Back to Journals » Clinical Ophthalmology » Volume 16
Unmet Need for ROP Screening in Peripheral Rural Areas
Authors Meena S , Bhatnagar K , Sheemar A, Gupta N , Tandon M, Agrawal N
Received 17 January 2022
Accepted for publication 3 May 2022
Published 16 June 2022 Volume 2022:16 Pages 1963—1969
DOI https://doi.org/10.2147/OPTH.S357591
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Seema Meena, Kavita Bhatnagar, Abhishek Sheemar, Neeraj Gupta, Manjari Tandon, Nikhil Agrawal
Department of Ophthalmology, AIIMS, Jodhpur, Rajasthan, India
Correspondence: Seema Meena, Department of Ophthalmology, AIIMS, Jodhpur, Rajasthan, India, Tel +91 9953572984, Email [email protected]
Aim: To study the incidence and risk factors associated with retinopathy of prematurity (ROP) at a tertiary care centre in Western India.
Methods: A retrospective review of records of both referred and babies born in our hospital who were screened for ROP within the last 21 months at a tertiary care centre was done. The newborns were screened as per National Neonatology Forum of India guidelines. ROP was classified according to the International Classification for Retinopathy of Prematurity criteria.
Results: A total of 167 neonates were screened with an incidence of ROP of 26.9%. The mean gestational age (GA) and mean birth weight (BW) were 31.89 ± 2.824 weeks and 1538.11 ± 530.84 gm. The mean BW of neonates having any ROP was significantly lower (1296.98gm, p < 0.001), and the mean GA was also significantly lower in ROP babies (30.67 weeks, p < 0.001). O2 supplementation, RDS, IVH, and NEC were the systemic risk factors that significantly correlated with ROP p-value < 0.001. On analysis of the correlation of stage of ROP with BW and GA, a significant correlation of − 0.307 (p < 0.001) and − 0.283 (p < 0.001) was found.
Conclusion: The incidence of ROP in this study is similar to that reported in the literature from other regions. Other than LBW and GA, oxygen supplementation, RDS, IVH, and NEC were significant risk factors associated with ROP.
Keywords: retinal neovascularization, screening, risk factors of ROP, patterns of ROP
Introduction
Retinopathy of prematurity (ROP) is a vascular proliferative disorder of the retina first described in premature infants by T.L.Terry in 1942.1,2 Since then, it has been increasingly diagnosed in premature babies and identified as a significant cause of blindness in children. In India, the reported incidence is 20% to 30%, which is high.3–5 Initially, high supplemental oxygen saturation was considered to be the sole reason for ROP. Still, now other factors including low birth weight, prematurity, respiratory distress syndrome (RDS), anaemia, sepsis, and blood transfusion were also found to have a significant association with ROP.6 Timely fundus screening of a neonate is the most important intervention to prevent ROP. However, its incidence is still quite high in developing countries like India mostly because of unawareness and lack of speciality care services at a rural level.
Many studies identify the incidence and risk factors of ROP from different regions of the world. However, there are very scarce data on the profile of ROP patients in western India. A lot of research is currently undergoing in this field to understand various risk factors. Here, our main goal was to evaluate the risk factors, incidence and profile of ROP patients in western India.
Methods
A retrospective review of records of babies screened for at neonatal intensive care unit and ophthalmology OPD of a tertiary care institute AIIMS, Jodhpur, Rajasthan, was done from January 2020 to September 2021. The study adhered to the declaration of Helsinki, and Institutional Ethics Committee approval was obtained from the All India Institute of Medical Sciences, Jodhpur (Reference no. AIIMS/IEC/2021/3903). As the study was retrospective, parental consent was not required for reviewing the medical records as per the ethics committee, and personal details of patients were not revealed. The inclusion criteria consisted of ROP screening guidelines followed in India.7 All babies born in our hospital admitted to the neonatal intensive care unit and referred babies from other health-care centres with the following criteria were examined by binocular indirect ophthalmoscopy and 28D lens: all infants born at 34 weeks or less gestational age; all infants weighing <2000 g or less at birth; all infants born at more than 34 weeks gestational age with associated risk factors (cardiorespiratory support; prolonged oxygen requirement; respiratory distress syndrome; chronic lung disease; fetal hemorrhage; blood transfusion; sepsis; exchange transfusion; interventricular hemorrhage; apnea; poor postnatal weight gain); other preterm infants based on the discretion of the paediatrician or neonatologist.
ROP screening was done by a vitreoretinal consultant, and the data was entered in a designated form. ROP was classified according to the International Classification of Retinopathy of Prematurity (ICROP) criteria and managed and followed up as per the Early Treatment of Retinopathy of Prematurity (ETROP) study.8,9 Aggressive Posterior ROP (APROP), plus, and pre-plus diseases were diagnosed according to revised ICROP criteria.9 The screening was terminated when either full retinal vascularization was complete or regression of ROP was noted. The data was entered in the Microsoft Excel sheet and analysed using SPSS software version 23 (IBM Corp, Armonk, NY, USA). The zone, stage, plus, pre-plus and APROP were calculated using the basic frequency table. The bivariate analysis of the association of ROP with birth weight (BW), gestational age (GA), and other systemic risk factors was done using independent t and chi-square tests. The correlation of the stage of ROP with BW and GA was analysed by using Spearman’s rho test. In the case of small sample size, Fisher’s Exact Test was used. A p-value of <0.05 was considered significant.
Results
A total of 167 neonates were screened for ROP from January 2020 to September 2021 including both inborn and outborn out of which 80 were females and 87 were males. The mean gestational age (GA) of infants was 31.89 ± 2.824 weeks with 18.7% >34 weeks and 81.3% <34 weeks. The mean birth weight (BW) was 1538.11 ± 530.84 ranging from 580 to 3500gm (Table 1). Any form of ROP was present in 45 neonates with incidence of 26.9% (Table 2). The ROP stage and zones were distributed as follows (Table 3): 20% stage 1 (n = 9), 33.3% stage 2 (n = 15), 28.9% stage 3 (n = 13), 2.2% stage 4 (n = 1), 8.9% stage 5 (n = 4), 8.9% zone 1 (n = 4), 40% zone 2 (n = 18), and 53.3% zone 3 (n = 24). APROP was present in 20% (n = 9) whereas 11.11% (n = 5) and 15.6% (n = 7) had plus and pre-plus.
 |
Table 1 Demographic Data of Screened Babies |
 |
Table 2 Comparison of Mean Gestational Age and Mean Birth Weight Between ROP Positive and Negative Cases by t-Test |
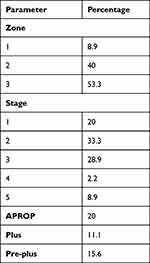 |
Table 3 Pattern of ROP |
The mean BW of neonates having any ROP was significantly lower, 1296.98gm (p < 0.001), as compared to other babies screened for ROP (1626.35 gm). Similarly, the mean GA was also significantly lower in ROP babies, 30.67 weeks (p < 0.001), compared to other babies without ROP with a mean GA of 32.33 weeks.
In a bivariate analysis for correlation of ROP with other risk factors, which included mode of delivery (vaginal or caesarean), O2 supplementation, respiratory distress syndrome (RDS), sepsis, blood transfusion, multiple births, necrotising enterocolitis (NEC), intraventricular haemorrhage (IVH), apnoea, anaemia, thrombocytopenia, patent ductus arteriosus, cardiovascular defects other than PDA and maternal risk factors (HTN, anaemia, assisted conception/premature rupture of membranes), only O2 supplementation, RDS, IVH and NEC were significantly correlated with ROP, p-value <0.001 (Table 4). On analysis of the correlation of stage of ROP with BW and GA, a significant correlation of −0.307 (p < 0.001) and −0.283 (p < 0.001) was found. The laser was done for stage 3 babies (31.4%), surgery for stages 4a and 4b (11.1%). Regression of ROP was noted in 34.7%, and 31.4% did not follow up.
 |
Table 4 Correlation of ROP with Risk Factors by Bivariate Analysis |
Discussion
A total of 167 neonates were screened at our centre for 21 months, with ROP present in 45 babies. The incidence of ROP in our study was 26.9%, comparable with other studies. In a study by Thakre et al, the incidence of ROP was 27.73%, with a mean birth weight of (1390 ± 310 g; P = 0.012) and the mean gestational age (31.12 ± 2.67wk; P = 0.0000).10 In another study by Paranjpe et al from Maharashtra, the reported incidence of ROP was 35.62%.11 A similar incidence with a slightly higher incidence has been reported in other studies (Table 5). 12–19 The mean BW of screened babies was 1538.11gm range (580–3500), and the mean GA was 31.89 weeks range (26–39) weeks. So very premature babies with very low birth weight (LBW) were part of this retrospective cohort, mostly inborn and screened in a neonatal intensive care unit (NICU).
 |
Table 5 Incidence of ROP in Recent Studies from India |
On the other hand, the mean GA of ROP babies at 30.67 weeks was significantly lower than the mean GA of screened babies without ROP at 32.33 weeks, p < 0.001. Similarly, the mean BW of ROP babies (1296.98gm) was significantly less than non-ROP babies (1626.35gm, p < 0.001). So, both these parameters suggest that LBW and prematurity are significant risk factors for ROP development. In a premature delivery, these factors are unavoidable, so a mandatory ROP screening assumes utmost importance in these babies. This collaborates with other studies.12–19
In a bivariate analysis of risk factors for ROP, oxygen supplementation, RDS, IVH, and NEC were the systemic factors significantly associated with ROP with a p-value <0.001. Several studies have evaluated the role of supplemental oxygen in ROP; the prominent ones being STOP-ROP (Supplemental Therapy with Oxygen to Prevent ROP), ELGAN Extremely Low Gestational Age Newborn), SUPPORT (Surfactant, Positive Airway Pressure, Pulse Oximetry Randomized Trial), and BOOST (Benefits of Oxygen Saturation Targeting Study).20–23 The STOP-ROP showed no difference in the incidence of ROP between 96–99% SaO2 and 89–94% SaO2 groups; the SUPPORT and BOOST studies showed higher mortality in lower oxygen saturation group though with lower incidence of ROP. Some of the authors have found that lower oxygen saturation targets at young post-gestational ages with higher targets at older post gestational ages reduced the incidence of severe ROP.24–26 So, supplemental oxygen must be carefully titrated against its disadvantages. Similarly, RDS leads to hypoxia requiring assisted ventilation and supplemental oxygen, which can independently lead to ROP. The role of RDS in ROP has been evaluated, and very few studies have shown it to be a risk factor for ROP.27 NEC was significantly associated with ROP in our study. Tomlinson et al have reported NEC to be an independent risk factor for ROP.28 ROP were noted in 79% of infants with NEC in their study, with early NEC more strongly correlated with ROP than late NEC. Ford et al also reported that NEC might increase the risk of more advanced disease and the risk of laser treatment for ROP.29 IVH was another factor in our study which was significantly associated with ROP. In a study of 324 eyes, a similar significant association of ROP with INH was noted by Chang et al.30 The incidence of IVH in premature infants with a BW less than 1500gm is around 20% to 40%.31 A similarity in pathogenesis between IVH and ROP has been proposed, with immature vasculature as the underlying cause.32,33
The other factors that were evaluated but were insignificant were the mode of delivery, sepsis, blood transfusion, multiple births, apnoea, anaemia, thrombocytopenia, patent ductus arteriosus, cardiovascular defects other than PDA and maternal risk factors, though individually they have been found associated with ROP in other studies.27
Correlation of Stages of ROP with BW and GA by a bivariate analysis shows a significantly negative correlation of −0.371 (p < 0.0001) and −0.283 (p < 0.0001), suggesting an increase in the stage of ROP with lowering of BW and GA. This is a significant correlation that signifies the need for early screening for very premature and very low birth weight babies and their early referral to specialized centres for ROP management in case of stage progression. On quantitative analysis of ROP parameters, stage 3 was present in 28.9%. In contrast, stages 4 and 5 were noted in 2.2% and 8.9%, respectively, which required treatment. The babies having stages 4 and 5 were referred babies, suggesting an unmet need for ROP screening in peripheral rural areas. This is primarily due to a lack of awareness and non-availability of specific treatment in rural regions. These cases can be managed at the earliest, thereby reducing severe morbidity.
The incidence of type 1 ROP is variable in different studies. The presence of APROP was noted in 21.1% of ROP babies in zone 1 and 2 in our study, which is quite high and indicates the severity of ROP. A similar incidence of APROP of 28% was noted by Anamika et al in eastern Madhya Pradesh.14 However, other authors have reported a lower incidence of APROP, ranging from 9% to 13%.34,35 In our study, treatment was required in 42.5% of ROP babies; these patients required laser therapy/surgery or both. The spontaneous regression of ROP was noted in 22.8% of babies. In contrast, it has been around 90% in the CRYO-ROP study. More evaluation needs to be done to see an actual rate of regression in the eastern part of the world.36 About 31.4% of patients did not come for follow-up as was advised. This is significant as ROP can progress rapidly and is difficult to treat later.
Initially, for ROP screening, babies less than 1750gm birth weight and 34 weeks gestational age were examined, but this has been modified to cover at-risk infants. The criteria of <1500gm and <30 weeks as followed in the USA were found to be inadequate to cover all babies in developing countries like India.38 On comparing ROP screening criteria with other countries worldwide, the birth weight limit in India is now higher at 2000gm, and the gestational age limit is also increased to 34–36 weeks.
The barriers to regular follow-up in a peripheral area need to be addressed, and regular counselling of parents is required in this regard. This can be achieved with proper planning and involving different welfare departments at the state and national levels through IEC (information, education and communication) activities.
Conclusion
The incidence of ROP in our study was similar to that reported in the literature from other regions. In addition to LBW and GA, RDS, IVH, and NEC were significant risk factors associated with ROP. The babies having stages 4 and 5 were referred babies suggesting an unmet need for ROP screening in peripheral rural areas.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Terry TL. Extreme prematurity and fibroblastic overgrowth of persistent vascular sheath behind each crystalline lens. I. Preliminary report. Am J Ophthalmol. 1942;25:203–204. doi:10.1016/S0002-9394(42)92088-9
2. Terry TL. Fibroblastic overgrowth of persistent tunica vasculosa lentis in premature infants. II. Report of cases – clinical aspects. Arch Ophthalmol. 1943;29:36–53. doi:10.1001/archopht.1943.00880130054003
3. Aggarwal R, Deorari AK, Azad RV, et al. Changing profile of retinopathy of prematurity. J Trop Pediatr. 2002;48:239–242. doi:10.1093/tropej/48.4.239
4. Kumar P, Sankar MJ, Deorari A, et al. Risk factors for severe retinopathy of prematurity in preterm low birth weight neonates. Indian J Pediatr. 2011;78:812–816. doi:10.1007/s12098-011-0363-7
5. Blencowe H, Lawn JE, Vazquez T, et al. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res. 2013;74(Suppl 1):35–49. doi:10.1038/pr.2013.205
6. Murthy KR, Murthy PR, Shah DA, Nandan MR, Benakappa N, Benakappa N. Comparison of profile of retinopathy of prematurity in semi urban/rural and urban NICUs in Karnataka, India. Br J Ophthalmol. 2013;97:687–689. doi:10.1136/bjophthalmol-2012-302801
7. Shukla R, Murthy GVS, Gilbert C, Vidyadhar B, Mukpalkar S. Operational guidelines for ROP in India: a summary. Indian J Ophthalmol. 2020;68(Suppl 1):S108–S114. doi:10.4103/ijo.IJO_1827_19
8. International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol Chic Ill. 2005;123:991–999.
9. Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol Chic Ill. 2003;121:1684–1694.
10. Thakre S, Deshmukh P, Kalyanshetti G, Mishrikotkar J. Incidence, severity, and risk factors of retinopathy of prematurity in Central Maharashtra, India. Perinatology. 2017;18:50.
11. Paranjpe DG, Sarwate DR, Shetty DN. Risk factor and outcome of retinopathy of prematurity among premature babies admitted to Tertiary Care Hospital: a retrospective observational study. J Curr Med Res Opin. 2019;02. doi:10.15520/jcmro.v2i11.226
12. Sachan A, Chandra P, Agarwal R, et al. Profile of retinopathy of prematurity in outborn and inborn babies at a tertiary eye care hospital. Indian Pediatr. 2020;57(11):1020–1022. doi:10.1007/s13312-020-2027-z
13. Anudeep K, Srikanth K, Sindal MD, Jha KN. Study of incidence, risk factors, and treatment outcomes in retinopathy of prematurity in a tertiary care center. TNOA J Ophthal Sci Res. 2019;57:24–26. doi:10.4103/tjosr.tjosr_10_19
14. Dwivedi A, Dwivedi D, Lakhtakia S, Chalisgaonkar C, Jain S. Prevalence, risk factors and pattern of severe retinopathy of prematurity in eastern Madhya Pradesh. Indian J Ophthalmol. 2019;67:819–823. doi:10.4103/ijo.IJO_1789_18
15. Patel SS, Shendurnikar N. Retinopathy of prematurity in India: incidence, risk factors, outcome and the applicability of current screening criteria. Int J Contemp Pediatr. 2019;6:2235–2241. doi:10.18203/2349-3291.ijcp20194698
16. Sathar A, Abbas S, Nujum ZT, Benson JL, Sreedevi GP, Saraswathyamma SK. Visual outcome of preterm infants screened in a tertiary care hospital. Middle East Afr J Ophthalmol. 2019;26(3):158–162. doi:10.4103/meajo.MEAJO_64_17
17. Kulkarni S, Kadam S, Patil A, Gilbert C. Retinopathy of prematurity: Maharashtra state model. Indian J Ophthalmol. 2020;68(Suppl 1):S121–S123. doi:10.4103/ijo.IJO_1867_19
18. Padhi TR, Pradhan L, Padhy SK, et al. Retinopathy of prematurity care in peripheral districts in Odisha, India: pilot for a sustainable model. Indian J Ophthalmol. 2020;68(Suppl 1):S124–S127. doi:10.4103/ijo.IJO_1914_19
19. Agarwal K, Balakrishnan D, Rani PK, Jalali S. Changing patterns of early childhood blinding conditions presenting to a tertiary eye center: the epidemic of retinopathy of prematurity in India. Indian J Ophthalmol. 2019;67(6):816–818. doi:10.4103/ijo.IJO_709_18
20. SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med. 2010;362:1959–1969. doi:10.1056/NEJMoa0911781
21. The STOP-ROP Multicenter Study Group. Supplemental therapeutic oxygen for prethreshold retinopathy of prematurity (STOP-ROP), a randomized, controlled trial. I: primary outcomes. Pediatrics. 2000;105:295–310. doi:10.1542/peds.105.2.295
22. Hauspurg AK, Allred EN, Vanderveen DK, et al. Blood gases and retinopathy of prematurity: the ELGAN study. Neonatology. 2011;99:104–111. doi:10.1159/000308454
23. Stenson B, Brocklehurst P, Tarnow-Mordi W. Increased 36-week survival with high oxygen saturation target in extremely preterm infants. N Eng J Med. 2011;364:1680–1682. doi:10.1056/NEJMc1101319
24. Gaynon MW. Rethinking STOP-ROP: is it worthwhile trying to modulate excessive VEGF levels in prethreshold ROP eyes by systemic intervention? A review of the role of oxygen, light adaptation state, and anemia in prethreshold ROP. Retina. 2006;26:S18–23. doi:10.1097/01.iae.0000244292.86627.1e
25. Wallace DK, Veness-Meehan KA, Miller WC. Incidence of severe retinopathy of prematurity before and after a modest reduction in target oxygen saturation levels. J AAPOS. 2007;11:170–174. doi:10.1016/j.jaapos.2006.08.012
26. Vanderveen DK, Mansfield TA, Eichenwald EC. Lower oxygen saturation alarm limits decrease the severity of retinopathy of prematurity. J AAPOS. 2006;10:445–448. doi:10.1016/j.jaapos.2006.04.010
27. Kim SJ, Port AD, Swan R, Campbell JP, Chan RVP, Chiang MF. Retinopathy of prematurity: a review of risk factors and their clinical significance. Surv Ophthalmol. 2018;63(5):618–637. doi:10.1016/j.survophthal.2018.04.002
28. Tomlinson L, Fundora J, Donohue P, et al. Association of surgical necrotizing enterocolitis with retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2020;61(7):2180.
29. Ford GS, Davis RM, Cheeseman E, Wee D. Evaluating necrotizing enterocolitis as a potential risk factor for advanced-stage retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2009;50(13):3150.
30. Chang JW, Hansen RM. Risk factor analysis for the development and progression of retinopathy of prematurity. PLoS One. 2019;14(7):e0219934. doi:10.1371/journal.pone.0219934
31. Philip AG, Allan WC, Tito AM, Wheeler LR. Intraventricular hemorrhage in preterm infants: declining incidence in the 1980s. Pediatrics. 1989;84(5):797–801. doi:10.1542/peds.84.5.797
32. Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. 2010;67(1):1–8. doi:10.1203/PDR.0b013e3181c1b176
33. Procianoy RS, Garcia-Prats JA, Hittner HM, Adams JM, Rudolph AJ. An association between retinopathy of prematurity and intraventricular hemorrhage in very low birth weight infants. Acta Paediatr Scand. 1981;70(4):473–477. doi:10.1111/j.1651-2227.1981.tb05725.x
34. Hungi B, Vinekar A, Datti N, et al. Retinopathy of prematurity in a rural Neonatal Intensive Care Unit in South India–a prospective study. Indian J Pediatr. 2012;79:911–915. doi:10.1007/s12098-012-0707-y
35. Sanghi G, Dogra MR, Das P, Vinekar A, Gupta A, Dutta S. Aggressive posterior retinopathy of prematurity in Asian Indian babies: spectrum of disease and outcome after laser treatment. Retina Phila Pa. 2009;29:1335–1339. doi:10.1097/IAE.0b013e3181a68f3a
36. Repka MX, Palmer EA, Tung B. Involution of retinopathy of prematurity. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol. 2000;118:645–649. doi:10.1001/archopht.118.5.645
37. Manjunathaswamy R, Rao AH, Hegade VP, Kumar P, Patil RB. A study of the incidence of retinopathy of prematurity in a tertiary care centre in Karnataka. Int J Contemp Pediatr. 2021;8:1033–1037. doi:10.18203/2349-3291.ijcp20212043
38. Sen P, Wu WC, Chandra P, Vinekar A, Manchegowda PT, Bhende P. Retinopathy of prematurity treatment: Asian perspectives. Eye. 2019;34. doi:10.1038/s41433-019-0643-4
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
