Back to Journals » Cancer Management and Research » Volume 12
Upregulated Long Non-Coding RNA LL22NC03-N64E9.1 Promotes the Proliferation and Migration of Human Breast Cancer Cells by Silencing Kruppel-Like Factor 2 Expression
Authors Lian W, Jiang X, Li L, Wang Q, Hong C, Yang P, Chen D
Received 21 June 2020
Accepted for publication 5 October 2020
Published 29 October 2020 Volume 2020:12 Pages 10763—10770
DOI https://doi.org/10.2147/CMAR.S268725
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Weibin Lian,1,* Xiaohua Jiang,2,* Liangqiang Li,1,* Qinglan Wang,1 Chengye Hong,1 Peidong Yang,1 Debo Chen1
1Department of Breast Surgery, Quanzhou First Hospital Affiliated to Fujian Medical University, Quanzhou, Fujian, People’s Republic of China; 2Department of Orthopedics, Xiang’an Hospital of Xiamen University, School of Medicine, Xiamen University, Xiamen, Fujian, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Weibin Lian; Debo Chen Tel +86-15260819892; +86-13600738668
Email [email protected]; [email protected]
Introduction: Recently, the significant regulatory effects of lncRNAs on the oncogenesis and growth of tumor have been demonstrated by an increasing number of research projects. A previous study showed that LL22NC03-N64E9.1 could promote the development of colorectal cancer, especially via enhanced cell proliferation. Similarly, this lncRNA should have comparable functions in breast cancer (BC), which requires in-depth investigation. Therefore, this study was designed to explore the correlation of LL22NC03-N64E9.1 with BC.
Methods: qRT-PCR was used to assess the relative expression of LL22NC03-N64E9.1 in BC tissues. Cell viability examination and colony formation experiments were performed to investigate the role of LL22NC03-N64E9.1 in BC cell’s proliferation. Transwell assays were used to explore the effects of LL22NC03-N64E9.1 on BC cell’s migration. RNA immunoprecipitation, chromosome immunoprecipitation assay and rescue experiments were performed to analyze the association of LL22NC03-N64E9.1 with target proteins and genes in BC cells.
Results: We identified that LL22NC03-N64E9.1 is an oncogene, upregulated in BC, which was verified in a cohort of 48 pairs of BC tissues. Based on the loss-of-function experiments, silencing LL22NC03-N64E9.1 expression significantly inhibited malignancy progression. In terms of the mechanism, LL22NC03-N64E9.1 acted on the enhancer of zeste homolog 2 (EZH2) by direct binding, which promoted BC cell growth. Furthermore, in the promoters of KLF2, the trimethylation of H3K27 could be regulated by LL22NC03-N64E9.1 as the mediator.
Conclusion: Relying on the LL22NC03-N64E9.1/EZH2/KLF2 pathway, the lncRNA LL22NC03-N64E9.1 was significantly associated with BC development and could, therefore, be a potential therapeutic target to block BC growth.
Keywords: breast cancer, lncRNA, LL22NC03-N64E9.1, H3K27me3, KLF2
Background
Breast cancer (BC) has been reported as one of the most threatening diseases due to its high prevalence and mortality.1,2 In addition, there are also an increased number of reports which anticipate that the social burden caused by BC will continue to increase in most regions,3 for example China, in particular amongst the urban population.4,5 Recently, BC treatment strategies have been improved due to various remarkable achievements, although the overall survival is still expected to be further extended.6,7 Therefore, researchers are committed to identify and probe into novel therapeutic targets, specifically, oncogenes, thus the regulatory mechanisms at the genomic level still need to be explored.8
Non-coding sequences occupy 98% of the human genome and although they do not directly translate to proteins, they are designated to translate to various types of RNA transcripts, such as long non-coding RNAs (lncRNAs).9,10 The most definite characteristic of a lncRNA is the length, which is more than 200 nucleotides.11 Although the existence of lncRNAs was previously mistaken for useless, accumulating evidence has revealed the clinical significance of lncRNAs as biomarkers and therapeutic targets.12,13 To our knowledge, several lncRNAs associated with BC prognosis have been identified, including NEAT1, which regulates cancer cell proliferation and invasion14,15 and MALAT1, which has been correlated to BC metastasis.16 However, there is a general consensus that multiple lncRNAs which have vital roles in the pathogenetic mechanisms of BC still need to be discovered, in particular the molecular details.
LL22NC03-N64E9.1, located at chromosome 22q11.1, has been reported as an oncogenic lncRNA and affects aggressive phenotypes in human lung and colorectal cancer.17,18 To investigate its relevance to BC, the expression profile of lncRNA LL22NC03-N64E9. 1 in human BC samples was compared with normal tissues. Subsequently, experiments were conducted to assess the association between LL22NC03-N64E9.1, and BC cell proliferation and migration. By rough estimation, 24% of lncRNAs could be physically associated with the polycomb repressive complex 2(PRC2).19,20 To clarify whether LL22NC03-N64E9. 1 could bind to the PRC2 complex, EZH2, a core subunit of the PRC2 complex, which catalyzed the trimethylation of histone H3 at lysine 27 (H3K27me3) was selected as the candidate target for the lncRNA.21 Finally, the downstream pathway regulated by altered LL22NC03-N64E9. 1 expression and EZH2 was identified, and the underlying mechanisms of the lncRNA in BC cells were investigated.
Methods
Tissue Collection and Ethics Statement
The sample collection took place at Quanzhou First Hospital Affiliated to Fujian Medical University. After the surgical procedures to remove primary BC, 48 BC tissue samples were acquired. The experiment protocol was reviewed and approved by the Ethics Committee of the Fujian Medical University (Fuzhou, Fujian, PR China), which followed the Declaration of Helsinki Principles. Accordingly, patient samples could only be used after obtaining signed informed consents.
Cell Lines and Cell Culture
MCF-7 and BT549, human cell lines of BC, were provided by the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich, MO), 100 U/mL penicillin and 100 g/mL streptomycin was used for cell culture in a humidified incubator set at 37°C with 5% CO2.
RNA Extraction and qRT-PCR Assays
All the experimental procedures were conducted following the manufacturers’ instructions. Trizol reagent (Invitrogen) was used to extract total RNA from the cell culture. PrimeScript RT reagent Kit (TaKaRa, Dalian, China) was utilized to reverse transcribe 1 μg of extracted total RNA into 20 μL product containing cDNA. SYBR Premix Ex Taq (TaKaRa, Dalian, China) was applied to quantify LL22NC03-N64E9.1 expression and its downstream targets, and the data were normalized to GAPDH level. The PCR primers had the following sequences: LL22NC03-N64E9.1 (Forward) 5′- AAGCCATGTAAAGGGGGCTC-3′; LL22NC03-N64E9.1 (Reverse) 5′- TGGTAGTCTGACCATTCTGCAT-3′; EZH2 (Forward) 5′- TGCACATCCTGACTTCTGTG-3′, EZH2 (Reverse) 5′- AAGGGCATTCACCAACTCC −3′; GAPDH (Forward) 5′- GAAGAGAGAGACCCTCACGCTG −3′; GAPDH (Reverse) 5′- ACTGTGAGGAGGGGAGATTCAGT-3′; kruppel-like factor 2 (KLF2) (Forward) 5′- CTGCACATGAAACGGCACAT-3′; KLF2 (Reverse) 5′-CAGTCACAGTTTGGGAGGGG-3′.
Cell Transfection
Lipofectamine 2000 (Invitrogen, USA) was the reagent for transfecting BC cell samples with the specific siRNAs. LL22NC03-N64E9.1 siRNA and the siRNA of scrambled negative control (si-NC) were provided by Invitrogen. The siRNA sequences were as follows: LL22NC03-N64E9.1 siRNA: UAGCUGGAGCAGUACAUCUUCAAUU; si-NC: UUCUCCGAACGUGUCACGUTT; EZH2 siRNA: GAGGUUCAGACGAGCUGAUUU. After transfection and 48h of incubation, the transfected samples were usable for subsequent analyses.
Cell Proliferation Analysis
Cell viability was examined using the MTT kit (Sigma). To conduct the colony formation assay, transfected cell samples were placed in standard six-well cell culture plates and maintained in DMEM containing 10% FBS for 2 weeks. The medium was replaced every 5 days. After 2 weeks, samples were fixed with methanol and stained for 15 min with 0.1% crystal violet (Sigma) in PBS. The number of stained colonies was counted and compared to evaluate colony formation.
Transwell Assay
A 24-well chamber (Corning, NY, USA) was embedded with polycarbonate membrane of 8 μm pore size. To assess cell invasion and migration, the membrane was either coated with Matrigel (Becton Dickinson, NJ, USA) or not coated, respectively, and subsequently placed into the upper side of the chamber and incubated for 24h. Cells remaining on the upper side were gently wiped with cottons swabs, while cells that passed through the membrane onto the lower side were fixed and stained with 0.5% crystal violet. Five fields in each well were randomly selected, and the number of migrant cells were counted. Each test was conducted in triplicate.
RNA Immunoprecipitation (RIP) Assays
Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore, USA) was used according to manufacturer’s protocol. The antibody against EZH2 was provided by Millipore.
Chromatin Immunoprecipitation (ChIP) Assays
The EZ-CHIP KIT (Millipore, USA) was used to perform the CHIP assay. EZH2 was provided by Abcam and the H3 trimethyl Lys 27 antibody, Histone H3, and Acetyl-Histone H3 Lys27 were provided by Millipore. The ChIP primer sequences were: KLF2 (Forward) 5′- ACGGGCTTATTGAGGTTGG-3′ and KLF2 (Reverse) 5′- GCCTGGGTGACAGAGGAGAC-3′. The immunoprecipitated DNA was quantitated by qPCR. The formula, 2[Input Ct − Target Ct] × 100 (%), was used to interpret the raw data. Each test was conducted independently in triplicate.
Statistical Analysis
Statistical analyses were performed using SPSS software version 17.0 (SPSS, IL, USA). The Student’s t-test or the chi-square test was used to compare different data sets and determine the significance. The data were expressed as means ± SD. Differences were considered as significant if P < 0.05. “*” indicates P < 0.05.
Results
LL22NC03-N64E9.1 is Upregulated in Human BC Tissue Samples
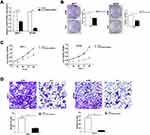
By interpreting the raw microarray data for human breast tissues obtained from the Cancer Genome Atlas (TCGA), the expression profile of LL22NC03-N64E9.1 could be compared between the groups with or without BC. Compared with noncancerous tissues, LL22NC03-N64E9.1 expression was obviously upregulated in BC tissues (Figure 1A). To further validate this result by qRT-PCR, we investigated 48 clinical BC samples and found that LL22NC03-N64E9.1 expression was also upregulated in the majority of BC samples (36 out of 48) compared with the adjacent noncancerous tissues (P < 0.001; Figure 1B).
LL22NC03-N64E9.1 Regulates BC Cell Proliferation and Migration
We examined whether LL22NC03-N64E9.1 was functionally involved in BC progression and discovered that LL22NC03-N64E9.1 expression was silenced after transfection with short interfering RNA (siRNA) in MCF-7 and BT549 cells (Figure 2A). To further assess the role of LL22NC03-N64E9.1 in the BC cell phenotype, loss-of-function assays were conducted. The findings provided by colony formation assay confirmed that cell colonies had significantly impeded growth in the LL22NC03-N64E9.1 knockdown group, compared with the si-NC group in MCF-7 and BT549 cells (Figure 2B). In addition, MTT assay revealed that LL22NC03-N64E9.1 knockdown significantly inhibited BC cell viability (Figure 2C). Transwell assay was conducted to determine whether LL22NC03-N64E9.1 expression was associated with BC cell migration and the results showed that LL22NC03-N64E9.1 knockdown significantly reduced the quantity of migratory cells among MCF-7 and BT549 BC cell lines, compared with the si-NC group (Figure 2D). Overall, the silenced LL22NC03-N64E9.1 expression reduced BC cell proliferation and migration.
LL22NC03-N64E9.1 Exerts Oncogene Function via Interacting with EZH2
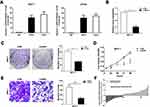
Our subsequent task was to clarify whether LL22NC03-N64E9. 1 could bind to the PRC2 complex. RNA–protein interaction prediction (RPISeq) analysis determined the score for LL22NC03-N64E9.1/EZH2 interaction, which was 0.8 with SVM classifier (data were not showed). With the probabilities of >0.5, the predictions were assigned to be positive, and accuracy for the predictions ranged from 57% to 99% for individual datasets of RNA–protein interactions.22 To verify the predictive results, RIP assays demonstrated the enrichment of endogenous LL22NC03-N64E9.1 in the anti-EZH2 fraction relative to the input and compared to the IgG fraction (Figure 3A). To measure the biological features of EZH2in BC, endogenous EZH2 expression in MCF-7 cells was knocked down by siRNA (Figure 3B). Colony formation and MTT assays showed that downregulated EZH2 weakened the proliferation ability of MCF-7 cells (Figure 3C and D). Additionally, the migration ability of BC cells was also strongly decreased due to EZH2 knockdown (Figure 3E). These results summarize the oncogenic role of EZH2 in promoting malignant BC cell proliferation and migration, which corresponds with the carcinogenesis of LL22NC03-N64E9.1 in BC. Furthermore, we detected EZH2 expression in 48 paired human BC tissues and the 48 paired normal tissues, and proved that EZH2 was indeed overexpressed in BC (Figure 3F).
LL22NC03-N64E9.1 Epigenetically Suppresses KLF2 Transcription by Interacting with EZH2
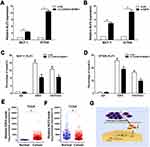
To identify the candidate genes targeting LL22NC03-N64E9.1 in BC, the expression profile for the genes downstream of LL22NC03-N64E9.1 in colorectal cancer cells, which was constructed by Lian et al,17 were reviewed. A qRT-PCR assay was conducted of the KLF2 as mentioned earlier in the study. The results showed that LL22NC03-N64E9.1 expression was effectively knocked down, while mRNA levels of KLF2 were upregulated (Figure 4A). Similarly, EZH2 knockdown led to the same consistent results (Figure 4B). Moreover, the results of ChIP assays showed that EZH2 could directly bind to the KLF2 promoter regions, thereby inducing H3K27me3 modification in MCF-7 and BT549 cells (Figure 4C and D). Knockdown of LL22NC03-N64E9.1 led to reduced EZH2 binding, and the H3K27me3 occupancy of the KLF2 promoter locus was also reduced (Figure 4C and D). Additionally, it was found that EZH2 expression was obviously increased in BC tissues, while KLF2 expression was lowly expressed in BC tissues (Figure 4E and F). Taken together, LL22NC03-N64E9.1 promoted BC cell proliferation and migration partly through epigenetically silencing KLF2 transcription (Figure 4G).
Discussion
LL22NC03-N64E9.1 has attracted considerable interest from researchers, which increased even more due to its recent discovered role in lung and colorectal cancer.17,18 For instance, Jing et al found that LL22NC03-N64E9.1 was upregulated in lung cancer samples, and it showed a positive association with key clinical parameters of lung cancer, such as overall survival (OS), tumor size, and advanced tumor stage, which could anticipate a poor prognosis.18 In addition, Lian et al found that LL22NC03-N64E9.1 was upregulated in colorectal cancer specimens, while its knockdown suppressed malignant cell proliferation.17 As suggested by previous research, the tumorigenesis and development of BC could be facilitated by abnormally expressed lncRNAs, while other specific lncRNAs have the potential of being candidate diagnostic markers and therapeutic targets against BC. Therefore, proved by this study, LL22NC03-N64E9.1 expression was remarkably enhanced in BC tissues. Referring to the effects, LL22NC03-N64E9.1 knockdown suppressed cell proliferation and migration of BC cells via the LL22NC03-N64E9.1/EZH2 pathway. Moreover, the lncRNA transcripts could mediate H3K27 trimethylation in KLF2 promoters. Thus, LL22NC03-N64E9.1 could modulate the tumor development in BC, thereby serving as a novel therapeutic target.
To interact with the downstream targets, most lncRNAs directly bind to specific RNA-binding proteins (RBPs). The RNA protein interaction might activate or inactivate downstream gene expression via various mechanisms, including RNA decay, histone protein modification, DNA methylation or chromosome reprogramming.23–26 This study demonstrated that LL22NC03-N64E9.1 could reduce KLF2 expression in BC cells while EZH2 was targeted by the lncRNA. As the core subunit within a PRC2 complex, EZH2 was the catalyst for the trimethylation of lysine 27 of histone H3 (H3K27me3), which facilitated tumor growth by deactivating the transcription for downstream tumor suppressor genes.27 According to various recent research, dysregulation of EZH2 is a tumor-related regulatory factor in different types of malignancies.28,29 Our study also revealed that EZH2 was oncogenic in BC cells. KLF2 is a transcription factor that belongs to the zinc-finger family.22,30 Notably, KLF2 is considered a tumor suppressor gene in a variety of tumors, including BC.31,32 Our data showed that LL22NC03-N64E9.1 could mediate H3K27 trimethylation in KLF2 promoters, thus suppressing its expression.
In summary, this study demonstrated that LL22NC03-N64E9.1 overexpression enhanced BC proliferation and metastasis partially through inhibiting KLF2 transcription. LL22NC03-N64E9.1 could be a novel target for antitumor chemotherapy. The identification of the LL22NC03-N64E9.1/EZH2/KLF2 axis broadened our knowledge and could serve as an important reference in improving BC treatment strategies by considering epigenetic modification. Apart from the achievements, the scope of the study was still limited in some aspects. If the sample size could be increased beyond the current range, the validation of the conclusions would be more creditable, and as follow-up research on the oncogenic functions of LL22NC03-N64E9.1, it would be valuable to include in-vivo tests conducted on animal models. In addition, it cannot be excluded that other candidate targets of LL22NC03-N64E9.1 might also participate in BC development. Thus, follow-up research should be designed to solve the abovementioned shortages, as well as identify the upstream regulatory pathways of LL22NC03-N64E9.1 overexpression in BC.
Data Sharing Statement
The data that support the findings of this study are available from Weibin Lian upon reasonable request.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed on the journal to which the article will be submitted; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study was funded by the Science and Technology Plan Project of Quanzhou (Grant No. 2018N073S) and the Startup Fund for scientific research, Fujian Medical University (Grant No. 2019QH1251).
Disclosure
The authors have declared that no conflict of interest exists.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
2. Barroso-Sousa R, Jain E, Cohen O, et al. Prevalence and mutational determinants of high tumor mutation burden in breast cancer. Ann Oncol. 2020;31(3):387–394. doi:10.1016/j.annonc.2019.11.010
3. Ranganathan K, Singh P, Raghavendran K, et al. The global macroeconomic burden of breast cancer: implications for oncologic surgery. Ann Surg. 2020.
4. Li N, Deng Y, Zhou L, et al. Global burden of breast cancer and attributable risk factors in 195 countries and territories, from 1990 to 2017: results from the Global Burden of Disease Study 2017. J Hematol Oncol. 2019;12:140.
5. Zheng Y, Zhang M. Disparities of breast cancer burden between China and western countries and its implication. Zhonghua Wai Ke Za Zhi. 2015;53:905–909.
6. Dobovisek L, Krstanovic F, Borstnar S, Debeljak N. Cannabinoids and hormone receptor-positive breast cancer treatment. Cancers. 2020;12:525.
7. Ferraiuolo RM, Wagner KU. Regulation and new treatment strategies in breast cancer. J Life Sci. 2019;1:23–38.
8. Panda S, Banerjee N, Chatterjee S. Solute carrier proteins and c-Myc: a strong connection in cancer progression. Drug Discov Today. 2020;25(5):891–900. doi:10.1016/j.drudis.2020.02.007
9. Haemmig S, Simion V, Yang D, Deng Y, Feinberg MW. Long noncoding RNAs in cardiovascular disease, diagnosis, and therapy. Curr Opin Cardiol. 2017;32(6):776–783. doi:10.1097/HCO.0000000000000454
10. Lu S, Zhang J, Lian X, et al. A hidden human proteome encoded by ‘non-coding’ genes. Nucleic Acids Res. 2019;47(15):8111–8125. doi:10.1093/nar/gkz646
11. Huang Y, Guo Q, Ding XP, Wang X. Mechanism of long noncoding RNAs as transcriptional regulators in cancer. RNA Biol. 2020;1–13.
12. Chen B, Li Y, He Y, Xue C, Xu F. The emerging roles of long non-coding RNA in gallbladder cancer tumorigenesis. Cancer Biomarkers. 2018;22:359–366.
13. Jiang MC, Ni JJ, Cui WY, Wang BY, Zhuo W. Emerging roles of lncRNA in cancer and therapeutic opportunities. Am J Cancer Res. 2019;9:1354–1366.
14. Li X, Deng S, Pang X, et al. LncRNA NEAT1 silenced miR-133b promotes migration and invasion of breast cancer cells. Int J Mol Sci. 2019;20(15):3616.
15. Pang Y, Wu J, Li X, et al. NEAT1/miR124/STAT3 feedback loop promotes breast cancer progression. Int J Oncol. 2019;55:745–754.
16. Arun G, Spector DL. MALAT1 long non-coding RNA and breast cancer. RNA Biol. 2019;16(6):860–863. doi:10.1080/15476286.2019.1592072
17. Lian Y, Yan C, Ding J, et al. A novel lncRNA, LL22NC03-N64E9.1, represses KLF2 transcription through binding with EZH2 in colorectal cancer. Oncotarget. 2017;8(35):59435–59445. doi:10.18632/oncotarget.19738
18. Jing H, Qu X, Liu L, Xia H. A novel long noncoding RNA (lncRNA), LL22NC03-N64E9.1, promotes the proliferation of lung cancer cells and is a potential prognostic molecular biomarker for lung cancer. Med Sci Monit. 2018;24:4317–4323. doi:10.12659/MSM.908359
19. Davidovich C, Cech TR. The recruitment of chromatin modifiers by long noncoding RNAs: lessons from PRC2. RNA. 2015;21:2007–2022.
20. Xue J-Y, Huang C, Wang W, Li H-B, Sun M, Xie M. HOXA11-AS: a novel regulator in human cancer proliferation and metastasis. Onco Targets Ther. 2018;11:4387–4393. doi:10.2147/OTT.S166961
21. Ma A, Stratikopoulos E, Park K-S, et al. Discovery of a first-in-class EZH2 selective degrader. Nat Chem Biol. 2020;16(2):214–222. doi:10.1038/s41589-019-0421-4
22. Yang J, Lian Y, Yang R, et al. Upregulation of lncRNA LINC00460 facilitates GC progression through epigenetically silencing CCNG2 by EZH2/LSD1 and indicates poor outcomes. Mol Ther Nucleic Acids. 2020;19:1164–1175. doi:10.1016/j.omtn.2019.12.041
23. Wang Z, Qiu H, He J, et al. The emerging roles of hnRNPK. J Cell Physiol. 2020;235(3):1995–2008. doi:10.1002/jcp.29186
24. He R-Z, Luo D-X, Mo -Y-Y. Emerging roles of lncRNAs in the post-transcriptional regulation in cancer. Genes Dis. 2019;6(1):6–15. doi:10.1016/j.gendis.2019.01.003
25. Yan J, Dutta B, Hee YT, Chng W-J. Towards understanding of PRC2 binding to RNA. RNA Biol. 2019;16(2):176–184. doi:10.1080/15476286.2019.1565283
26. Creamer KM, Lawrence JB. XIST RNA: a window into the broader role of RNA in nuclear chromosome architecture. Philos Trans R Soc Lond B Biol Sci. 2017;372:20160360.
27. Laugesen A, Hojfeldt JW, Helin K. Molecular mechanisms directing PRC2 recruitment and H3K27 methylation. Mol Cell. 2019;74:8–18.
28. Kim KH, Roberts CW. Targeting EZH2 in cancer. Nat Med. 2016;22:128–134.
29. Roche J, Gemmill RM, Drabkin HA. Epigenetic regulation of the epithelial to mesenchymal transition in lung cancer. Cancers. 2017;9(12):72. doi:10.3390/cancers9070072
30. Wang Q, He Y, Kan W, et al. microRNA-32-5p targets KLF2 to promote gastric cancer by activating PI3K/AKT signaling pathway. Am J Transl Res. 2019;11:4895–4908.
31. Hu CC, Liang YW, Hu JL, Liu LF, Liang JW, Wang R. LncRNA RUSC1-AS1 promotes the proliferation of breast cancer cells by epigenetic silence of KLF2 and CDKN1A. Eur Rev Med Pharmacol Sci. 2019;23:6602–6611.
32. Taniguchi H, Jacinto FV, Villanueva A, et al. Silencing of kruppel-like factor 2 by the histone methyltransferase EZH2 in human cancer. Oncogene. 2012;31(15):1988–1994. doi:10.1038/onc.2011.387
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.