Back to Journals » Research and Reports in Tropical Medicine » Volume 12
Undernutrition and Associated Factors Among Adult Tuberculosis Patients in Jigjiga Public Health Facilities, Somali Region, East, Ethiopia
Authors Muse AI , Osman MO , Ibrahim AM , Wedajo GT , Daud FI, Abate KH
Received 8 April 2021
Accepted for publication 26 May 2021
Published 17 June 2021 Volume 2021:12 Pages 123—133
DOI https://doi.org/10.2147/RRTM.S311476
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mario Rodriguez-Perez
Abdilahi Ibrahim Muse,1 Mohamed Omar Osman,2 Ahmed Mohammed Ibrahim,2 Girma Tadesse Wedajo,2 Fuad Ismail Daud,1 Kalkidan Hassen Abate3
1Department of Nursing, College of Medicine and Health Science, Jigjiga University, Jigjiga, Ethiopia; 2Department of Public Health, College of Medicine and Health Science, Jigjiga University, Jigjiga, Ethiopia; 3Department of Population and Family Health, Jimma University, Jimma, Ethiopia
Correspondence: Mohamed Omar Osman
Department of Public Health, College of Medicine and Health Science, Jigjiga University, P.O. Box: 1020, Jigjiga, Ethiopia
Tel +251921424748
Fax +251 25 775 5976
Email [email protected]
Background: Tuberculosis and undernutrition are the public health concerns of people living in middle and low-income countries. When patient develops TB, undernutrition is not only a risk factor for progression of latent TB infection to active disease, but also intensifies the risk of drug toxicity, relapse and death. Nutritional supplementation in patients with TB is associated with faster sputum conversion, higher cure and treatment completion rates, and body-weight gain.
Objective: To find out the magnitude of undernutrition and associated factors among adult tuberculosis patients in jigjiga public health facilities.
Methods and Materials: A facility-based cross-sectional study design was applied. Data were collected using a structured questionnaire while anthropometric measurements were collected in their scale measurements. The data were entered into an Epi-data version 3.1, then were exported and analyzed using SPSS v20. Bivariate logistic regression was done to assess the association between the outcome variable and the independent variables, value < 0.25 was considered as a candidate for multivariate logistic regression at 95% CI. In multivariable logistic regression analysis, the level of statistical significance was declared at a p-value less than 0.05.
Results: The magnitude of undernutrition was 44.3% [95% CI (38.2, 49.7)]. Sex (female) [AOR=1.769, CI=1.035, 3.024], educational status [AOR=3.939, CI=2.285, 6.792] and being Bedridden [AOR=3.718, CI=1.115, 12.394) were predictors of Undernutrition among adult tuberculosis patients.
Conclusion: The magnitude of undernutrition among adult patients with TB was high in the area. Overall routine appropriate nutrition assessment and support should be given to undernourished patients with TB. The level of education about nutrition should be improved by counseling on a balanced diet to all patients with TB and particularly for female patients. Appropriate nutrition support should be provided to undernourished TB patients, and more focused on those who are bedridden.
Keywords: under-nutrition, TB, adult, public health facilities, Jigjiga town, Ethiopia
Introduction
Background
Undernutrition is defined as a condition in which the nutrient and energy intake is inadequate to meet an individual’s needs to sustain good health.1
Individuals become undernourished when their diet does not provide them with adequate calories and proteins for maintenance and growth. Illness can further result in decreased intestinal absorption.2
Undernutrition and TB have a complex association. Undernutrition weakens the human immune system and this can result in latent TB developing into active infection. In turn, TB exacerbates undernutrition by increasing metabolic demand and decreasing appetite.3 Patients with tuberculosis face a condition characterized by decreased appetite, nutrient intake and micronutrient malabsorption resulting from the altered metabolic system of the patients, which is caused by the disease process. Undernourished patients with TB have delayed recovery and increased death rates in comparison to patients with TB with normal nutrition.4
Appetite-regulatory hormones are altered in TB patients (elevations in Peptide YY2, ghrelin, and resistin and reductions in plasma leptin). As hormones normalize during treatment, appetite is restored and nutritional status improves5 may be associated with low BMI and may account for the poor nutrition linked with TB.6
According to WHO estimate every year, 10 million people fall ill with tuberculosis (TB). Despite being a preventable and curable disease, it causes the death 1.5 million each year – making it the world’s top infectious killer.7
More than one-third of the world’s population is infected by M. tuberculosis. The global annual death due to TB is estimated to be three million.8 The relationship between undernutrition and TB is evident globally in high, middle and low income countries. In the United States of America, adults who have low Body Mass Index (BMI), reduced subcutaneous fat or reduced skeletal muscle mass of have been shown to be at increased risk of developing TB compared to those with a normal nutritional status.9
Globally it is estimated that undernutrition causes about one-quarter of all new TB cases and tuberculosis is thought to be one of the most frequent underlying causes of wasting.10
In India, undernutrition, and tuberculosis are co-epidemics that are viewed as interconnected public health problems; 55% of TB incidence is estimated to be attributable to the effect of undernutrition.11 In Sri Lanka, nutritional status is much lower in adult patients with TB than healthy controls and undernutrition doubles the probability of acquiring active TB.12 Sub-Saharan countries are among the 30 high TB burden countries in the world; the number of adults infected by TB infection ranges from 10,103 in Congo to 219,156 in South Africa. 90% of patients with TB are adults of working age, resulting in lost days of work and a consequent economic burden on these countries.13 In Malawi patients with TB and undernutrition were twice as likely to die early and develop progressive lung disease; undernutrition also increased the risk of developing TB three-fold.14
In Ethiopia, a country-wide rapid nutrition assessment performed in 2015 found that 2 out of 3 registered adult patients with TB had a BMI lower than 18.5 kg/m2.15
Nutritional status is important for health and immunity. Cell-mediated immunity is the primary host defense against TB; TB is, therefore, a vital risk factor for the development of undernutrition. Reactivation of previously sub-clinical TB infection is often related to worsening nutritional status. Therefore, the effective management of TB requires a detailed assessment of nutritional status since this can help to manage disease complications and also understand how nutritional status is likely to impact the disease’s clinical course.16
Addressing undernutrition alongside TB treatment has been shown to improve treatment outcomes, and reduce the risk of recurrence. Nutritional supplement counseling helps to ensure adequate energy intake, resulting in improved which gives a significant improvement in body weight, total lean mass, and physical functioning.17
TB programs exist that deliver treatment services to patients freely in order to control the spread of disease. Despite these extensive efforts, there are still many undernourished adult patients with TB in Ethiopia.15 A recent finding of meta-analysis in Ethiopia showed that more than half of TB patients in were undernourished 50.8% which implicates undernutrition of TB patients was noticeably high.18
In Ethiopia, there are very few reports available on the prevalence, severity and implications of undernutrition in adult patients with TB and no reports in Somali regional state, where the population is geographically, ethnically and culturally different from the population where the available reports were performed. This study aimed to fill this gap by studying the magnitude of undernutrition and associated factors among adult patients with TB in Jigjiga public health facilities. This information will be used to design targeted interventions that effectively address the problems of undernutrition in patients with TB in Jigjiga town.
Methods and Materials
Study Area and Period
Jigjiga is the capital city of the Somali Regional State, Ethiopia and situated about 630 km east of Addis Ababa. Jigjiga has 20 kebeles (Ethiopia’s smallest administration units) in the town and 10 rural kebeles surrounding the town. There is a regional, hospital, a referral hospital and two health centers which are owned by the government, one private hospital and fifty-eight private clinics. Government institutions deliver TB services: a total of 830 adult patients with TB were on follow-up, ranging from 145 in Ayardaga health centre to 300 in Jigjiga City health centre. According to the town’s municipal report of 2019, Jigjiga has a total population of 884,660, split between the urban (population = 516,052) and rural (population = 368,608) kebeles. 97% of the population identify as Somali.19 The study was conducted between 1st and 30th April, 2020.
Study Design
Facility-based cross-sectional study design was used.
Inclusion and Exclusion Criteria
Inclusion Criteria
All adult TB patients, who started anti-TB drugs and had a follow-up in Jigjiga public health facilities during the study period.
Exclusion Criteria
All adult TB patients with a dietary restrictions, or had severely mentally ill and critically ill patients who were unable to communicate.
Sample Size Determination
Sample size was determined based on the double population proportion formula and one of three key predictors from the previous study selected10 and computed by EP INFO version 7. Since the study population is <10,000 a correction formula was used. The final sample size was = 302 after adding a 10% non-response rate.
Sampling Technique
Consecutive sampling technique was used.
Sampling Procedure
Study participants were interviewed consecutively by considering proportional allocations on the number of patient flow in each health facility.
Data Collection Techniques and Tools
A structured questionnaire was prepared using the conceptual framework and information from the literature. Anthropometric measurements were made using the MUAC measuring tape, stadiometer and digital weighing scales made and utilized by UNICEF. Data collectors with previous relevant experience were recruited from health facilities and collected data under close supervision by a supervisor in each health facility.
Data Collection Procedure
Socio-demographic, dietary intake and general health information was collected from all eligible participants using a structured questionnaire administered by nurses under the close supervision of their supervisors. Bodyweight in kilograms (kg) and height in meters (m) were recorded from all participants except pregnant and lactating mothers; their nutritional status was measured using MUAC in millimeters (mm) by the nurses, under the close supervision of the respective supervisors. Weight was measured using a portable weighing scale and recorded to the nearest 0.1 kg. During the procedure, the subjects wore light clothes and were barefoot. Height was measured by using a stadiometer and recorded to the nearest 0.1 cm.20 MUAC was measured for pregnant and lactating mothers by using a non-stretch tape on the non-dominant hand halfway between the olecranon process and acromion process.
BMI was calculated by dividing weight (in kg) by height (in m2). “Underweight” was defined if BMI <18.5 kg/m2; “Normal weight” as BMI between 18.5 and 24.9 kg/m2; “Overweight” as BMI between 25 and 29.9 kg/m2; “Obese class I” as BMI between 30 and 34.9 kg/m2, “Obese class II” as BMI between 35 and 39.9 kg/m2; and “Obese class III” as BMI > 40kg/m2.21 Patient records were reviewed for type and severity of disease, HIV/AIDS status, stage of TB, and type of anti-TB medication, duration of anti-TB medication, duration of cough and other symptoms of TB.
Study Variables
Dependent Variable
Undernutrition (BMI<18.5 kg/m2 and, or MUAC <210mm for pregnant and lactating mothers).
Independent Variables
Behavior and lifestyle factors, food Intake factors, Disease factors, Eating problems, Functional status.
Data Quality Control
The data collectors and supervisors were trained to ensure familiarity with the questionnaire, and MUAC, weight and height measurements. Instruments were calibrated prior to use to ensure functionality, accuracy and consistency. Patient weight was measured twice and the average of the two measurements taken.
The questionnaire was prepared in English and pretested with 2% participants in Kabribayah health centre, which was not part of the study, and modifications were made as required. To improve the data quality of the data, the data collectors were closely supervised and each completed questionnaire was checked to ascertain that all questions were properly filled and corrected by the supervisors.
Operational Definitions
Undernutrition: This is a condition in which an adult person’s BMI <18.5 kg/m2 or MUAC< 210mm for pregnant and lactating mothers.21
The FANTA (Food and nutrition technical assistance) Guide to Anthropometry is a user-friendly reference that provides update information on anthropometry, the measurement of the human body and how to use it to assess and understand the nutritional status of individuals and populations in low resource settings.22 According to FANTA anthropometry, International classification of adult nutritional status as (mild, moderate, severe, normal, and overweight).
Dietary counseling: This is a process by which a health professional with special training in nutrition helps people make healthy food choices and form healthy eating habits.10
Nutritional support: Nutritional support is having numerous components like nutrition education and counseling in health facilities, water, and hygiene or food safety interventions to avert diarrhea as well as provision of sufficient quantity/quality of food and food aid by any organization.10
Duration of cough and other TB symptoms: The number of days that a cough or other TB symptoms (such as accidental weight loss, night sweats, loss of appetite or fever) has lasted before the diagnosis of TB.4
Functional status: Ability to carry out normal daily activities.10
Working: Able to carry out normal daily activities and no special care needed.
Not working: Unable to work but able to live at home and able to care for most personal needs but requires occasional assistance.
Bedridden: Unable to care for self, requiring institutional or hospital care.
Wealth index: Families are given scores based on the amount and types of consumer goods they own, extending from a television to a bicycle or car, in addition to housing features such as the source of drinking water, toilet facilities and flooring materials. These scores are calculated using principal component analysis.23
Severe thinness, moderate thinness, and mild thinness is a BMI< 16kg/m2, 16–16.99 kg/m2 and 17–18.49 kg/m2.21
Data Processing and Analysis
All collected data were cleaned manually for incompleteness, coded and entered into Epidata version 3.1 and then exported to SSPS version 20. Descriptive statistics (frequency distribution, proportion, mean and standard deviation) was used to summarize variables. All Continuous variables were first transformed into categorical variables before univariate analyzed. Bivariate and multivariable logistic regression was performed to assess the association of variables with undernutrition; variables with a p-value of less than 0.25 in the bivariate analysis were entered into the final model. By calculating odds ratios, their 95% confidence limits and P-value less than or equal to 0.05 were taken as statistically significant. All the assumptions of regression analysis (model adequacy and multi-co linearity of independent variables) were checked to be satisfied using appropriate methods. The absence of multi-co-linearity was checked by using VIF/tolerance. The model adequacy was checked by ensuring Hosmer Lemeshow’s goodness of fit test had P-value >0.05.
Ethical Consideration
Ethical clearance was obtained from the Institutional Review Board (IRB) of Jigjiga University based on Helsinki declaration and an official letter was given to the respective institutions. Study participants gave written consent confirming that they were informed about the purpose of the study.
Results
Socio-Demographic Characteristics of Adult Patients with TB
Out of 302 study participants initially sampled in the study, a total of 296 participated, resulting in a response rate of 98%. Mean age of the study participants was 32 (±SD = 13.243; range 18–70). The majority of participants were male 153 (51.7%); Somali 277 (93.6%) and Muslim 282 (95.3%). Most participants 181; (61.1%) lived in an urban area with a family head of father 242 (81.8%). 151 (51%) of participants were married and 155 (52.4%) were illiterate. 78 (26.4%) participants were students and 158 (53.4%) households had a family size of between 6 and 10 (Table 1).
 |
Table 1 Socio-Demographic Characteristics of Adult Patients with TB in Jigjiga Public Health Facilities, Somali Region, Eastern Ethiopia, April 2020 |
Nutrition Information for Adult Patients with TB
Two hundred and sixteen participants (73%) had received dietary counseling from a health professional that was treating them and only 5 (1.7%) participants had received nutritional support. The majority of respondents 209 (70.6%) consumed a meal three times daily while 22 (7.4%), 52 (17.6%), and 13 (4.4%) consumed a meal once daily, twice daily or more than four times daily, respectively. Based on the source of food consumed, 7 (2.4%) received their food as a gift, 21 (7.1%) ate their own product and 268 (90.5%) purchased their food from the market. Most participants 183 (61.8%) did not practice dietary diversity (Table 2).
 |
Table 2 Nutrition Information of Adult Patients with TB in Jigjiga Public Health Facilities, Somali Region, Eastern Ethiopia, April, 2020 |
Behavior and Lifestyle of Adult Patients with TB
Almost eighty percent of participants did not use substances (chew Khat, smoke cigarettes or drink alcohol) while 7%, 12% and 1% were currently cigarette smokers, Khat chewers and alcohol drinkers respectively (Figure 1).
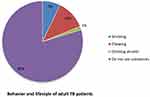 |
Figure 1 Behavior and lifestyle of adult patients with TB. |
Health Status of Adult Patients with TB
The overall prevalence of undernutrition among adult TB patients was 44.3% [95% CI (38.2, 49.7)] Ninety-eight (34.1%) participants had a cough for more than four weeks before being diagnosed with TB and 89 (30.1%) had concurrent breathing difficulties. Moreover, 52 (17.6%) had problems with eating from which 35 (11.8%), 13 (4.4%) and 1 (0.3%) were caused by poor appetite, nausea or vomiting and mouth ulcer, respectively. For the last two weeks or a month before data collection, 33 (11.1%) of the respondents have been ill other than TB, 20 (6.8%), 9 (3.0%) and 4 (1.4%) had gastritis, diarrheal disease and diabetes mellitus, respectively. According to the functional status of the participants 210 (70.9%), 70 (23.6%) and 16 (5.4%) were working, ambulatory and bedridden, respectively (Table 3).
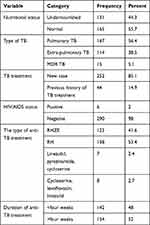 |
Table 3 Health Status of Adult Patients with TB in Jigjiga Public Health Facilities, Somali Region, Eastern Ethiopia, April, 2020 |
Records from Health Facilities
Over half 167 (56.4%) of the respondents were patients with pulmonary TB and 252 (85.1%) were new cases. Over half 158 (53.4%) had been prescribed rifampicin and isoniazid, and 154 (52%) patients had been taking anti-TB medication for more than four weeks. Six (2%) were HIV positive (Table 4).
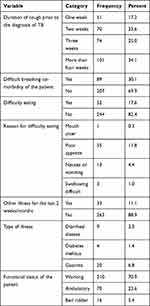 |
Table 4 Record Review of Adult Patients with TB in Jigjiga Public Health Facilities, Somali Region, Eastern Ethiopia, April, 2020 |
The Magnitude of Undernutrition Among Adult Patients with TB Based on FANTA Classification
According to FANTA anthropometry, 2018 International classification of adult nutritional status (mild, moderate, severe, normal, and overweight), the current result revealed that out of those undernourished, 25 (8.4%) were severe, 26 (8.8%) were moderate and 80 (27%) were mildly undernourished but 13 (4.4%) of the respondents were overweight ie BMI > 25kg/m2 (Figure 2).
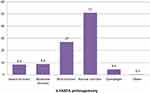 |
Figure 2 Magnitude of undernutrition among adult patients with TB based on FANTA classification. |
Multivariate Logistic Regression Analysis of Factors Associated with Undernutrition Among Adult Patients with TB
In a multivariable logistic regression analysis, female patients with TB were approximately two times more likely to be undernourished than male patients [AOR=1.769, CI=1.035, 3.024]. Respondents who were illiterate were nearly four times more likely to be undernourished compared to those who were literate [AOR=3.939, CI=2.285, 6.792]. Bedridden patients with TB were nearly four times more likely to have undernutrition relative to those who were working and ambulatory [AOR=3.718, CI=1.115, 12.394] (Table 5).
 |
Table 5 Factors Associated with Undernutrition Among Adult Patients with TB in Jigjiga Public Health Facilities, Somali Region, Eastern Ethiopia, April, 2020 |
Discussion
This study found that more than forty percent of participants with TB were undernourished. Moreover, gender, educational status and functional status were factors significantly associated with undernutrition among adult patients with TB. The number of undernourished adults in this study (44.3%) was less than from studies from Brazil (50%)24 and Sri-Lanka (51%).12 This may be due to socio-economic, behavioral and lifestyle differences. In addition, the methodological approaches of these studies may also contribute to the disparity. For example, in the study in Brazil, patients with TB over 60 years’ old were excluded; while in the Sri Lanka study participants were aged between 80 and 100; these disparities in age groups may have a confounding effect on nutrition. The prevalence from our study was also lower than seen in West Tripura (59.1%) and north Karnataka in India (55.8%).25,26 This may be due to the socio-cultural difference of the respondents; moreover, participants of studies in India had higher rates of co morbidityfor example, HIV/AIDS and diabetes mellitus.
Compared to other studies in Africa, prevalence was also shown to be lower than that of studies conducted in Zambia (57%)27 and Malawi (51%).28 This could be due to socio-cultural, behavioral and lifestyle differences. Additionally, these studies included only newly diagnosed patients with TB whereas our study included patients who were on anti-TB medication and had to follow up. Anti-TB medication may have an impact on the nutritional status of patients.
Two studies in Ethiopia have also reported higher rates of concurrent undernutrition among adult patients with TB (57.2%)29 and (63.2%)30 which again are higher than we report. This could be due to the high number of participants from rural areas, where there may be less access to health care and nutrition-rich food. In addition, over half of the respondents of these studies had TB symptoms for more than four weeks prior diagnosis and (11.6%) had co morbidities, for example kidney disease, cardiovascular disease and asthma, which might have an effect on their nutrition.
Two studies performed in Hosanna and Addis Ababa public health facilities identified a prevalence of undernutrition in TB patients of 38.90%4 and 39.7%,10 respectively, which are in line with this study’s results. This may be due to the similar methods used. Furthermore, these studies were carried out in health centers and hospitals that might deliver similar services to the participants as in our study.
Female patients with TB were 1.77 times more likely to be undernourished when compared to male patients with TB. This is in line with another study done in Adama, Ethiopia.31
This could a higher number of female patients who were subjected to social bias compared with male patients, which could compromise their ability to put nutritional counseling given to them by their treating clinicians or health professionals into action. This argument strengthened by
The WHO report in 2019 which indicated that, worldwide, the male to female ratio was 2:1 among patients with TB.32 In addition, more female patients were excluded in social-economic and health opportunities in the African context which might lead to less access to health care, balanced nutrition, safe water and sanitation.
This study found an association between undernutrition and education, as illiterate patients with TB were nearly four times more likely to be undernourished compared to those who were literate. This finding is supported by a study conducted in north Karnataka, India that declared illiterate patients with TB were more likely to be undernourished than those who were literate.25,33 Those with lower levels of literacy may have less understanding about dietary diversity and anti-TB adherence.
Our study stated that there is a significant association between undernutrition and functional status which is consistent with another similar study done in Addis Ababa.10 Bedridden patients with TB were nearly four times more likely to be undernourished compared to those who were working. The functional status of patients is commonly associated with their primary medical situation, and patients with worse functional status often have a poorer health status.34 This situation may result in reduced eating of food which may in turn cause undernutrition.
Limitation of the Study
There were questions allocated to assess behavior and lifestyle factors that might subject to social desirability bias. Moreover, the study was unable to relate undernourished patients with TB to the nutrition centre due to lack of nutrition supply.
Appetite mediators were not evaluated. Half of the patients had been on therapy for >1 month yet therapy improves nutritional status; this could underestimate the prevalence of undernutrition.
Conclusion
The study reports a high prevalence of undernutrition among adult patients with TB in Jigjiga public health facilities. Factors significantly associated with undernutrition were sex, educational status and functional status. Education about nutrition should be improved to include knowledge and dietary counseling of balanced diet. These programs should be targeted for female patients. Appropriate nutrition support or intervention should be given to undernourished patients with TB, female patients and especially those who are bedridden TB patients, routinely. Based on these findings, we recommend nutrition education, prevention of TB transmission at the community level and increasing awareness about the risk factors of undernutrition and TB. Future research should focus on the attitude of adult patients with TB about consuming nutrition-rich foods while on anti-TB treatment.
Disclosure
The authors reported no conflicts of interest for this work.
References
1. Maleta K. Undernutrition. Malawi Med J. 2006;18(4):189–205.
2. Teshome MS, Gissa SB, Tefera BZ, et al. Undernutrition and its predictors among people living with HIV/AIDS attending antiretroviral therapy clinic in Jimma University Specialized Hospital. Int J Nutr Metab. 2017;9(8):67–74.
3. WHO. Nutritional Care and Support for Patients with Tuberculosis. WHO Library Cataloguing; 2013.
4. Geberemeskel T, Woldeyohannes D, Demisie M, Demisie M. Undernutrition and associated factors among adult tuberculosis patients in hossana town public health facilities, Southern Ethiopia. J Trop Dis. 2018;06(01). doi:10.4172/2329-891X.1000253
5. Chang SW, Pan WS, Lozano Beltran D, et al. Gut hormones, appetite suppression and cachexia in patients with pulmonary TB. PLoS One. 2013;8(1):e54564. doi:10.1371/journal.pone.0054564
6. Zheng Y, Ma A, Wang Q, et al. Relation of leptin, ghrelin and inflammatory cytokines with body mass index in pulmonary tuberculosis patients with and without type 2 diabetes mellitus. PLoS One. 2013;8(11):e80122. doi:10.1371/journal.pone.0080122
7. WHO. Global Tuberculosis Report 2020: Executive Summary; 2020.
8. França TG, Ishikawa LL, Zorzella-Pezavento SF, et al. Impact of malnutrition on immunity and infection. J Venom Anim Toxins Incl Trop Dis. 2009;15(3):374–390.
9. Phan MN, Guy ES, Nickson RN, et al. Predictors and patterns of weight gain during treatment for tuberculosis in the United States of America. Int J Infect Dis. 2016;53:1–5. doi:10.1016/j.ijid.2016.09.006
10. Dargie B, Tesfaye G, Worku A. Prevalence and associated factors of undernutrition among adult tuberculosis patients in some selected public health facilities of Addis Ababa, Ethiopia: a cross-sectional study. BMC Nutr. 2016;2(1):7. doi:10.1186/s40795-016-0046-x
11. WHO. Nutritional Care and Support for Patients with Tuberculosis in India. World Health Organization; 2001.
12. Jayasuriya NA, Nanayakkara L, Iddamalgoda N, Derore K. Food security and nutrition among the tuberculosis infected patients. A case study among patients at the chest clinic in Sri lanka. 2014.
13. Annabel B, Anna D, Hannah M.Global Tuberculosis Report; Geneva: World Health Organization; 2019.
14. Papathakis P, Piwoz E. Nutrition and Tuberculosis: A Review of the Literature and Considerations for TB Control Programs. United States Agency for International Development, Africa’s Health 2010 Project; 2008:1.
15. Bekele A. Guidelines for management of TB, DR-TB and Leprosy in Ethiopia. Federal Democratic Republic of Eethiopia Ministry of Health; 2018.
16. Dodor EA. Evaluation of nutritioal status of tuberculosis patiets at the effia-kwata regioal hospital. Ghana Med J. 2008;42(1):22–28.
17. Kanta S, Gupta H, Ahluwalia S. Significance of nutrition in pulmonary tuberculosis. Crit Rev Food Sci Nutr. 2015;55(7):955–963. doi:10.1080/10408398.2012.679500
18. Wondmieneh A, Gedefaw G, Getie A, et al. Prevalence of undernutrition among adult tuberculosis patients in Ethiopia: a systematic review and meta-analysis. J Clin Tuberc Other Mycobact Dis. 2021;22:100211. doi:10.1016/j.jctube.2020.100211
19. Jigjiga city Adminstration office.Estimate Population of Jigjiga City; 2019.
20. Katsilambros N, Dimosthenopoulos C, Kontogianni MD, Manglara E, Poulia KA. Clinical Nutrition in Practice. United Kingdom: John Wiley & Sons; 2010.
21. Cashin K, Oot L. Guide to anthropometry. A Practical Tool for Program Planners, Managers, and Implementers. USAID and FHI36; 2018.
22. Cogill BAIMG. Anthropometric Indicators Measurement Guide. Washington, DC: FHI 360/FANTA; 2003.
23. Yigezu B. Ethiopian Demographic and Health Survey. Central Statistical Agency; 2016.
24. Piva SGN, Costa MDCN, Barreto FR, et al. Prevalence of nutritional deficiency in patients with pulmonary tuberculosis. J Bras Pneumol. 2013;39(4):476–483. doi:10.1590/S1806-37132013000400012
25. Indupalli AS, Sirwar SB, Shaikh K. Nutritional status of tuberculosis cases registered under tuberculosis unit of Gulbarga city, North Karnataka, India. Int J Bioassays. 2013;2(3):616–619.
26. Sarkar M, Baidya S, Bhattacharya H. Undernutrition among pulmonary tuberculosis patients in West Tripura: a Cross Sectional Study. World journal pharmaceutical research. 2016;6(3):782-789.
27. Zachariah R, Spielmann MP, Harries AD, et al. Moderate to severe malnutrition in patients with tuberculosis is a risk factor associated with early death. Trans R Soc Trop Med Hyg. 2002;96(3):291–294. doi:10.1016/S0035-9203(02)90103-3
28. Van Lettow M, Harries AD, Kumwenda JJ, et al. Micronutrient malnutrition and wasting in adults with pulmonary tuberculosis with and without HIV co-infection in Malawi. BMC Infect Dis. 2004;4(1):61. doi:10.1186/1471-2334-4-61
29. Feleke BE, Feleke TE, Biadglegne F. Nutritional status of tuberculosis patients, a comparative cross-sectional study. BMC Pulm Med. 2019;19(1):182. doi:10.1186/s12890-019-0953-0
30. Hussien B, Hussen MM, Seid A, et al. Nutritional deficiency and associated factors among new pulmonary tuberculosis patients of Bale Zone Hospitals, southeast Ethiopia. BMC Res Notes. 2019;12(1):751. doi:10.1186/s13104-019-4786-y
31. Guadie FF. Assessment of nutritional status and associated factors among adult TB patients on directly observed treatment of short course in health facilities at Adama Town, East Shewa Zone, Ethiopia. Scholar Pract Pract J. 2016;1(1).
32. WHO. Global Tuberculosis Report 2019. Geneva: World Health Organization; 2019.
33. Dodor E. Evaluation of nutritional status of new tuberculosis patients at the effia-nkwanta regional hospital. Ghana Med J. 2008;42(1):22.
34. Madebo T, Lindtjørn B. The impact of functional performance, HIV status, malnutrition, and clinical features on treatment outcomes of patients with pulmonary tuberculosis. Ethiop J Health Dev. 2000;14(2):177–182. doi:10.4314/ejhd.v14i2.9918
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
