Back to Journals » Journal of Experimental Pharmacology » Volume 12
Up-and-Coming Experimental Drug Options for Metastatic Colorectal Cancer
Received 1 October 2020
Accepted for publication 27 October 2020
Published 11 November 2020 Volume 2020:12 Pages 475—485
DOI https://doi.org/10.2147/JEP.S259287
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bal Lokeshwar
Sarah K Cimino,1 Cathy Eng2
1Department of Pharmacy, Vanderbilt University Medical Center, Nashville, Tennessee, USA; 2Department of Medicine: Hematology/Oncology, Vanderbilt University Medical Center, Nashville, Tennessee, USA
Correspondence: Sarah K Cimino
Department of Pharmacy, Vanderbilt University Medical Center, 1301 Medical Center Drive, Nashville, TN 37232, USA
Tel +1 615 875 6967
Fax +1 615 343 8668
Email [email protected]
Abstract: Colorectal cancer is one of the top causes of cancer and cancer-related deaths worldwide. The prognosis of metastatic colorectal cancer is poor and treatment options are limited. Many patients will run out of treatment options before they become medically unfit for therapy. As such, there is a need to expand upon the current understanding of disease biology as well as drug resistance mechanisms in order to create new approaches for therapy. In this review article, we will discuss the mechanistic rationale and clinical data for new drugs and therapeutic combinations under development for metastatic colorectal cancer.
Keywords: colon cancer, rectal cancer, drug resistance, novel combinations
Introduction
Colorectal cancer is the fourth leading cause of cancer and the second leading cause of cancer death in the United States.1 New cases and death from colorectal cancer have decreased over the past 20 years.1 However, prognosis is heavily correlated to the stage of disease. Patients presenting with Stage I disease have 5-year survival rates >90%, whereas patients with metastatic disease have a 5-year survival rate of <15%.1 Approximately 20% of patients present with metastatic disease and at least 20% of patients with localized disease will go on to develop metastatic disease, highlighting the need to improve survival in the metastatic population.1,2 The drug armamentarium for metastatic colorectal cancer is limited, and many patients run out of treatment options before they become medically unfit for therapy. Thus, a more sophisticated understanding of disease physiology and drug resistance mechanisms is needed to guide development of more effective therapies. Recently, research in this area has become more promising; however realistic optimism is warranted because with new drugs come more complex treatment paradigms, potential new toxicities, and economic considerations. This article will focus on some of the promising new targeted drug therapies and drug combinations for metastatic colorectal cancer.
The Vascular Endothelial Growth Factor (VEGF) Pathway
The development of VEGF pathway inhibitors introduced targeted drug therapy to the treatment paradigm for metastatic colorectal cancer (mCRC). The VEGF family consists of ligands VEGF-A, -B, -C, and -D and placental growth factor, which bind to tyrosine kinase receptors VEGFR-1, VEGFR-2, and VEGFR-3 on vascular endothelial cells.3,4 This pathway is responsible for tumor angiogenesis. Increased levels of VEGF, as seen in mCRC, increase vascular permeability and create leaky blood vessels which allow tumors to recruit new blood supplies and also enhance the ability for tumor migration and metastases.5,6 VEGFR-2 is thought to be the primary mediator for angiogenesis in mCRC.3,4 Inhibiting the VEGF pathway in mCRC reduces the formation of new blood supplies to tumors, and also normalizes blood vessel architecture to facilitate the delivery of chemotherapy.4 All patients with mCRC are potential candidates for VEGF inhibition. Currently US Food and Drug Administration (FDA)-approved VEGF pathway inhibitors and their specific targets include bevacizumab (VEGF), ramucirumab (VEGFR-2), ziv-aflibercept (VEGF), and regorafenib (VEGFR-1,2,3). Each of these agents has been shown to improve survival.7–10 In clinical practice, bevacizumab is the preferred parenteral agent because ramucirumab and ziv-aflibercept are more expensive and lack additional clinical benefit beyond what bevacizumab may offer. However, patients may ultimately develop resistance to bevacizumab rendering the agent ineffective in some instances.11,12 Regorafenib is beneficial in that is the only FDA-approved orally bioavailable VEGF pathway inhibitor, however its use is limited due to off-target side effects. A number of new orally bioavailable molecules, including rivoceranib, fruquintinib, and donafenib are under development to address some of these challenges (NCT04073615, NCT04067986, NCT03397199, NCT04322539, NCT03977090, NCT04179084, and NCT02870582).
Rivoceranib (Apatinib, YN968D1)
Rivoceranib is approved in China for gastric cancer. It is a small-molecule receptor tyrosine kinase inhibitor that selectively inhibits VEGFR-2, and to a lesser degree, it also inhibits the RET, c-kit, and c-Src tyrosine kinases.3,4 It has been hypothesized that intracellular targeting of VEGFR-2 may overcome resistance to bevacizumab, which solely targets the ligand.13 Rivoceranib was studied in a Phase I trial of 46 patients with advanced solid malignancies naïve to VEGF inhibition.3 Of the 36 evaluable patients for response, 7 (18.9%) had a partial response (PR), including 3 patients with colon cancer.3 The largest prospective study to date of rivoceranib in patients with mCRC was conducted by Wang and colleagues.13 In this study, 48 patients with mCRC who had failed standard chemotherapies received rivoceranib 500 mg daily.13 Four (8.3%) patients had a PR and 29 (60.4%) patients had stable disease (SD), respectively.13 Median progression-free survival (PFS) was 4.8 months and overall survival (OS) was 9.1 months.13 Notably, receipt of prior anti-angiogenic therapy had no impact on PFS and OS, supporting the theory that rivoceranib may be effective when resistance has developed to other anti-angiogenic therapies.13 Additionally, rivoceranib exceeded the historical median PFS (1.7 months) and OS (6.3 months) for best supportive therapy in the third-line and beyond for mCRC.13 These results are corroborated by additional smaller studies in mCRC including Guo and colleagues, (PFS 3.8 months, OS not reached), Liang and colleagues, (PFS 4.8 months, OS 10.1 months), and Li and colleagues, (PFS 3.7 months, OS 7.3 months).13–16 Adverse effects from rivoceranib were consistent with inhibitors of the VEGF pathway and included hypertension, proteinuria, and hand-foot syndrome.3,13 A multicenter phase I/II trial is underway in the United States further evaluating the rivoceranib in mCRC (NCT04073615).
Fruquintinib (HMPL-013)
Another agent under development and currently approved in China for mCRC is fruquitinib. Unlike rivoceranib, fruquintinib is a small-molecule inhibitor of VEGFR-1, −2, and −3.17 Fruquintinib was approved in China based on the results of the Phase III FRESCO trial. In this trial, 416 Chinese patients with mCRC who had received at least two prior lines of treatment were randomized to receive fruquintinib 5 mg daily for 3 weeks on and 1 week off or placebo.18 The primary endpoint was OS.18 The median OS was 9.3 months in the fruquitinib group and 6.6 months in the placebo group (P<0.001).18 There was also significant improvement in median PFS with fruquitinib, 3.7 vs 1.8 months (P<0.001).18 Similar to rivoceranib, this study also found that OS was similar among patients who had previously received VEGF inhibitors compared to those who had not.18 There were no new safety signals in this study with the expected adverse effects of hypertension, proteinuria and hand-foot syndrome.18 While this study made great strides for the Chinese population, the results are not generalizable to other parts of the world where VEGF inhibitors are typically incorporated earlier in treatment compared to practice in China. Notably, the FRESCO-2 trial is underway which is a global phase III trial in patients with mCRC who have exhausted all standard treatment options comparing fruquintinib to best supportive care (NCT04322539). In June 2020, the FDA granted Fast Track Designation for the development of fruquintinib, based on the currently available positive data and pending the additional results from FRESCO-2.
Donafenib (CM4307)
The final novel VEGF pathway inhibitor, also being studied in China, is donafenib. Donafenib is a small molecule inhibitor of multiple tyrosine kinases including VEGFR, PDGFR, and Raf.19 It is an analog of sorafenib, developed by substituting a trideuteriomethyl group for a methyl group, which enhances the pharmacokinetic profile.19 The first-in-human phase I trial included 25 patients, 8 of whom had colorectal cancer.19 One colorectal cancer patient experienced a PR and one experienced SD.19 Adverse effects included hand-foot syndrome, rash, and diarrhea.19 The investigation of donafenib in colorectal cancer was expanded via a phase III trial in China which randomized patients with mCRC with no further treatment options to donafenib 300 mg twice daily on Days 1–21 of a 28-day cycle versus placebo (NCT02870582). This trial has been completed, but results are pending.
The Epidermal Growth Factor Receptor (EGFR) Pathway
Inhibitors of the EGFR pathway were the second class of targeted drugs introduced into the mCRC treatment paradigm. The EGFR family consists of four receptor tyrosine kinases, EGFR, HER2, HER3 and HER4 (Figure 1).20 EGFR can be activated by a number of ligands, including epidermal growth factor (EGF), transforming growth factor-α, betacellulin, and heparin binding EGF-like growth factor.20 Once activated, there are a number of downstream signaling cascades including the MAPK (RAS/RAF/MEK/ERK), mTOR (PI3K/AKT/mTOR), JAK/STAT3, and Wnt (APC/β-catenin).20,21 These downstream signaling cascades ultimately stimulate tumor cell growth and survival.20 The initial pharmacologic target on the EGFR pathway for mCRC was the EGFR receptor, with the advent of the EGFR inhibitors cetuximab and panitumumab. Although these anti-EGFR antibodies have been shown to improve survival, not all patients with mCRC are candidates for anti-EGFR antibodies. Approximately 40% of patients with mCRC have KRAS mutations, which confer primary resistance to cetuximab and panitumumab by constitutively activating RAS downstream of EGFR.22,23 As such, use of panitumumab or cetuximab is not recommended in patients with KRAS mutations.24,25 Other potential mechanisms for primary resistance to anti-EGFR antibodies include alterations in EGFR and EGFR ligands, NRAS mutations, BRAF mutations, and PIK3CA mutations, all of which propagate signaling despite EGFR blockade.26,27 In addition to primary resistance, many patients will ultimately develop acquired resistance to anti-EGFR antibodies. Acquired resistance may occur due to secondary mutations in the signaling pathway or activation of parallel signaling pathways. The MAPK (RAS/RAF/MEK/ERK) pathway is the most researched anti-EGFR antibody escape pathway. Up to 50% of patients treated with anti-EGFR antibodies will develop acquired resistance due to secondary KRAS mutations.26,27 Secondary NRAS and BRAF mutations have also been implicated in acquired resistance.26,27 Tumors may also take advantage of parallel signaling pathways to survive. These pathways include the type 1 insulin-like growth factor receptor (IGF-1R), mesenchymal-epithelial transition factor receptor (MET receptor), and the human epidermal growth factor receptor-2 (HER2).26,27 Upon activation by their respective ligands, these pathways are able to signal cell effectors downstream of EGFR to stimulation cell proliferation despite the fact that EGFR is not activated.26,27 Current research is focused on quelling these resistance mechanisms in order to restore sensitivity to EGFR inhibition.
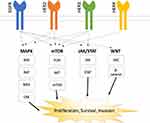 |
Figure 1 EGFR Pathways. |
Sotorasib (AMG 510)
As mentioned previously, RAS mutations cause sustained proliferative signaling regardless of ligand binding to EGFR, which confers primary resistance to currently available anti-EGFR therapies.28 Unfortunately, multiple attempts to inhibit RAS pharmaceutically have failed.29 Luckily, new promise is emerging for patients with the Kirsten RAS (KRAS) p.G12C mutation, comprising 1–4% of colorectal cancers.30,31 Sotorasib is a novel small molecule that irreversibly inhibits KRAS p.G12C, locking it in the inactive guanosine diphosphate-bound state.31,32 In the first-in-human phase I CodeBreaK100 trial, sotorasib was studied in 129 patients with the KRAS p.G12C mutation, including 42 patients with advanced colorectal cancer.31,32 In the colorectal cancer cohort, the overall response (ORR) and disease control (DCR) rates were 7.1% and 73.8%, and the median duration of stable disease was 4 months.31 Adverse effects included diarrhea, fatigue, and nausea.31,32 Overall, the results from this study was disappointing for the colorectal cancer cohort. One of the explanations for these results is that KRAS pG12C-mutant colorectal cancer cells may still become activated upstream by EGFR despite RAS inhibition.31,33 Future trials combining sotorasib with an EGFR inhibitor may be warranted to adequately treat patients with RAS mutations.33
Encorafenib (LGX818)
The RAF protein lies downstream of RAS in the MAPK signaling pathway, and mutations in RAF also confer primary resistance to currently available anti-EGFR therapies. Mutations in the BRAF isoform are present in 5–10% of colorectal cancers.22 The majority of BRAF mutations are caused by a substitution of valine with glutamic acid at codon 600 (BRAF V600E).22,34 Patients with the BRAF V600E mutation generally respond poorly to standard therapies and have a worse overall prognosis.34 BRAF inhibition alone in colorectal cancer is ineffective.35 Resistance to BRAF inhibition develops upstream via activation of EGFR and downstream via activations in MEK and ERK.35 Recently, the phase III BEACON CRC trial showed improved overall survival with both the doublet of cetuximab and encorafenib (a small molecule inhibitor of BRAF V600E, wild-type BRAF, and CRAF), and the triplet combination of cetuximab, encorafenib, and binimetinib (a small molecule inhibitor of MEK1/2) compared to cetuximab plus chemotherapy in the second-line and later setting.35,36 Although the doublet and triplet arms were not directly compared in this trial, clinical endpoints were very similar between these two arms. The ORR as 26% with triplet therapy and 20% with doublet therapy.35 The median PFS was 4.3 months with triplet therapy and 4.2 months with doublet therapy and the median OS was 9.0 months with triplet therapy and 8.4 months with doublet therapy.35 The rate of adverse events were also similar between doublet and triplet therapy.35 Adverse effects included diarrhea, nausea, vomiting, acneiform rash, and fatigue.35 This trial led to FDA-approval of the doublet, and marked the first available BRAF-targeted therapy for mCRC. However, the short PFS of approximately 4 months highlights that further research is needed to optimize the treatment of these patients. The Phase II ANCHOR-CRC trial is underway examining cetuximab, encorafenib, and binimetinib in the first-line setting for mCRC to see if these agents given earlier in treatment will help improve outcomes (NCT03693170).
Ulixertinib (BVD-523)
One proposed escape mechanism to explain the modest PFS in the BEACON CRC trial may be the reactivation of MEK or ERK signaling. To date, MEK inhibitors alone have been unsuccessful in the treatment of colorectal cancer.28,37,38 ERK, however, the terminal kinase of the MAPK pathway, presents a new target for inhibition. Inhibition of ERK may be effective in patients with primary resistance to EGFR inhibitors via constitutively active RAS or RAF mutations and may also halt upstream escape routes.39 Currently, there are no approved drugs that inhibit ERK. Ulixertinib is a reversible, small molecule ERK1/2 inhibitor under investigation.39 It was studied in a phase I trial of 162 patients with MAPK mutant advanced solid tumors.39 Twenty-six (19%) patients had colorectal cancer, and 17 (13%) of those patients had a BRAF mutation.39 In the 101 patients who were evaluable for response, no patients had a CR and 14 patients had a PR; responses in colorectal cancer patients were not specifically reported.39 Patients with responses had NRAS, BRAFV600E, and non-V600E BRAF mutant cancers. Adverse effects included rash, diarrhea, nausea, and fatigue. A phase II trial is underway with pre-specified cohorts for BRAF- or MEK1/2-mutated colorectal cancer (NCT04488003).
Besides the MAPK (RAS/RAF/MEK/ERK) pathway, parallel pathways such as mTOR (PI3K/AKT/mTOR), JAK/STAT3, and Wnt (APC/β-catenin) have also been implicated in anti-EGFR resistance. Investigations are underway to identify the best way to inhibit EGFR signaling in mCRC patients (NCT03355066, NCT04303403, NCT01351103)
The Human Epidermal Growth Factor Receptor 2 (HER2) Pathway
A second member of the EGFR signaling kinase receptors is HER2. Approximately 5% of patients with colorectal cancer have HER2 alterations, including mutations and amplifications.40 While there is no clear prognostic role associated with HER2 amplification; it may be predictive of resistance to anti-EGFR monoclonal antibodies.40,41 HER2 has no known ligands and instead relies upon dimerization with either EGFR, HER3, or HER4 to activate downstream signaling.20,40 Similar to EGFR, HER2 activates similar downstream signaling cascades that ultimately promote cell proliferation and survival.40,41 Interestingly, HER2 alterations tend to be mutually exclusive with mutations in the MAPK (RAS/RAF/MEK/ERK) and mTOR (PI3K/AKT/mTOR) signaling pathways.40 Multiple phase II trials have shown favorable results for anti-HER2 therapies in mCRC, including trastuzumab, pertuzumab, lapatinib, and trastuzumab-emtansine.42–44 However, there are no currently FDA-approved therapies for HER2 positive colorectal cancer.
Tucatinib (ONT-380)
Tucatinib is an orally-administered small-molecule that inhibits phosphorylation of both HER2 and HER3, resulting in downstream inhibition of MAPK and mTOR signaling pathways.45 Tucatinib is an analog of lapatinib, but distinguishes itself from lapatinib in that it is highly specific for HER2, whereas lapatinib inhibits both HER2 and EGFR.46 Tucatinib is FDA-approved for metastatic HER2 positive breast cancer, although research is underway to evaluate tucatinib in HER2 positive colorectal cancer. In a phase I study of 50 patients with advanced HER2 positive cancers, 6 patients (12%) had colorectal cancer.47 Of the 35 patients evaluable for response, 3 (9%) of patients had a PR and 20 (57%) of patients had SD.47 The MOUNTAINEER trial was a phase II trial that included 26 patients with HER2 positive mCRC who had not received prior anti-HER2 therapy. In this trial, tucatinib 300 mg daily was combined with trastuzumab (a humanized anti-HER2 monoclonal antibody) 8 mg/kg on day 1 of cycle 1, then 6 mg/kg every 3 weeks thereafter.41 Trastuzumab synergizes with tucatinib by binding HER2 extracellularly.48 Twenty-two patients were evaluable, and 12 (55%) of patients had a PR or CR.49 The most common treatment-related adverse events were AST and/or ALT elevation and diarrhea.49 There is a phase I study underway examining the impact of tucatinib plus trastuzumab and oxaliplatin-based chemotherapy for HER2 positive Gastrointestinal Cancers (NCT04430738).
Trastuzumab Deruxtecan (T-DXd, DS-8201)
Another drug indicated for metastatic breast cancer that is being explored for colorectal cancer is trastuzumab deruxtecan. This is an antibody-drug conjugate composed of trastuzumab, a cleavable linker, and DXd, a topoisomerase I inhibitor payload.50 The phase II DESTINY-CRC01 trial included patients with RAS-wild type, HER2-expressing mCRC, who had received at least two prior lines of therapy.50 Patients received trastuzumab deruxtecan 6.4 mg/kg every 3 weeks in 3 cohorts: (A: HER2 IHC 3+ or IHC 2+/ISH+; B: IHC 2+/ISH−; C: IHC 1+).50 Seventy-four patients were enrolled, 53 in cohort A, 7 in cohort B, and 18 in cohort C.50 The overall response rate was 45.3% (24/53 patients) in cohort A, including 1 CR.50 The median PFS was 6.9 months.50 Notably, 16 patients had received prior anti-HER2 therapy, and of those, 7 (43.8%) had a PR or CR. This shows that trastuzumab deruxtecan can overcome trastuzumab resistance, likely due to less reliance on the homogeneity of HER2 positivity and the ability to kill neighboring HER2 negative cells (known as the “bystander effect”).50,51 No responses were observed in cohorts B or C.50 Adverse effects included neutropenia, anemia, and interstitial lung disease.50
Overall, there are many challenges but steady progress is being made to refine our understanding of the best therapeutic application of medications to inhibit the VEGF, EGFR, and HER2 pathways to improve outcomes in mCRC patients.
New Targets
Berzosertib (M6620, VX-970, VE-822)
Berzosertib is a first-in-class ataxia–telangiectasia and Rad3 related (ATR) inhibitor.52 ATR, along with ataxia-telangiectasia mutated (ATM), are members of the phosphatidylinositol-3 kinase-related kinase (PIKK) family of protein kinases, which regulate the DNA damage response (DDR).53 ATR is recruited to damaged single-stranded DNA, whereas ATM is recruited to damaged double-stranded DNA.53 Upon recruitment, ATR and ATM alert the cell of the DNA damage and subsequently promote homologous recombination repair.53 The DDR pathway is integral to maintain a healthy genome and defects in the DDR pathway can promote oncogenesis.53 One of these defects may include loss of ATM.53 Loss of ATM places higher reliance on ATR for DNA repair and cell survival.52 This led to the hypothesis that ATR inhibition in ATM-deplete tumors may sensitize those tumors to conventional chemotherapy.52 A phase I trial included 40 patients with advanced solid tumors and examined berzosertib monotherapy (17 patients) and berzosertib in combination with carboplatin (23 patients).52 All 17 patients were evaluable in the monotherapy arm.52 One patient with mCRC and ATM loss achieved CR with berzosertib monotherapy and maintained this response for 29 months.52 Five patients on monotherapy achieved SD.52 Twenty-one patients were evaluable who received combination therapy.52 One patient in the combination therapy group had a PR lasting 6 months and notably, this patient was previously platinum-resistant.52 Fifteen patients in the combination therapy group had SD.52 Approximately 7% of patients with colorectal cancer have ATM mutations, so this represents an exciting new target and also introduces a novel means to overcome platinum resistance.54
Onvansertib (NMS-P937, NMS-1,286,937, PCM-075)
Onvansertib is a novel orally bioavailable polo-like kinase 1 (PLK1) inhibitor.55 Polo-like kinases (PLK) are responsible for regulating mitosis within the cell cycle.55,56 There are five PLK isoforms, PLK-1, −2, −3, −4, and −5.57 PLK1 is uniquely integral to carrying out proper mitotic functions such as: mitotic entry, spindle formation, cytokinesis, and mitotic exit.55,57 Deregulated PLK1 leads to mitotic errors, genetic instability, and tumorigenesis.56,57 Overexpression of PLK1 has been described in multiple tumor types, including colon cancer, and is associated with poor prognosis.55,56 PLK1 inhibition induces a G2/M cell cycle block and subsequent cell death.56 The first-in-human phase I study evaluating onvansertib included 19 adults with advanced or metastatic tumors, 4 of whom had colorectal cancer.56 Of the 16 patients evaluable for efficacy, 5 (31.2%) patients achieved SD, including 2 patients with colorectal cancer and no patients achieved a response.56 Interestingly, 3 of the 5 patients with SD had KRAS-mutations.56 Adverse effects included myelosuppression, nausea, hypokalemia, hypocalcemia, and hypophosphatemia.56 A phase Ib/II trial is currently underway to examine onvansertib in combination with FOLFIRI and bevacizumab for mCRC patients with a KRAS mutation (NCT03829410). In May 2020, the FDA granted Fast Track designation for onvansertib in KRAS-mutated mCRC.
Masitinib (AB1010)
Masitinib is an inhibitor of multiple tyrosine kinases, predominantly c-Kit, but to a lesser extent, it also inhibits platelet-derived growth factor receptor α and β, Lyn, and fibroblast growth factor receptor 3.58 C-Kit, also known as the stem cell factor receptor, is responsible for regulating hematopoiesis, and also plays a role in mast cell activation.59 Increased mast cell activity is linked to poor prognosis in colorectal cancer, however the role of c-Kit in colorectal cancer is less clear.58,60,61 A phase Ib/II trial studied masitinib plus FOLFIRI in 18 irinotecan-naïve patients with mCRC.61 The initial masitinib dose was 9 mg/kg, but that was later reduced to 6 mg/kg to minimize the risk for toxicity.61 The ORR was 28%, including 1 patient with a CR.61 Median PFS was 6.2 months and median OS was 17.6 months.61 Adverse effects were not reported in this trial, but a prior phase I study noted nausea, vomiting, and diarrhea as the most common adverse effects.58 A phase II/III study is currently underway to compare masitinib plus FOLFIRI to best supportive care in patients with mCRC who have received at least 3 prior treatments (NCT03556956).
Adavosertib (AZD1775, MK-1775)
Adavosertib is a first-in-class inhibitor of the WEE1 kinase.62 WEE1 works along the G2/M checkpoint and prohibits cells with DNA damage from proceeding into mitosis.63 More specifically, WEE1 inactivates cyclin-dependent kinase 1 (CDK1) in response to DNA damage, resulting in cell cycle arrest to allow for DNA repair.63 Inhibition of WEE1, therefore, leads to increased CDK1 activity which allows for cells with aberrant DNA to enter mitosis, ultimately leading to lethal DNA damage.63 A phase I study examined adavosertib in 25 adult patients with refractory or metastatic solid tumors.62 Twenty-one patients were evaluable for response, and 4 (19%) patients had a PR.62 Adverse effects included myelosuppression, nausea, and vomiting.62 A Phase I study is currently underway exploring adavosertib in RAS or BRAF mutated mCRC (NCT02906059).
Novel Combinations
VEGF Inhibitor Combinations
There is a great need to improve outcomes in patients with mCRC. Currently, the two last line agents mCRC are trifluridine/tipiracil and regorafenib. Although both of these agents were shown to statistically improve OS, the PFS is poor at ~1.5 months for both agents, and the ORR is even lower at ~1% of patients.64 There are multiple investigations underway examining novel VEGF pathway inhibition with conventional chemotherapy, including trifluridine/tipiracil, to improve outcomes in mCRC. Pfeiffer and colleagues reported a phase II trial of 93 patients who received bevacizumab plus trifluridine/tipiracil or trifluridine/tipiracil alone in the last-line setting for mCRC.65 Median OS was significantly improved with the combination, 9.4 months vs 6.7 months, P = 0.028.65 Median PFS was also longer with the combination, 4.6 months vs 2.6 months, P = 0.001.65 However, response rates were still low, only one (2%) patient in the combination group had a PR, and no patients in the monotherapy group had a PR.65 Regardless, this combination approach does seem to be promising and there are a few ongoing trials examining the role of VEGF pathway inhibitors in conjunction with either conventional chemotherapy or trifluridine/tipiracil in hopes of improving outcomes in this last line setting. Additionally, the FOLFIRINOX-R trial is looking at the combination of regorafenib and FOLFIRINOX in the first-line setting for mCRC. Results from each of these trials will be very insightful and will hopefully approve outcomes for these patients [Table 1].
 |
Table 1 VEGF Inhibitor and Chemotherapy Combinations Under Investigation |
Immunotherapy Combinations
Immunotherapy is another attractive area for research in mCRC. Results from the KEYNOTE-177 trial were practice-changing after showing that pembrolizumab doubled PFS compared to chemotherapy plus bevacizumab or cetuximab in the first-line treatment of microsatellite deficient mismatch repair/microsatellite instability-high (dMMR/MSI-high) mCRC.66 Similarly, the CheckMate 142 trial, which combined nivolumab with ipilimumab in the first-line treatment of dMMR/MSI-H mCRC, also found a high ORR of 60%.67 While this is a huge success for the approximately 5% of patients with mCRC who are dMMR/MSI-H, immunotherapeutic options are still lacking for patients with proficient mismatch repair/microsatellite stable (pMMR/MSS) mCRC.68,69
One novel combination being explored for the pMMR/MSS mCRC population is VEGF inhibition plus immune checkpoint inhibition. It’s been proposed that VEGF inhibitors may enhance lymphocyte activation by reducing tumor-associated macrophages and regulatory T cells which typically block lymphocyte activation.69,70 Therefore, by blocking the VEGF pathway, sensitivity to immunotherapy may be restored in pMMR/MSS tumors.69 The REGONIVO and REGOMUNE trials both examined regorafenib in combination with a checkpoint inhibitor, and both had promising ORR >30%.70,71 Gou and colleagues published preliminary results from their phase II trial in China of fruquintinib plus sintilimab (a programmed death 1, PD-1, inhibitor), and described a lower ORR of 15%.72 A phase Ib trial of fruquintinib and genolimzumab (a PD-1 inhibitor) is ongoing and it will be interesting to see if this trial corroborates these results.72 Finally, the phase II AtezoTRIBE study is examining the combination of bevacizumab, atezolizumab, and FOLFOXIRI in the first line setting for mCRC irrespective of microsatellite status.69 A list of the ongoing trials exploring immunotherapy combinations for mCRC is in Table 2.
 |
Table 2 VEGF Inhibitor and Immunotherapy Combinations Under Investigation |
Besides the VEGF pathway, the combination of the MAPK pathway inhibition and immunotherapy is also being evaluated. The rationale behind this is that MAPK inhibition increases antigen expression and lymphocyte infiltration and also reduces immunosuppressive cytokines in the tumor microenvironment.73 The combination of atezolizumab, vemurafenib, and cobimetinib (a PD-L1 inhibitor, BRAF inhibitor, and MEK inhibitor) was recently studied in 514 patients with advanced BRAF V600E mutated melanoma and was shown to improve PFS compared to vemurafenib and cobimetinib alone.74 On the basis of this and other pre-clinical trials, ongoing studies are exploring a similar approach for mCRC. In addition, the phase II AVETUX-CRC trial examined the combination of FOLFOX, cetuximab, and avelumab (a PD-L1 inhibitor) in patients with RAS/BRAF-wildtype mCRC. The intention to treat protocol included 39 patients, and the ORR was found to be 79.5%, with 6 complete and 25 partial responses.75 Ongoing trials examining the combination of MAPK inhibition and immunotherapy are listed in Table 3.
 |
Table 3 MAPK Inhibitor and Immunotherapy Combinations Under Investigation |
Conclusion
In general, we are encouraged by the fact that mCRC patients are living longer; however, there are still many opportunities to enhance therapeutic outcomes for these patients. A better understanding of disease biology, drug resistance patterns, escape pathways, and optimal treatment combinations will help elicit better responses for these patients. Multiple studies are underway with many promising strategies to improve outcomes for patients with mCRC.
Disclosure
CE serves on advisory boards for Foundation of Medicine, Merck and Pfizer. The authors report no other conflicts of interest in this work.
References
1. National Cancer Institute. Cancer stat facts: colorectal cancer SEER data. Available from: https://seer.cancer.gov/statfacts/html/colorect.html.
2. Riihimaki M, Hemminki A, Sundquist J, Hemminki K. Patterns of metastasis in colon and rectal cancer. Sci Rep. 2016;6:29765. doi:10.1038/srep29765
3. Li J, Zhao X, Chen L, et al. Safety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignancies. BMC Cancer. 2010;10:529. doi:10.1186/1471-2407-10-529
4. Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011;102(7):1374–1380. doi:10.1111/j.1349-7006.2011.01939.x
5. Bogenrieder T, Herlyn M. Axis of evil: molecular mechanisms of cancer metastasis. Oncogene. 2003;22:6524–6536. doi:10.1038/sj.onc.1206757
6. Bendardaf R, Buhmeida A, Hilska M, et al. VEGF-1 expression in colorectal cancer is associated with disease localization, stage, and long-term disease-specific survival. Anticancer Res. 2008;28:3865–3870.
7. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi:10.1056/NEJMoa032691
8. Tabernero J, Yoshino T, Cohn AL, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomized, double-blind, multicenter, Phase 3 study. Lancet Oncol. 2015;16:499–508.
9. Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30(28):2499–2506.
10. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicenter, randomized, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–312.
11. Cidon EU, Alonso P, Masters B. Markers of response to antiangiogenic therapies in colorectal cancer: where are we now and what should be next? Clin Med Insights Oncol. 2016;10(S1):41–55.
12. Rossini D, Moretto CC, Cremolini C, et al. Treatments (tx) after progression to first-line FOLFOXIRI plus bevacizumab (bev) in metastatic colorectal cancer (mCRC) patients (pts): A pooled analysis of TRIBE and MOMA studies by GONO group. J Clin Oncology. 2017;35(15):Abstract 3542. doi:10.1200/JCO.2017.35.15_suppl.3542
13. Wang F, Yuan X, Jia J, et al. Apatinib monotherapy for chemotherapy-refractory metastatic colorectal cancer: a multi-centre, single-arm, prospective study. Sci Rep. 2020;10:6058. doi:10.1038/s41598-020-62961-5
14. Liang L, Wang L, Zhu P, et al. A pilot study of apatinib as third-line treatment in patients with heavily treated metastatic colorectal cancer. Clinical Colorectal Cancer Gastrointestinal Malignancies. 2018;17(3):E443E449. doi:10.1016/j.clcc.2018.02.011
15. Gou M, Haiyan S, Zhang Y, et al. Efficacy and safety of apatinib in patients with previously treated metastatic colorectal cancer: a real-world retrospective study. Sci Rep. 2018;8:4602. doi:10.1038/s41598-018-22302-z
16. Li A, Wang K, Xu A, et al. Apatinib as an optional treatment in metastatic colorectal cancer. Medicine. 2019;98(35):e16919. doi:10.1097/MD.0000000000016919
17. Cao J, Zhang J, Peng W, et al. A phase I study of safety and pharmacokinetics of fruquintinib, a novel selective inhibitor of vascular endothelial growth factor receptor-1, −2, and −3 tyrosine kinases in Chinese patients with advanced solid tumors. Cancer Chemother Pharmacol. 2016;78:259–269. doi:10.1007/s00280-016-3069-8
18. Li J, Qin S, Xu R, et al. Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer the FRESCO randomized clinical trial. JAMA. 2018;319(24):2486–2496.
19. Li X, Giu M, Wang S, et al. A Phase I dose‑escalation, pharmacokinetics and food‑effect study of oral donafenib in patients with advanced solid tumours. Cancer Chemother Pharmacol. 2020;85:593–604. doi:10.1007/s00280-020-04031-1
20. Seshacharyulu P, Ponnusamy MP, Haridas D, et al. Targeting the EGFR signaling pathway in cancer. Expert Opin Ther Targets. 2012;16(1):15–31. doi:10.1517/14728222.2011.648617
21. Xie Y, Chen Y, Fang J. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduction Targeted Therapy. 2020;5:22.
22. Bylsma LC, Gillezeau C, Garawin TA, et al. Prevalence of RAS and BRAF mutations in metastatic colorectal cancer patients by tumor sidedness: A systematic review and meta‐analysis. Cancer Med. 2020;9(3):1044–1057. doi:10.1002/cam4.2747
23. Van Cutsem E, Köhne C, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29(15):2011–2019. doi:10.1200/JCO.2010.33.5091
24. Vectibix® (Panitumumab) Injection for Intravenous Use [Package Insert]. Amgen Inc; 2017.
25. Erbitux® (Cetuximab) Injection, for Intravenous Use [Package Insert]. Eli Lilly and Company; 2019.
26. Zhao B, Wang L, Qiu H, et al. Mechanisms of resistance to anti-EGFR therapy in colorectal cancer. Oncotarget. 2017;8(3):3980–4000. doi:10.18632/oncotarget.14012
27. Li Q, Wang Y, Tu J, et al. Anti-EGFR therapy in metastatic colorectal cancer: mechanisms and potential regimens of drug resistance. Gastroenterology Report. 2020;8(3):179–191. doi:10.1093/gastro/goaa026
28. Bendell JC, Javie M, Bekaii-Saab TS, et al. A Phase 1 dose-escalation and expansion study of binimetinib (MEK162), a potent and selective oral MEK1/2 inhibitor. Br J Cancer. 2017;116(5):575–583.
29. Cox AD, Fesik SW, Kimmelman AC, et al. Drugging the undruggable Ras: mission possible? Nat Rev Drug Discov. 2014;13(11):828–851.
30. Schirripa M, Nappo F, Cremolini C, et al. KRAS G12C metastatic colorectal cancer: specific features of a new emerging target population. Clin Colorectal Cancer. 2020;19(3):219–225. doi:10.1016/j.clcc.2020.04.009
31. Hong DS, Fakih MG, Strickler JH, et al. KRASG12C inhibition with sotorasib in advanced solid tumors. N Engl J Med. 2020;383:1207–1217. doi:10.1056/NEJMoa1917239
32. Fakih M, Desai J, Kuboki Y, et al. CodeBreak 100: activity of AMG 510, a novel small molecule inhibitor of KRASG12C, in patients with advanced colorectal cancer. J Clinical Oncology. 2020;38(15):4018. doi:10.1200/JCO.2020.38.15_suppl.4018
33. Amodio V, Yaeger R, Archella P, et al. EGFR blockade reverts resistance to KRAS G12C inhibition in colorectal cancer. Cancer Discov. 2020;10:1129–1139. doi:10.1158/2159-8290.CD-20-0187
34. Corcoran RB, Atreya CE, Falchook GS, et al. Combined BRAF and MEK inhibition with dabrafenib and trametinib in BRAF V600–mutant colorectal cancer. J Clinical Oncology. 2015;33(34):4023–4031. doi:10.1200/JCO.2015.63.2471
35. Kopetz S, Grothey A, Yaeger R, et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E–mutated colorectal cancer. N Engl J Med. 2019;381:1632–1643. doi:10.1056/NEJMoa1908075
36. Braftovi® (encorafenib) capsules for oral use [Package Insert]. Pfizer Oncology. 2020.
37. Bennouna J, Lang I, Valladares-Ayerbes M, et al. A Phase II, open-label, randomised study to assess the efficacy and safety of the MEK1/2 inhibitor AZD6244 (ARRY-142886) versus capecitabine monotherapy in patients with colorectal cancer who have failed one or two prior chemotherapeutic regimens. Invest New Drugs. 2011;29:1021–1028. doi:10.1007/s10637-010-9392-8
38. Infante JR, Fecher LA, Falchook GS, et al. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13(8):773–781. doi:10.1016/S1470-2045(12)70270-X
39. Sullivan RJ, Infante JR, Janku F, et al. First-in-class ERK1/2 inhibitor ulixertinib (BVD-523) in patients with MAPK mutant advanced solid tumors: results of a phase I dose-escalation and expansion study. Cancer Discov. 2018;8(2):184–195. doi:10.1158/2159-8290.CD-17-1119
40. Nowak JA. HER2 in colorectal carcinoma. Surg Pathol Clin. 2020;13(3):485–502. doi:10.1016/j.path.2020.05.007
41. Strickler JH, Niedzwiecki D, Zemla T, et al. A Phase II, open label study of tucatinib (ONT-380) combined with trastuzumab in patients with HER2+ metastatic colorectal cancer (mCRC)(MOUNTAINEER). J Clin Oncol. 2016;Abstract TPS3624.
42. Sartore-Bianchi A, Trusolino L, Martino C, et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, Phase 2 trial. Lancet Oncol. 2016;17(6):738–746. doi:10.1016/S1470-2045(16)00150-9
43. Meric-Bernstam F, Hurwitz H, Raghav KPS, et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019;20(4):518–530. doi:10.1016/S1470-2045(18)30904-5
44. Sartore-Bianchi A, Martino C, Lonardi S, et al. Phase II study of pertuzumab and trastuzumab-emtansine (T-DM1) in patients with HER2-positive metastatic colorectal cancer: the HERACLES-B (HER2 amplification for colo-rectal cancer enhanced stratification, cohort B) trial. Annals Oncology. 2019;30(5):V869870. doi:10.1093/annonc/mdz394.024
45. TukysaTM (Tucatinib) Tablets for Oral Use [Package Insert]. Seattle Genetics, Inc; 2020.
46. Tykerb (Lapatinib) Tablets [Package Insert]. Novartis; 2018.
47. Moulder SL, Borges VF, Baetz T, et al. Phase I study of ONT-380, a HER2 inhibitor, in patients with HER2+-advanced solid tumors, with an expansion cohort in HER2+ metastatic breast cancer (MBC). Clinical Cancer Research. 2017;23(14):3529–3536. doi:10.1158/1078-0432.CCR-16-1496
48. Kulukian A, Lee P, Taylor J, et al. Preclinical activity of HER2-selective tyrosine kinase inhibitor tucatinib as a single agent or in combination with trastuzumab or docetaxel in solid tumor models. Mol Cancer Ther. 2020;19(4):976–987. doi:10.1158/1535-7163.MCT-19-0873
49. Strickler JH, Zemla T, Ou F-S, et al. Trastuzumab and tucatinib for the treatment of HER2 amplified metastatic colorectal cancer (mCRC): initial results from the MOUNTAINEER trial. Annals Oncology. 2019;30(5):V200. doi:10.1093/annonc/mdz246.005
50. Siena S, Di Bartolomeo M, Raghav KPS, et al. A phase II, multicenter, open-label study of trastuzumab deruxtecan (T-DXd; DS-8201) in patients (pts) with HER2-expressing metastatic colorectal cancer (mCRC): DESTINY-CRC01. J Clin Oncol. 2020;38(15):Abstract 4000. doi:10.1200/JCO.2020.38.15_suppl.4000
51. Mitani S, Kawakami H. Emerging targeted therapies for HER2 positive gastric cancer that can overcome trastuzumab resistance. Cancers. 2020;12(2):400. doi:10.3390/cancers12020400
52. Yap T, O’Carrigan B, Penney MS, et al. Phase I trial of first-in-class ATR inhibitor M6620 (VX-970) as monotherapy or in combination with carboplatin in patients with advanced solid tumors. J Clin Oncol. 2020;38(27):3195–3204. doi:10.1200/JCO.19.02404
53. Reaper PM, Griffiths MR, Long JM, et al. Selective killing of ATM- or p53-deficient cancer cells through inhibition of ATR. Nat Chem Biol. 2011;7:428–430. doi:10.1038/nchembio.573
54. The Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(330337). doi:10.1038/nature11252
55. Valsasina B, Beria I, Alli C, et al. NMS-P937, an orally available, specific small-molecule polo-like kinase 1 inhibitor with antitumor activity in solid and hematologic malignancies. Mol Cancer Ther. 2012;11(4):1006–1016. doi:10.1158/1535-7163.MCT-11-0765
56. Weiss GJ, Jameson G, Von Hoff DD, et al. Phase I dose escalation study of NMS-1286937, an orally available polo-like kinase 1 inhibitor, in patients with advanced or metastatic solid tumors. Invest New Drugs. 2018;36:85–95. doi:10.1007/s10637-017-0491-7
57. Kumar S, Kim J. PLK-1 targeted inhibitors and their potential against tumorigenesis. Phytochemicals Cancer Prevention Therapy. 2015;2015:705745.
58. Soria JC, Massard C, Magné N, et al. Phase 1 dose-escalation study of oral tyrosine kinase inhibitor masitinib in advanced and/or metastatic solid cancers. Eur J Cancer. 2009;45(12):2333–2341.
59. Dubreuil P, Letard S, Ciufolini M, et al. Masitinib (AB1010), a potent and selective tyrosine kinase inhibitor targeting KIT. PLoS One. 2009;4(9):e7258.
60. Gavert N, Shvab A, Sheffer M, et al. c-Kit is suppressed in human colon cancer tissue and contributes to L1-mediated metastases. Cancer Res. 2013;73(18):5754–5763. doi:10.1158/0008-5472.CAN-13-0576
61. Taieb J, Borg C, Lecomte T, et al. Masitinib plus FOLFIRI for second line treatment of metastatic colorectal cancer: an open label phase Ib/II trial. J Clin Oncol. 2015;33(15):3526. doi:10.1200/jco.2015.33.15_suppl.3526
62. Do K, Wilsker D, Ji J, et al. Phase I study of single-agent AZD1775 (MK-1775), a Wee1 kinase inhibitor, in patients with refractory solid tumors. J Clin Oncol. 2015;33(30):3409–3415. doi:10.1200/JCO.2014.60.4009
63. Webster PJ, Littlejohns AT, Gaunt HJ, et al. AZD1775 induces toxicity through double-stranded DNA breaks independently of chemotherapeutic agents in p53-mutated colorectal cancer cells. Cell Cycle. 2017;16(22):2176–2182. doi:10.1080/15384101.2017.1301329
64. Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909–1919. doi:10.1056/NEJMoa1414325
65. Pfeiffer P, Yilmaz M, Möller S, et al. TAS-102 with or without bevacizumab in patients with chemorefractory metastatic colorectal cancer: an investigator-initiated, open-label, randomised, phase 2 trial. Lancet Oncol. 2020;21:412–420. doi:10.1016/S1470-2045(19)30827-7
66. Andre T, Shiu K, Kim TW, et al. Pembrolizumab versus chemotherapy for microsatellite instability-high/mismatch repair deficient metastatic colorectal cancer: the phase 3 KEYNOTE-177 study. J Clin Oncol. 2020;38(18):Abstract LBA4. doi:10.1200/JCO.2020.38.18_suppl.LBA4
67. Lenz H, Lonardi S, Zagonel V, et al. Nivolumab plus low-dose ipilimumab as first-line therapy in microsatellite instability-high/DNA mismatch repair deficient metastatic colorectal cancer: clinical update. J Clin Oncol. 2020;38(suppl4; abstr 11).
68. Battaglin F, Naseem M, Lenz H, et al. Microsatellite instability in colorectal cancer: overview of its clinical significance and novel perspectives. Clinical Advances Hematol Oncol. 2018;16(11):735–747.
69. Antoniotti C, Borelli B, Rossini D, et al. AtezoTRIBE: a randomised phase II study of FOLFOXIRI plus bevacizumab alone or in combination with atezolizumab as initial therapy for patients with unresectable metastatic colorectal cancer. BMC Cancer. 2020;20(683):4. doi:10.1186/s12885-020-07169-6
70. Fukuoka S, Hara H, Takahashi N, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase Ib trial (REGONIVO, EPOC1603). J Clin Oncol. 2020;38(18):2053–2061. doi:10.1200/JCO.19.03296
71. Cousin S, Bellera CA, Guégan JP, et al. REGOMUNE: A phase II study of regorafenib plus avelumab in solid tumors—Results of the non-MSI-H metastatic colorectal cancer (mCRC) cohort. J Clin Oncol. 2020;38(15):Abstract 4019. doi:10.1200/JCO.2020.38.15_suppl.4019
72. Gou M, Yan H, E L T, et al. Fruquintinib combination with sintilimab in refractory metastatic colorectal cancer patients in China. J Clin Oncol. 2020;38(15):Abstract 4028. doi:10.1200/JCO.2020.38.15_suppl.4028
73. Hughes PE, Caenepeel S, Wu LC. Targeted therapy and checkpoint immunotherapy combinations for the treatment of cancer. Trends Immunol. 2016;37(7):462–476. doi:10.1016/j.it.2016.04.010
74. Gu tzmer R, Stroyakovskiy D, Gogas H, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAF V600 mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;395(10240):1835–1844. doi:10.1016/S0140-6736(20)30934-X
75. Stein A, Binder M, Goekkurt E, et al. Avelumab and cetuximab in combination with FOLFOX in patients with previously untreated metastatic colorectal cancer (MCRC): final results of the phase II AVETUX trial (AIO-KRK-0216). J Clin Oncol. 2020;38(suppl4; abstr 96):96. doi:10.1200/JCO.2020.38.4_suppl.96
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
