from each ventilator, performed calculations, and transmitted the data to a tablet computer-based mobile application carried by each RCP. The mobile application contained (a) a dashboard screen showing all patient respiratory parameters and alarms, (b) a detailed screen for each patient, and (c) a sequence of clinical handoff pages (Figure 2). The mobile application was designed to provide RCPs with continuous, real-time Vt, f, pressure/volume waveforms, plateau pressures, and indications of alarms from the ventilators, all displayed on an 8-inch tablet (Samsung, Galaxy Tab A, San Jose, CA) during the clinical simulation. The tablet user interface was designed after an online survey of over 175 practicing RCPs determined minimum data practitioners wanted the ability to access, and the optimal information required for clinical handoff. The mobile application provided efficient data entry to record medication administration and clinical information (blood gases, chest radiographs, breath sounds, suctioning, etc.) via check boxes, radio buttons, and pop-up selections, which could be accomplished within 15 seconds. Parameter changes and patient data were automatically downloaded from the patient’s ventilator. All information was available at all times for all patients simultaneously and used to populate the clinical handoff template defined by the team.
 |
Figure 1 Wi-Fi dongle connected to the serial port of a Puritan Bennett 840 Ventilator (A). One room of the simulated ICU (B). |
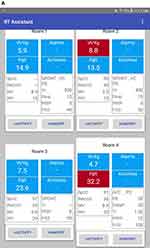 |
Figure 2 Continued. |
 |
Figure 2 Continued. |
To develop the clinical handoff template, ten different RCPs and RT Managers collaborated to reach a consensus on the minimum information that should be included (Figure 3). This template was then used to create an electronic shift report guide within the tablet (RT Assistant, Convergent Engineering, Gainesville, FL).
 |
Figure 3 A consensus shift report template containing minimum information required. |
Next, a mock intensive care unit (ICU) was created (Santa Fe College, Gainesville, FL). This ICU was populated by four simulated patients requiring MV in a control group, and 4 similar patients in the study group. After obtaining informed consent, eight RCPs participated in this simulation study, performing care for both the control and experimental group. The essential qualities of the scenarios (e.g., changing compliance, airway resistance, f, Vt) were maintained between the two groups but were disguised with different demographics, admission diagnosis, and clinical interventions. Each set of simulations was designed to be an accelerated clinical shift, including three rounds of bedside patient assessments, aerosolized bronchodilator administration, endotracheal suctioning, patient transport, and other activities that could be completed within four hours.
For the control group, each of eight RCPs, from three different hospitals, were provided with a scripted clinical handoff report from one of the primary investigators and asked to care for the simulated patients as they would in their hospital practice. The investigator reviewed the significance of LPV guidelines with the RCP before the study. The Wi-Fi dongles attached to the ventilators and respiratory monitors collected data continuously throughout the control study, but the RCPs were not aware of these devices. After completion of the simulated shift, the RCPs were asked to provide a shift report to two senior clinicians who graded the shift report using checklists and a 9-question 5-point Likert scale questionnaire. The checklists were created by compiling information from the scenarios and the minimum information shift report described above to provide a simple method for evaluating the amount of critical information transmitted during the shift report. The RCP also filled out a 5-point Likert scale questionnaire to self-evaluate their performance.
For the experimental group, the same 8 RCPs who participated in the control group were presented with scenarios similar to the previous exercise. The RCPs were first trained on the use of the RT Assistant mobile application and again reminded of the LPV practice guidelines. The training required approximately 15–20 minutes to complete. All RCPs demonstrated proficiency in use of the software. After their simulated shift, the study group used the RT Assistant to provide a structured shift-report to the same senior clinicians. As previously noted, data were collected from the ventilators and respiratory monitor and surveys were filled out by the receiving clinicians and RCP. Additional questions were asked of the RCP to evaluate the effectiveness and ease-of-use of the RT Assistant interface.
All research-related activities were approved by the Western Institutional Review Board (WIRB). Written and informed consent was obtained from all active participants. The anonymous online survey received a waiver of informed consent.
Description of Scenarios
The simulated ICU environment consisted of eight simulated patients with severe respiratory failure of different etiologies who were intubated and mechanically ventilated. Demographics, initial conditions, and required treatments and interventions were also different. Table 1 shows the scenarios studied for the control group. The experimental group (Table 2) consisted of similar patients disguised with different demographics to minimize the RCP learning effect but still allow comparison.
 |
Table 1 Virtual Scenarios Presented to the Control Group |
Results
LPV Improvement
Although all RCPs were reminded of the LPV guidelines (Pplat below 30 cmH2O and Vt between 4 and 8 mL/kg/PBW) before the beginning of each phase of the study, the Pplat was above the LPV guideline 26.6 ± 7.1% of the time and the Vt was outside the range 19.5 ± 5.3% of the time during the control group testing. With the use of the RT Assistant (experimental group), by providing the RCP more LPV awareness, even when not in the patient’s room, the time above the Pplat dropped by 74% (to 7 ± 4.1%) and the time outside the Vt range dropped by 60% (to 7.8 ± 3.3%). The Wilcoxon Signed Rank Test indicated both values were significant at p-values of 0.007 and 0.015, respectively.
Clinical Handoff Improvement
The percentage of data transferred during the shift change – as quantified by the checklists – increased significantly during the experimental group from 45% (control) to 63% (experimental), with a p-value of essentially zero at all possible alpha levels (two proportion z-test).
The average shift report score – average of 9-question Likert questionnaire evaluated by staff clinicians – improved significantly with the use of the RT Assistant from 2.8 to 1.6 on a scale of 1 to 5 with 1 being best. The Wilcoxon Signed Rank Test resulted in a p-value of 0.03 with an alpha level of 0.05.
The mean time required to complete the handoff increased from 7.8 ± 2.0 minutes during the control study to 12.8 ± 32.9 minutes with the RT Assistant. This increase was expected due to the increased quantity and quality of the information transmitted and, possibly, due to initial unfamiliarity with the tool. Anecdotally, we noticed that without the RT Assistant, most RCPs spent at least 5 minutes collecting and organizing their data for the shift-report. No time was spent preparing for the shift report with the RT Assistant.
RCP Satisfaction Scores
The RCP’s evaluation of their experience with the tool during the experimental group was positive (Table 3). On a scale of 1 to 5 using a 10-item questionnaire (1 indicating definitely satisfied), 8 of the 9 questions resulted in a score of 1.4 or less.
 |
Table 3 Subset of the Results of the Satisfaction Survey During the Experimental Group. The Full Results of the Survey are Provided in the Online Supplement |
Discussion
The main finding of this study was that an electronic platform adapted to a hand-held tablet receiving real-time ventilatory parameters can increase RCP’s awareness of two key LPV parameters: Pplat <30 cmH2O and Vt between 4 and 8 mL/kg PBW. Furthermore, an electronic checklist can improve the quality of RCP handoffs. We chose to apply the intervention to a group of experienced RCPs who functioned as their own control, changing the scenarios between the control and experimental phases, including change of demographics, evolution of the scenarios, and recommended interventions. Despite the group’s seniority, LPV in the control group was out of recommended range for 19.5 to 26.6% of the time. It is quite conceivable that this number would be more significant for less experienced RCPs as, almost 20 years from the initial recommendation to set mechanical ventilation in ARDS, LPV remains underutilized. There is a clear need to explore innovative alternatives to improve compliance to evidence-based medicine in this matter, and we submit that well-designed electronic tools might be the answer. LPV is an obvious choice for improving compliance, but many other “nudges” or context-sensitive reminders could be implemented easily (spontaneous breathing testing, oxygenation, driving pressure, clinical protocol adherence, and extubation readiness).
In the experimental group, the time above the Pplat limit dropped by 74% (to 7 ± 4.1%), and the time outside the recommended Vt range decreased by 60% (to 7.8 ± 3.3%) when the electronic system was used. Continuous, instead of intermittent, awareness resulted in more frequent ventilator adjustments in the right direction to more closely follow the LPV guidelines. Based on the remarkable increase in the use of LPV in the experimental group, it is very likely that this approach would also benefit other monitored parameters such as driving pressure.
Fragmentation of care is an unfortunate, but real, phenomenon of longitudinal hospital care. While mnemonics may help, they often miss details of patient–ventilator interaction without a systematic checklist. The tested software was designed to not only improve compliance with the LPV guidelines but also enhance communication between RCPs.
Using the automated checklist, we noted a significant increase (absolute change of 18%) of handoff data transmitted between the control and the experimental group. In our study, the mean time to deliver the shift report increased from 7.8 ± 2 minutes during the control study to 12.8 ± 2.9 minutes when the RT Assistant was used. We expected a more detailed report to take more time than a memory checklist, as there is often a trade-off between quality and time. However, the increased time needed to transmit the electronic handoff was offset by a faster preparation of the shift report, which was automatically prepopulated with information obtained by the system.
We hypothesized that the electronic system increased the RCPs’ level of LPV awareness by continuous, real-time feedback. This electronic application integrated well with the RCP workload. This highly demanding workflow often creates difficulties obtaining appropriate situational awareness and causes personnel dissatisfaction. In a 2016 survey of RCPs, the most significant source of dissatisfaction amongst RCPs was that they were overburdened due to staffing shortages, high patient-to-clinician ratio and the increasing workload without additional staffing resources.19 A potential advantage for the handheld system is improved situational awareness that is achieved from the mobile data delivery from all patients to the clinician. Situational awareness and response times can suffer when RCPs are overburdened.
In a survey of 130 managers, 30% indicated they do not have adequate staff, and 21% noted that they have significant and chronic understaffing; 30% of these directors felt that patient safety issues resulted from the understaffing.20 Staffing problems will likely continue since the Bureau of Labor Statistics indicates that the need for new RCPs will grow 23% over the next ten years and 30,000 more RCPs will be needed.21 The COVID-19 pandemic stretched clinician staffing further and highlighted the need for improved efficiency tools.
We consider the simplicity and cost-effectiveness of this stand-alone hardware-software as an advantage. The system consists of electronic dongles on the medical devices, a dedicated server controlled by the hospital, and a mobile application developed in Java and running on Android. These are open platforms, easily adaptable to meet local needs and logistics. We believe this system is a logical first step towards future platforms that can provide additional decision support, including the Artificial Intelligence and Internet of Things (AIOT) framework.
Limitation
Our study represents an in vitro, preliminary observation and presents several limitations. The application of mechanical ventilation is complex with many variables. We focused our monitoring on 2 LPV parameters, Vt and Pplat. Further investigation is required for other settings and parameters such as PEEP, FiO2, respiratory rate, and driving pressures used in mechanical ventilation.
Secondly, we appreciate implementation of electronic prompters can be complex and not well received by healthcare professionals. Loss of autonomy by increasingly relying on remote monitoring and alarms, in particular, is often perceived as intrusive. Behavioral economic strategies are introduced in the handheld display, with menu choices and color code to prioritize LPV tasks.18,22 These alarms may be preset or allowed to be determined by the individual practitioner.
Conclusion
We have demonstrated that using simple electronic monitoring system, LPV awareness and patient clinical information during electronic handoff significantly improves. This introduces a tool that may be helpful for clinicians in providing safe care for patients receiving mechanical ventilation. Their actual feasibility and effectiveness will need to be evaluated in vivo – that is in a multicentered, randomized setting – before instituting a systemic implementation of process learning and measuring its effect on the outcome. We are currently in the process of verifying these results in a patient study at two academic medical centers in Florida.
Code Availability
Code is proprietary.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon request.
Ethics
All research methods were carried out in accordance with relevant guidelines and regulations. No animals were used in this research. Oversight of this study was provided by the Western Institutional Review Board (WIRB).
Consent to Participate
Western Institutional Review Board (WIRB) approved and informed consent was obtained from all individual participants included in the study.
Acknowledgments
We thank Ms. Leah Carlson, Director of Clinical Education at Santa Fe College, for her tremendous support and efforts in designing and implementing the simulated clinical trial.
Funding
This work was supported by the National Institute of Health – National Heart, Lung, and Blood research grant # 1R43HL146012-01A1 “RT Assistant – handheld patient safety tool for mechanical ventilation”.
Disclosure
Neil R. Euliano is the president and owner of Convergent Engineering, Inc. Paul Stephan and Konstantinos Michalopoulos are employees at Convergent Engineering, Inc. The authors report no other conflicts of interest in this work.
References
1. Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi:10.1056/NEJM200005043421801
2. Checkley W, Brower R, Korpak A, et al. Effects of a clinical trial on mechanical ventilation practices in patients with acute lung injury. Am J Respir Crit Care Med. 2008;177(11):1215–1222. doi:10.1164/rccm.200709-1424OC
3. Haitsma JJ, Lachmann RA, Lachmann B. Lung protective ventilation in ARDS: role of mediators, PEEP and surfactant. Monaldi Arch Chest Dis. 2003;59(2):108–118.
4. Neto AS, Simonis FD, Barbas CSV, et al. Lung-protective ventilation with low tidal volumes and the occurrence of pulmonary complications in patients without acute respiratory distress syndrome: a systematic review and individual patient data analysis. Crit Care Med. 2015;43(10):2155–2163. doi:10.1097/CCM.0000000000001189
5. Young CC, Harris EM, Vacchiano C, et al. Lung-protective ventilation for the surgical patient: international expert panel-based consensus recommendations. Br J Anaesth. 2019;123(6):898–913. doi:10.1016/j.bja.2019.08.017
6. Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319(7):698–710. doi:10.1001/jama.2017.21907
7. Papazian L, Aubron C, Brochard L, et al. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care. 2019;9(1):69. doi:10.1186/s13613-019-0540-9
8. Spece LJ, Mitchell KH, Caldwell ES, et al. Low tidal volume ventilation use remains low in patients with acute respiratory distress syndrome at a single center. J Crit Care. 2018;44:72–76. doi:10.1016/j.jcrc.2017.10.021
9. Weiss CH, Baker DW, Weiner S, et al. Low tidal volume ventilation use in acute respiratory distress syndrome. Crit Care Med. 2016;44(8):1515–1522. doi:10.1097/CCM.0000000000001710
10. Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458–1465. doi:10.1001/jama.282.15.1458
11. Kalhan R, Mikkelsen M, Dedhiya P, et al. Underuse of lung protective ventilation: analysis of potential factors to explain physician behavior. Crit Care Med. 2006;34(2):300–306. doi:10.1097/01.CCM.0000198328.83571.4A
12. Mikkelsen ME, Dedhiya PM, Kalhan R, et al. Potential reasons why physicians underuse lung-protective ventilation: a retrospective cohort study using physician documentation. Respir Care. 2008;53(4):455–461.
13. Rubenfeld GD, Cooper C, Carter G, et al. Barriers to providing lung-protective ventilation to patients with acute lung injury. Crit Care Med. 2004;32(6):1289–1293. doi:10.1097/01.CCM.0000127266.39560.96
14. Bourdeaux CP, Thomas MJ, Gould TH, et al. Increasing compliance with low tidal volume ventilation in the ICU with two nudge-based interventions: evaluation through intervention time-series analyses. BMJ Open. 2016;6(5):e010129. doi:10.1136/bmjopen-2015-010129
15. Bagga S, Paluzzi DE, Chen CY, et al. Better ventilator settings using a computerized clinical tool. Respir Care. 2014;59(8):1172–1177. doi:10.4187/respcare.02223
16. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803–1812. doi:10.1056/NEJMsa1405556
17. Alert SE. Inadequate hand-off communication. Sentinel Event Alert. 2017;58:1–6.
18. Mehta M, Veith J, Szymanski S, et al. Clinicians’ perceptions of behavioral economic strategies to increase the use of lung-protective ventilation. Ann Am Thorac Soc. 2019;16(12):1543–1549. doi:10.1513/AnnalsATS.201905-410OC
19. Minnesota’s Respiratory Therapist Workforce, 2016. Minnesota department of health 2016; 2016. Available from: http://www.health.state.mn.us/divs/orhpc/workforce/rt/2016rt.pdf.
20. California Society for Respiratory Care. California Society for respiratory care safe staffing standards position statement and white paper; 2016. Available from: http://www.csrc.org/resources/Documents/Safe%20Staffing%20files/Final%20Safe%20Staffing%20Papers/CSRC%20Staffing%20Position%20Statement%20and%20White%20Paper%20V10112016.pdf.
21. Bureau of Labor Statistics 2018. Occupational outlook handbook respiratory therapists; 2018. Available from: https://www.bls.gov/ooh/healthcare/respiratory-therapists.htm.
22. Knighton AJ, Kean J, Wolfe D, et al. Multi-factorial barriers and facilitators to high adherence to lung-protective ventilation using a computerized protocol: a mixed methods study. Implement Sci Commun. 2020;1(1):67. doi:10.1186/s43058-020-00057-x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


