Back to Journals » Journal of Asthma and Allergy » Volume 9
Update on the role of alternatively activated macrophages in asthma
Received 18 January 2016
Accepted for publication 31 March 2016
Published 3 June 2016 Volume 2016:9 Pages 101—107
DOI https://doi.org/10.2147/JAA.S104508
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Frank Redegeld
Zhilong Jiang, Lei Zhu
Department of Pulmonary Medicine, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China
Abstract: Lung macrophages link innate and adaptive immune responses during allergic airway inflammatory responses. Alveolar macrophages (AMs) and interstitial macrophages are two different phenotypes that differentially exert immunological function under physiological and pathological conditions. Exposure to pathogen induces polarization of AM cells into classically activated macrophages (M1 cells) and alternatively activated macrophages (M2 cells). M1 cells dominantly express proinflammatory cytokines such as TNF-α and IL-1 β and induce lung inflammation and tissue damage. M2 cells are further divided into M2a and M2c subsets. M2a cells dominantly produce allergic cytokines IL-4 and IL-13, but M2c cells dominantly produce anti-inflammatory cytokine IL-10. M2a and M2c cells are differently involved in initiation, inflammation resolution, and tissue remodeling in the different stages of asthma. Microenvironment dynamically influences polarization of AM cells. Cytokines, chemokines, and immune-regulatory cells interplay and affect the balance between the polarization of M1 and M2 cells, subsequently influencing disease progression. Thus, modulation of AM phenotypes through molecular intervention has therapeutic potential in the treatment of asthma and other allergic inflammatory diseases. This review updated recent advances in polarization and functional specialization of these macrophage subtypes with emphasis on modulation of polarization of M2 cells in asthma of human subjects and animal models.
Keywords: asthma, macrophage polarization, alternatively activated macrophages, M2 cells, cytokines
Introduction
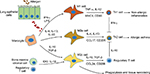
Lung macrophages are a heterogenic population of mononuclear phagocytes that are divided into alveolar macrophages (AMs) and interstitial macrophages (IMs).1,2 AMs reside in the lung inner surfaces and have both proinflammatory and anti-inflammatory properties, whereas IMs reside in the interstitial area, maintain immune homeostasis in the respiratory tract, and exert immune tolerance to harmless antigens.1,3 According to different cell surface markers and cytokine expression levels, AMs are further divided into two major subtypes M1 and M2 cells (Figure 1).4 M1 cells are classically activated phenotype cells, expressing high levels of proinflammatory cytokines such as inducible nitric oxide synthase (iNOS), IL-1 β, and TNF-α and responsible for inflammation and protection against invading pathogens5, whereas M2 cells are alternatively activated phenotype cells and can be further divided into alternative activated cells (M2a), type II alternatively activated cells (M2b), or acquired deactivated cells (M2c) and M2d cells (Table 1).6,7 However, identification and dynamic changes of these M2 subtypes are not well documented in the asthmatic mouse model and human subjects so far.8–10 These cell subtypes express specific cell surface markers and several anti-inflammatory mediators and chemokines, such as IL-10, IL-13, and CCL-17.11–13 They are critically involved in the initiation and resolution of lung inflammation during allergic immune responses. Their polarization and function are greatly influenced by the microenvironment, such as several cytokines and chemokines. This review updates the recent advances of polarization of lung macrophages and their specialized function in asthmatic animal models and patients with asthma.
Macrophages in asthma
Asthma is a heterogeneous lung allergic disorder and is divided into atopic and nonatopic phenotypes, which share common features of lung hypersensitivity. Atopic asthma is mediated by IgE and is usually caused by allergens, air pollution, and genetic factors; however, nonatopic asthma is not mediated by IgE and is usually caused by virus infection, drugs, chemical irritants, cigarette smoking, stress, etc. The activated Th2 cells and type 2 innate lymphoid cells together with basophils drive infiltration of eosinophils in asthmatic lungs, but in some cases, neutrophils and Th17 cells are largely present and are controlled by the Th17 cell subset.14 As a first line of the cell component, mononuclear macrophages are activated and proliferated during the early phase of disease and play a pivotal role in the clearance of pathogens, initiation of lung inflammation, and inflammation resolution during later phases. The study of Ji et al3 in bleomycin-induced lung injury mouse model showed that circulating Ly6C(hi) monocytes peaked on day 3 and their magnitude was positively associated with pulmonary inflammatory response, whereas M2-like AMs (F4/80+CD11c+CD206+) peaked on day 14 and were positively correlated with the magnitude of lung fibrosis. Although lung-resident macrophages are well investigated, the cell origin is still elusive. A recent study suggested that resident AMs are derived from Csf1r(+) erythro-myeloid progenitors and yolk sac but myeloid-derived macrophages cells originate and renew from bone marrow hematopoietic stem cells.15 Their development and renewal into a distinct macrophage phenotype require granulocyte-macrophage colony-stimulating factor16 and expression of discrete tissue-selective transcription factors such as MafB and c-Maf.17–21
Recent reports showed that lung macrophages in different compartments have proinflammatory and anti-inflammatory functions. Lung-resident macrophages are reported to have immune regulatory function because depletion of AMs by clodronate liposomes can cause Th2-type allergic immune responses in the mice sensitized by house dust mite (HDM)22 and adoptive transfer of AMs from naïve animal can completely abolish Th2 cell polarization and lung dendritic cell-mediated allergen capture and migration to the lymph,23 but the data are contradictory to the report by Lee et al24 showing that depletion of AMs in a mouse allergic asthma model attenuated Th2-type allergic lung inflammation and airway remodeling, accompanied by the enhanced Th1 immune responses. In addition, the number of circulating-derived monocytes increased in the inflamed lung and participated in lung allergic immune responses. Zaslona et al25 recently observed that depletion of circulating monocytes can attenuate allergic inflammation. Therefore, macrophages in different compartments exert distinct biological functions in the allergic responses. Further investigation should be performed to define the underlying molecular and immunological mechanisms.
Classically activated macrophages (M1 cells) in asthma
M1 cells play an important role in host defense against pathogen invasion via phagocytosis and release many proinflammatory cytokines and chemokines. This cell phenotype is characterized by expression of high levels of MHCII and CD86.5,26,27 It was reported that M1 cells were greatly increased in nonallergic lung inflammation after exposure to farm dust extract, in association with increased Th1 and Th17 cell population.28 The increased polarization of M1 cells has properties of antiallergic responses because patients with less severe asthma have more M1 cell population than those with severe asthma.29 Multiple factors affect polarization of M1 cells, from either naïve M0 or polarized M2 cells. In vitro studies showed that the polarized cells can be switched back to M0 state in a cytokine-deficient medium for 12 days or switched to another cell phenotype after culture in an alternative polarizing medium.30
The polarized M1 cells can efficiently activate Th1 cells by secreting IP-10, IFN-γ, IL-8, IL-23p40/p19, TNF-α, IL-1 β, and RANTES, but not IL-12 (p40/p35) after pathogen infection, including (myco)bacteria.30,31 Lipopolysaccharide, IFN-γ, and granulocyte-macrophage colony-stimulating factor are potent inducers for the polarization of M1 cells.32 Mice that lack IFN-γ have low M1 cells but have a large amount of M2 cells, with the decreased ratio of iNOS to arginase.33 Other mediators such as oxidized low-density lipoprotein, fatty acid, caveolin-1 (Cav-1), and high-mobility group box 1 (HMGB1) protein were also involved in M1 cell-biased polarization.34–37 van Tits et al34 reported that the oxidized low-density lipoprotein-loaded macrophages can enhance macrophage chemotactic protein expression via a downregulating Krüppel-like factor 2, a nuclear transcription factor.34 Cav-1, a membrane scaffolding protein, can promote the polarization of M1 cells. Shivshankar et al36 reported that Cav-1 null macrophages had a more pronounced M2 profile activation in response to IL-4 stimulation. HMGB1 protein is released from IMs and can significantly induce the expression of M1 marker iNOS, while decreasing M2 marker IL-10 in kidney injury and fibrosis animal model. However, it remains unknown whether there are similar effects in the asthma mouse model.
Alternatively activated macrophages (M2 cells) in asthma
M2 cells are potent macrophage subtypes and have multiple functions in different diseases and disease phases. The variable function is related to the distinct cytokine expression profile and activation status of the cells. It is reported that M1 cells are predominantly presented 1–3 days after the nitrogen mustard-induced lung injury, whereas M2 macrophages were significantly increased at 28 days.38 However, the dynamic changes of M2 cells in asthma is still not well identified in animal models and patients with asthma. In asthmatic animals, this cell phenotype is characterized by low expression of MHCII, CD86, and iNOS2 but high levels of arginase-1, family proteins chitinase-like Ym1/2 and Fizz1/RELM-α (found in inflammatory zone 1), and cell surface receptors such as macrophage mannose receptor, also called CD206. CD206 has an important function in the phagocytosis of M2 cells via increasing efferocytosis of invading pathogens and apoptotic cells.5,9,39–42 It is reported that CD206 facilitates the scavenging and degradation of ricin. CD206-deficient mice were more susceptible to toxin-induced death than wild-type mice due to compromised efferocytosis activity of M2 cells.43 Therefore, high levels of CD206 would be beneficial to phagocytosis and pathogen clearance of M2 cells; which may explain the underlying mechanisms of higher potency of M2 cells in the binding and more uptake of pathogens than the M1 cells.31 Among M2a and M2c subtypes, M2c cells have lower NF-κB activation and lower expression of antigen-presenting and costimulatory molecules (HLA-DR, CD86, and CD40)31 but greater expression of IL-10 than M2a cells in the renal injury animal model.44 Our previous results also revealed that lipoprotein-associated phospholipase A2 deficiency increased macrophage phagocytosis and IL-10 expression in M2c cells in Aspergillus fumigatus-sensitized mice.45,46 Similar results are also observed in the asthmatic mouse model with surfactant protein A deficiency, in which a high level of IL-13 was expressed in M2a cells.47 Thus, M2c cell subtype is considered a major subtype in the initiation of inflammation resolution. The upregulated CD163 (a member of hemoglobin scavenger receptor of cysteine-rich family) and CD206 on M2 cells might participate in the process. However, it was reported that lipopolysaccharide, IFN-γ, and TNF-α from M1 cells and other activated cells can suppress CD163 expression, whereas IL-6 and anti-inflammatory IL-10 can increase CD163 expression in monocytes and macrophages, indicating that the role of cytokine microenvironment affects polarization of M2 cells possibly through regulation of key cell scavenger receptors.48 Therefore, M2c cells can be a useful cell target in the treatment of lung inflammatory diseases such as asthma.
Different from M2c cells, M2a cells are characterized by expression of high levels of IL-13, a cytokine critically involved in allergic immune responses and mucus production.49 In addition, CCL-17, CCL-18, CCL-22, and eotaxin-2 (CCL-24) are highly expressed from M2 cells and facilitate Th2 and eosinophil infiltration into the inflamed lungs.12,30,50 However, recent findings reveal that these mediators and M2 cell-specific transcription factors are responsible for lung tissue remodeling and fibrosis. IL-13 can increase expression of MUC5AC and TGF-β 2 while decreasing beta IV Tubulin in human bronchial epithelial cells.51 Forced expression of recombinant Fizz1 in rat lung fibroblast cell line can enhance production of collagen type I and α-smooth muscle actin.52 Therefore, lung fibrosis can be controlled by modulation of M2 cell phenotype during the early stages of airway remodeling.
Polarization of M2 cells
Cytokines and other mediators
Owing to the distinct role of M1 and M2 cells in the pathogenesis of asthma, it has become important to maintain an optimal balance between the population of M1 and M2 cells. Modulation of polarization of M1 and M2 cells has therapeutic potential. It is documented that IL-13,30 IL-33,8 and M-CSF32 are potent inducers of M2 cell-biased polarization. IL-13 was greatly increased in M2 cell-dominant allergic mice, in association with upregulation of Fizz1/RELM-α and YM1.5,53,54 In IL-13 transgenic mice, a greater amount of M2 cells was also observed after Cryptococcus neoformans infection.55 In addition, IL-33 is involved in the polarization of M2 cells. Lung epithelial cells are a major source of IL-33 after the first allergen challenge, but after the third challenge, ∼20% and ∼10% respectively, of the IL-33-producing cells in the lungs were M2 macrophages and conventional dendritic cells.56 The increased M2 cell-biased polarization by IL-33 was possibly mediated by upregulation of IL-4, IL-5, IL-13, CCL-17, CCL-18, and CCL-24 after binding to the IL-33 receptor ST2.57,58 Moreover, there are elevated levels of serum IL-35, IL-17A, basophil activation marker basogranulin, and eosinophilic airway inflammation biomarker periostin in allergic asthmatic patients, but it is unclear whether or not they have direct effects on the polarization of M2 cells.59 Recently, Draijer et al reported that prostaglandin E2 (PGE2) can promote IL-10-expressing M2c cells in HDM-induced asthmatic mice. The effects were further confirmed by direct free PGE2 treatment or adoptive transfer of PGE2-treated macrophages, in which the treated mice had fewer infiltrating eosinophils in lungs.9 Therefore, it would be a promising strategy in asthma therapy to induce M2c-biased polarization through molecular intervention.
Transcription factors
Recent studies have indicated that transcription factors and intracellular proteins, such as tuberous sclerosis complex 1 (TSC1) ,60 stress-responsive activating transcription factor 7 (ATF 7) ,61 STIP1 homology and U-Box containing protein 1 (STUB1) ,62 ten eleven translocation (Tet) methylcytosine dioxygenase (Tet2),63 microRNA (MiR-511),64 docosahexaenoic acid, peroxisome proliferator-activated receptor gamma (PPARγ) ,42 and programmed cell death protein 4 (Pdcd4)40 can modulate polarization of M2 cells by influencing gene expression. NLRP3 (also known as Nacht, Lrp, and Pyd domain-containing protein 3, NALP3, or cryopyrin) is an intracellular protein and forms protein NALP3 inflammasome complexes with ASC and pro-caspase-1 that drive the activation of inflammatory caspases. Recent studies indicated that NLRP3 inflammasome has been implicated in the pathogenesis of several acquired inflammatory diseases including asthma. NLRP3 can promote IL-4 expression by Th2 cells via binding to IL-4 promoter in conjunction with the transcription factor IRF4.65,66 Although there is no report so far about the effects of NLRP3 on the polarization of M2 cells, we expect that NLRP3 may drive M2a cell-biased polarization through IL-4 upregulation. Downregulation of NLRP3 expression may attenuate allergic responses as well as suppress cell pyroptosis.67 In addition, jumonji domain containing-3 (Jmjd3; also known as Kdm6b) is a histone 3 Lys27 (H3K27) demethylase. Satoh et al previously reported that Jmjd3 is essential for polarization of M2 macrophages in response to helminth infection and chitin. The effects depend on demethylase activity of Jmjd3 and downstream Irf4, a key transcription factor. Overexpression or activation of Jmjd3 is beneficial to host defense against helminth infection and the alleviation of asthma.68 Therefore, Jmjd3-mediated H3K27 demethylation is crucial for regulating the development of M2 macrophages leading to anti-helminth host responses.
Immune-regulatory cells
In addition to Th2 cells, some immune-regulatory cells, such as CD4+CD25+ regulatory T (Treg) cells and stem cells, are able to drive M2 cell-biased polarization. For example, mice infused with syngeneic CD4+CD25+ Treg cells have more population of CD206+ peritoneal macrophages, with low levels of CD80 and MHCII.69 Macrophages cocultured with Treg cells have increased CD206, CD163, and CCL-18 as well as an enhanced phagocytic capacity. CD4+CD25+CD127(low)Foxp3+ Tregs produced IL-10, IL-4, and IL-13, partially responsible for the upregulation of CD163, CCL-18, and phagocytosis, respectively.70 Furthermore, it is well documented that bone marrow-derived mesenchymal stem cells (BM-MSCs) have immune-regulatory property. A recent study also indicated that BM-MSCs can induce M2 cell-biased polarization.71 Intravenous injection of BM-MSCs can normalize and stabilize lung function in the HDM-induced asthmatic mouse model. A further study indicated that the beneficial effects are associated with M2-biased polarization of resident macrophages after resident macrophages engulfed the injected MSC in vivo.72 Similarly, Yin et al also recently reported that polarization of M2 cells can be enhanced by adipose tissue-derived stromal cells. The macrophages have downregulated IL-6, TNF-α, iNOS, and CD86 but increased Arg1, CD206, Fizz1, Ym1/2, and IL-10 after coculture with adipose tissue-derived stromal cells.73 The effects might be mediated by the released immune-regulatory mediators, such as IL-10 and indoleamine 2,3-dioxygenase from stromal cells.74,75 Therefore, adoptive transfer MSCs have therapeutic potential in the treatment of inflammatory diseases, such as asthma, through increasing M2 cell-biased polarization.
Conclusion and therapeutic perspectives
Lung M1 and M2 cells are distinct cell subtypes and participate in the pathogenesis of asthma. M1 cells express high levels of proinflammatory cytokines, and M2 cells express high levels of Th2-type cytokines. Owing to their different cytokine expression profiles, M1 and M2 cells play different roles in the pathogenesis of asthma. A variety of regulatory cytokines, chemokines, mediators, and immune-regulatory cells affect polarization and chemotaxis of lung macrophages. These mediators interplay and influence disease duration and severity through the altered polarization of M1 and M2 cells. Therefore, modulation of phenotypes of lung macrophage has therapeutic potential in the treatment of asthma and other lung inflammatory diseases.
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China to LZ (81270137) and a research grant from Zhongshan Hospital, Fudan University, People’s Republic of China, to ZLJ (A654116001). The authors thank Kelly Yiting Jiang from Cornell University for her scientific editing assistance in the preparation of this manuscript.
Disclosure
The authors report no conflicts of interest in this work. None of the authors affiliated with this manuscript have any commercial associations that might pose a conflict of interest. The authors alone are responsible for the content and writing of the article.
References
Bedoret D, Wallemacq H, Marichal T, et al. Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice. J Clin Invest. 2009;119(12):3723–3738. | ||
Boorsma CE, Draijer C, Melgert BN. Macrophage heterogeneity in respiratory diseases. Mediators Inflamm. 2013;2013:769214. | ||
Ji WJ, Ma YQ, Zhou X, et al. Temporal and spatial characterization of mononuclear phagocytes in circulating, lung alveolar and interstitial compartments in a mouse model of bleomycin-induced pulmonary injury. J Immunol Methods. 2014;403(1–2):7–16. | ||
Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. | ||
Veremeyko T, Siddiqui S, Sotnikov I, Yung A, Ponomarev ED. IL-4/IL-13-dependent and independent expression of miR-124 and its contribution to M2 phenotype of monocytic cells in normal conditions and during allergic inflammation. PLoS One. 2013;8(12):e81774. | ||
Bai J, Adriani G, Dang TM, et al. Contact-dependent carcinoma aggregate dispersion by M2a macrophages via ICAM-1 and β2 integrin interactions. Oncotarget. 2015;6(28):25295–25307. | ||
Colin S, Chinetti-Gbaguidi G, Staels B. Macrophage phenotypes in atherosclerosis. Immunol Rev. 2014;262(1):153–166. | ||
Judd LM, Heine RG, Menheniott TR, et al. Elevated IL-33 expression is associated with pediatric eosinophilic esophagitis, and exogenous IL-33 promotes eosinophilic esophagitis development in mice. Am J Physiol Gastrointest Liver Physiol. 2016;310(1):G13–G25. | ||
Draijer C, Boorsma CE, Reker-Smit C, Post E, Poelstra K, Melgert BN. PGE2-treated macrophages inhibit development of allergic lung inflammation in mice. J Leukoc Biol. Epub 2016 Mar 1. | ||
Moreira AP, Hogaboam CM. Macrophages in allergic asthma: fine-tuning their pro- and anti-inflammatory actions for disease resolution. J Interferon Cytokine Res. 2011;31(6):485–491. | ||
Zdrenghea MT, Makrinioti H, Muresan A, Johnston SL, Stanciu LA. The role of macrophage IL-10/innate IFN interplay during virus-induced asthma. Rev Med Virol. 2015;25(1):33–49. | ||
Staples KJ, Hinks TS, Ward JA, Gunn V, Smith C, Djukanovic R. Phenotypic characterization of lung macrophages in asthmatic patients: overexpression of CCl17. J Allergy Clin Immunol. 2012;130(1404–1412):e1407. | ||
Chung Y, Hong JY, Lei J, Chen Q, Bentley JK, Hershenson MB. Rhinovirus infection induces interleukin-13 production from CD11b-positive, M2-polarized exudative macrophages. Am J Respir Cell Mol Biol. 2015;52(2):205–216. | ||
Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16(1):45–56. | ||
Perdiguero EG, Geissmann F. The development and maintenance of resident macrophages. Nat Immunol. 2015;17(1):2–8. | ||
Suzuki T, Arumugam P, Sakagami T, et al. Pulmonary macrophage transplantation therapy. Nature. 2014;514:450–454. | ||
Amit I, Winter DR, Jung S. The role of the local environment and epigenetics in shaping macrophage identity and their effect on tissue homeostasis. Nat Immunol. 2015;17(1):18–25. | ||
Becker M, De Bastiani MA, Parisi MM, et al. Integrated transcriptomics establish macrophage polarization signatures and have potential applications for clinical health and disease. Sci Rep. 2015;5:13351. | ||
Glass CK, Natoli G. Molecular control of activation and priming in macrophages. Nat Immunol. 2015;17(1):26–33. | ||
Rosas M, Davies LC, Giles PJ, et al. The transcription factor Gata6 links tissue macrophage phenotype and proliferative renewal. Science. 2014;344(6184):645–648. | ||
Soucie EL, Weng Z, Geirsdottir L, et al. Lineage-specific enhancers activate self-renewal genes in macrophages and embryonic stem cells. Science. 2016;351(6274):aad5510. | ||
Mathie SA, Dixon KL, Walker SA, et al. Alveolar macrophages are sentinels of murine pulmonary homeostasis following inhaled antigen challenge. Allergy. 2015;70(1):80–89. | ||
Lauzon-Joset JF, Marsolais D, Langlois A, Bissonnette EY. Dysregulation of alveolar macrophages unleashes dendritic cell-mediated mechanisms of allergic airway inflammation. Mucosal Immunol. 2014;7(1):155–164. | ||
Lee YG, Jeong JJ, Nyenhuis S, et al. Recruited alveolar macrophages, in response to airway epithelial-derived monocyte chemoattractant protein 1/CCl2, regulate airway inflammation and remodeling in allergic asthma. Am J Respir Cell Mol Biol. 2015;52(6):772–784. | ||
Zaslona Z, Przybranowski S, Wilke C, et al. Resident alveolar macrophages suppress, whereas recruited monocytes promote, allergic lung inflammation in murine models of asthma. J Immunol. 2014;193(8):4245–4253. | ||
Winkler C, Witte L, Moraw N, et al. Impact of endobronchial allergen provocation on macrophage phenotype in asthmatics. BMC Immunol. 2014;15:12. | ||
Rupil LL, de Bem AF, Roth GA. Diphenyl diselenide-modulation of macrophage activation: down-regulation of classical and alternative activation markers. Innate Immun. 2012;18(4):627–637. | ||
Robbe P, Draijer C, Borg TR, et al. Distinct macrophage phenotypes in allergic and nonallergic lung inflammation. Am J Physiol Lung Cell Mol Physiol. 2015;308(4):L358–L367. | ||
Draijer C, Robbe P, Boorsma CE, Hylkema MN, Melgert BN. Characterization of macrophage phenotypes in three murine models of house-dust-mite-induced asthma. Mediators Inflamm. 2013;2013:632049. | ||
Tarique AA, Logan J, Thomas E, Holt PG, Sly PD, Fantino E. Phenotypic, functional, and plasticity features of classical and alternatively activated human macrophages. Am J Respir Cell Mol Biol. 2015;53(5): | ||
Verreck FA, de Boer T, Langenberg DM, et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci U S A. 2004;101(13):4560–4565. | ||
Sierra-Filardi E, Vega MA, Sanchez-Mateos P, Corbi AL, Puig-Kroger A. Heme oxygenase-1 expression in M-CSF-polarized M2 macrophages contributes to LPS-induced IL-10 release. Immunobiology. 2010;215(9-10):788–795. | ||
Arora S, Hernandez Y, Erb-Downward JR, McDonald RA, Toews GB, Huffnagle GB. Role of IFN-γ in regulating T2 immunity and the development of alternatively activated macrophages during allergic bronchopulmonary mycosis. J Immunol. 2005;174(10):6346–6356. | ||
van Tits LJ, Stienstra R, van Lent PL, Netea MG, Joosten LA, Stalenhoef AF. Oxidized LDL enhances pro-inflammatory responses of alternatively activated M2 macrophages: a crucial role for Kruppel-like factor 2. Atherosclerosis. 2011;214(2):345–349. | ||
Tian S, Zhang L, Tang J, Guo X, Dong K, Chen SY. HMGB1 exacerbates renal tubulointerstitial fibrosis through facilitating M1 macrophage phenotype at the early stage of obstructive injury. Am J Physiol Renal Physiol. 2015;308(1):F69–F75. | ||
Shivshankar P, Halade GV, Calhoun C, et al. Caveolin-1 deletion exacerbates cardiac interstitial fibrosis by promoting M2 macrophage activation in mice after myocardial infarction. J Mol Cell Cardiol. 2014;76:84–93. | ||
Nomura M, Liu J, Rovira II, et al. Fatty acid oxidation in macrophage polarization. Nat Immunol. 2016;17(3):216–217. | ||
Venosa A, Malaviya R, Choi H, Gow AJ, Laskin JD, Laskin DL. Characterization of distinct macrophage subpopulations during nitrogen mustard-induced lung injury and fibrosis. Am J Respir Cell Mol Biol. 2016;54(3):436–446. | ||
Hong JY, Chung Y, Steenrod J, et al. Macrophage activation state determines the response to rhinovirus infection in a mouse model of allergic asthma. Respir Res. 2014;15:63. | ||
Zhong B, Yang X, Sun Q, et al. Pdcd4 modulates markers of macrophage alternative activation and airway remodeling in antigen-induced pulmonary inflammation. J Leukoc Biol. 2014;96(6):1065–1075. | ||
Bhatia S, Fei M, Yarlagadda M, et al. Rapid host defense against Aspergillus fumigatus involves alveolar macrophages with a predominance of alternatively activated phenotype. PLoS One. 2011;6(1):e15943. | ||
Chang HY, Lee HN, Kim W, Surh YJ. Docosahexaenoic acid induces M2 macrophage polarization through peroxisome proliferator-activated receptor gamma activation. Life Sci. 2015;120:39–47. | ||
Gage E, Hernandez MO, O’Hara JM, McCarthy EA, Mantis NJ. Role of the mannose receptor (CD206) in innate immunity to ricin toxin. Toxins. 2011;3(9):1131–1145. | ||
Lu J, Cao Q, Zheng D, et al. Discrete functions of M2a and M2c macrophage subsets determine their relative efficacy in treating chronic kidney disease. Kidney Int. 2013;84(4):745–755. | ||
Jiang Z, Ravaioli G, Fehrenbach ML, et al. Lipoprotein associated phospholipase A2 (LP-PLA2)/platelet activating factor acetyl hydrolase (PAF-AH) deficiency is associated with increased numbers of M2 macrophages in the lung during the allergic airway response in mice. Am J Respir Crit Care Med. 2012;185:A4302. | ||
Jiang Z, Kokalari B, Redai IG, Hanman N, Macphee CH, Haczku A. Lack of lipoprotein associated phospholipase A2 (Lp-PLA2) enhanced phagocytosis and IL-10 expression and decreased NF-kB activation in CD206+ M2 macrophages in gene deficient mice. Am J Respir Crit Care Med. 2014;189:A2485. | ||
Jiang Z, Kokalari B, Redai IG, et al. Lack of surfactant protein A (SP-A) enhances airway inflammation and hyperresponsiveness after ozone (O3) or Aspergillus fumigatus (Af) exposure in association with increased presence of IL-13+/CD206+ alternatively activated (M2) macrophages. Am J Respir Crit Care Med. 2013;187:A3554. | ||
Buechler C, Ritter M, Orso E, Langmann T, Klucken J, Schmitz G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol. 2000;67(1):97–103. | ||
Byers DE, Holtzman MJ. Alternatively activated macrophages and airway disease. Chest. 2011;140(3):768–774. | ||
Siddiqui S, Secor ER Jr, Silbart LK. Broncho-alveolar macrophages express chemokines associated with leukocyte migration in a mouse model of asthma. Cell Immunol. 2013;281(2):159–169. | ||
Malavia NK, Mih JD, Raub CB, Dinh BT, George SC. IL-13 induces a bronchial epithelial phenotype that is profibrotic. Respir Res. 2008;9:27. | ||
Dong L, Wang SJ, Camoretti-Mercado B, Li HJ, Chen M, Bi WX. FIZZ1 plays a crucial role in early stage airway remodeling of OVA-induced asthma. J Asthma. 2008;45(8):648–653. | ||
Nair MG, Cochrane DW, Allen JE. Macrophages in chronic type 2 inflammation have a novel phenotype characterized by the abundant expression of Ym1 and Fizz1 that can be partly replicated in vitro. Immunol Lett. 2003;85(2):173–180. | ||
Blease K, Mehrad B, Standiford TJ, et al. Enhanced pulmonary allergic responses to Aspergillus in CCR2-/- mice. J Immunol. 2000;165(5):2603–2611. | ||
Muller U, Stenzel W, Kohler G, et al. IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans. J Immunol. 2007;179(8):5367–5377. | ||
Nabe T, Wakamori H, Yano C, et al. Production of interleukin (IL)-33 in the lungs during multiple antigen challenge-induced airway inflammation in mice, and its modulation by a glucocorticoid. Eur J Pharmacol. 2015;757:34–41. | ||
Joshi AD, Oak SR, Hartigan AJ, et al. Interleukin-33 contributes to both M1 and M2 chemokine marker expression in human macrophages. BMC Immunol. 2010;11:52. | ||
Kurowska-Stolarska M, Stolarski B, Kewin P, et al. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol. 2009;183(10):6469–6477. | ||
Wong CK, Leung TF, Chu IM, Dong J, Lam YY, Lam CW. Aberrant expression of regulatory cytokine IL-35 and pattern recognition receptor NOD2 in patients with allergic asthma. Inflammation. 2015;38(1):348–360. | ||
Zhu L, Yang T, Li L, et al. TSC1 controls macrophage polarization to prevent inflammatory disease. Nat Commun. 2014;5:4696. | ||
Yoshida K, Maekawa T, Zhu Y, et al. The transcription factor ATF7 mediates lipopolysaccharide-induced epigenetic changes in macrophages involved in innate immunological memory. Nat Immunol. 2015;16(10):1034–1043. | ||
Wei Q, Sha Y, Bhattacharya A, et al. Regulation of IL-4 receptor signaling by STUB1 in lung inflammation. Am J Respir Crit Care Med. 2014;189(1):16–29. | ||
Zhang Q, Zhao K, Shen Q, et al. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature. 2015;525(7569):389–393. | ||
Karo-Atar D, Itan M, Pasmanik-Chor M, Munitz A. MicroRNA profiling reveals opposing expression patterns for miR-511 in alternatively and classically activated macrophages. J Asthma. 2015;52(6):545–553. | ||
Baroja-Mazo A, Martin-Sanchez F, Gomez AI, et al. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat Immunol. 2014;15(8):738–748. | ||
Bruchard M, Rebe C, Derangere V, et al. The receptor NLRP3 is a transcriptional regulator of TH2 differentiation. Nat Immunol. 2015;16(8):859–870. | ||
Vande Walle L, Van Opdenbosch N, Jacques P, et al. Negative regulation of the NLRP3 inflammasome by A20 protects against arthritis. Nature. 2014;512(7512):69–73. | ||
Satoh T, Takeuchi O, Vandenbon A, et al. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol. 2010;11(10):936–944. | ||
Liu G, Ma H, Qiu L, et al. Phenotypic and functional switch of macrophages induced by regulatory CD4+CD25+ T cells in mice. Immunol Cell Biol. 2011;89(1):130–142. | ||
Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A. 2007;104(49):19446–19451. | ||
Song X, Xie S, Lu K, Wang C. Mesenchymal stem cells alleviate experimental asthma by inducing polarization of alveolar macrophages. Inflammation. 2015;38(2):485–492. | ||
Braza F, Dirou S, Forest V, et al. Mesenchymal stem cells induce suppressive macrophages through phagocytosis in a mouse model of asthma. Stem Cells. Epub 2016 Feb 17. | ||
Yin X, Pang C, Bai L, Zhang Y, Geng L. [Adipose-derived stem cells promote the polarization from M1 macrophages to M2 macrophages]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi/Chin J Cell Mol Immunol. 2016;32(3):332–338. Chinese. | ||
Abomaray FM, Al Jumah MA, Kalionis B, et al. Human chorionic villous mesenchymal stem cells modify the functions of human dendritic cells, and induce an anti-inflammatory phenotype in CD1+ dendritic cells. Stem Cell Rev. 2015;11(3):423–441. | ||
Francois M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20(1):187–195. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.