Back to Journals » International Journal of Nanomedicine » Volume 17
Ultrasound Responsive Smart Implantable Hydrogels for Targeted Delivery of Drugs: Reviewing Current Practices
Authors Sun Y, Chen LG, Fan XM, Pang JL
Received 20 May 2022
Accepted for publication 31 August 2022
Published 22 October 2022 Volume 2022:17 Pages 5001—5026
DOI https://doi.org/10.2147/IJN.S374247
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mian Wang
Yi Sun,1,* Le-Gao Chen,2,* Xiao-Ming Fan,3 Jian-Liang Pang4
1Center for Plastic & Reconstructive Surgery, Department of Plastic & Reconstructive Surgery, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital, Hangzhou Medical College), Hangzhou, 310014, People’s Republic of China; 2General Surgery, Cancer Center, Department of Vascular Surgery, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital, Hangzhou Medical College), Hangzhou, 310014, People’s Republic of China; 3Cancer Center, Department of Ultrasound Medicine, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital, Hangzhou Medical College), Hangzhou, 310014, People’s Republic of China; 4Department of Vascular Surgery, Tiantai People’s Hospital of Zhejiang Province (Tiantai Branch of Zhejiang People’s Hospital), Taizhou, 317200, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiao-Ming Fan, Department of Ultrasound Medicine, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital, Hangzhou Medical College), No. 158 Shangtang Road, Hangzhou, Zhejiang, 310014, People’s Republic of China, Tel/Fax +86-571-85893290, Email [email protected] Jian-Liang Pang, Department of Vascular Surgery, Tiantai People’s Hospital of Zhejiang Province (Tiantai Branch of Zhejiang People’s Hospital), Kangning Middle Road, Shifeng Street, Tiantai County, Taizhou, Zhejiang, 317200, People’s Republic of China, Tel/Fax +86-576- 81302085, Email [email protected]
Abstract: Over the last two decades, the process of delivering therapeutic drugs to a patient with a controlled release profile has been a significant focus of drug delivery research. Scientists have given tremendous attention to ultrasound-responsive hydrogels for several decades. These smart nanosystems are more applicable than other stimuli-responsive drug delivery vehicles (ie UV-, pH- and thermal-, responsive materials) because they enable more efficient targeted treatment via relatively non-invasive means. Ultrasound (US) is capable of safely transporting energy through opaque and complex media with minimal loss of energy. It is capable of being localized to smaller regions and coupled to systems operating at various time scales. However, the properties enabling the US to propagate effectively in materials also make it very difficult to transform acoustic energy into other forms that may be used. Recent research from a variety of domains has attempted to deal with this issue, proving that ultrasonic effects can be used to control chemical and physical systems with remarkable specificity. By obviating the need for multiple intravenous injections, implantable US responsive hydrogel systems can enhance the quality of life for patients who undergo treatment with a varied dosage regimen. Ideally, the ease of self-dosing in these systems would lead to increased patient compliance with a particular therapy as well. However, excessive literature has been reported based on implanted US responsive hydrogel in various fields, but there is no comprehensive review article showing the strategies to control drug delivery profile. So, this review was aimed at discussing the current strategies for controlling and targeting drug delivery profiles using implantable hydrogel systems.
Keywords: smart hydrogels, ultrasound, acoustic energy, cavitation, on-demand drug delivery, localized therapy
Graphical Abstract:
Introduction
The field of controlled drug delivery has faced two significant challenges over the last two decades.1,2 One challenge has been to sustain a therapeutic agent’s zero-order release over an extended period. This objective has been accomplished using a variety of approaches, including osmotically powered pumps, matrices with adjustable swelling, diffusion, or erosion rates, multilayered matrices, and non-uniform profiles of drug loading. The second of these issues is the staggered or pulsatile administration of a therapeutic chemical or protein. Two distinct approaches have been extensively examined as potential solutions to these needs. One is the development of a delivery system capable of delivering a payload at a predetermined period or in pulses following a predetermined sequence.3 The other idea is to produce a system that is capable of responding to changes in the local environment.4 These systems have been demonstrated to alter their drug delivery rates in response to stimuli such as the absence or presence of a specific molecule, ultrasound, magnetic fields, temperature, light, electric fields, and mechanical forces.5,6 These systems are well-suited for the release of drugs whose plasma concentrations are not constant.
Implantable hydrogels are stable three-dimensional hydrogels in which the hydrogel’s polymeric chains (natural or synthetic) are cross-linked via physical and chemical interactions. Chemical crosslinking results in permanent junctions within the polymer network, whereas physical interactions occur as a result of polymer chain entanglements or as a result of physical interactions including hydrogen bonding, hydrophobic or ionic interactions. Due to hydrogels’ biomimetic properties, they have numerous applications in biomedical engineering, such as cell culture, drugs, and therapeutics delivery. Hydrogels are polymeric networks that absorb water and have been designed to imitate many of the inherent characteristics of soft tissue.7 Hydrogels, for example, can be designed to mimic the rheological, mechanical, and biochemical properties, as well as the stimulus responsiveness, of soft tissue.8,9 Smart hydrogels are capable of structure and volume phase transitions in response to external stimuli, opening up huge possibilities for scientific investigations and a variety of advanced multidimensional technology applications.10 The emergence of smart hydrogels (as functional materials) has revolutionized the field of study of stimuli-responsive hydrogels.11 In general, smart hydrogels are absorbent to superabsorbent materials that can respond to small changes in the environment, such as temperature, pH, ionic strength, chemical species, biological conditions, electric field, and ultrasound.12–16
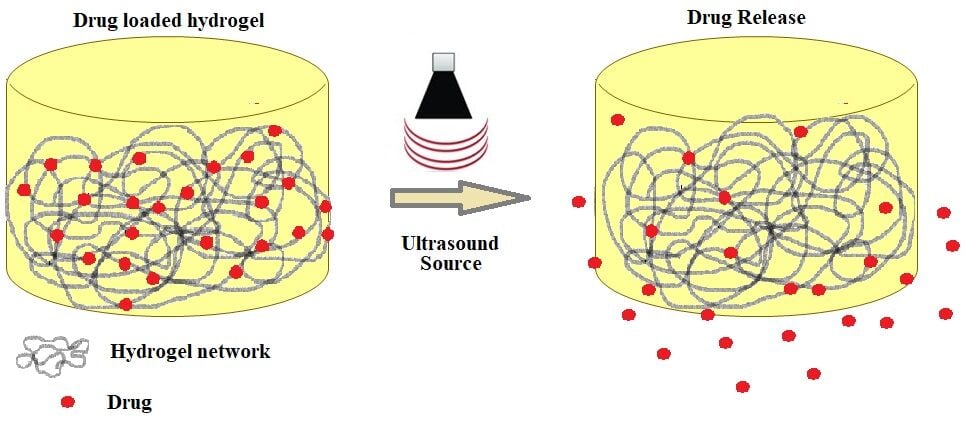
Ultrasound (US) is a term that refers to a sound that is beyond the limit of the human hearing range and has a variety of applications, including mixing, cleaning, and imaging.17 Since US waves are non-invasive and have a low-risk profile, they are frequently used as exogenous stimuli. Ultrasound-induced mechanical and thermal effects are speculated to be the primary mechanisms underlying drug release from carriers (Figure 1).18,19 In drug delivery, US allows for highly precise temporal control of release, typically occurring in real-time during the insonation period.20 Its uses with hydrogels are particularly prevalent in the biomedical field (eg, drug delivery, cancer treatments).21–24 Because of their interaction with ultrasonic waves, implanted hydrogels can be activated and let to burst, allowing drug loads to be released into targeted tissues.25 US has a great spatial resolution and is capable of delivering stimuli to extremely precise locations, including those deep within the body.26 In general, cavitation, including inertial cavitation, stable cavitation, and sonoporation, has been a primary cause of US-triggered drug release.27 Typically, activation of the US is irreversible, ie, it restricts the on-off control offered by other triggers.28 As a result, polymers capable of self-healing are beneficial when utilized with this trigger. Huang et al, for example, developed Fe3+-[PEG-Dopa] hydrogels based on coordination bonds between mussel–mimetic metals and catechol. These hydrogels have a moderate bonding strength, balancing powerful mechanical capabilities with the potential for disturbance by US.23 One network of the hydrogels with a double-network structure, used by Sun et al, provides mechanical stability, while another network, broken by US, triggers the drug release that is bound to polymers by dynamic covalent bonds.29 According to some studies, nanoparticles can act as temporary barriers, separating input stimuli from the components of an inactive hydrogel network till certain circumstances are met. For example, calcium-loaded liposomes can be ruptured by US, leading to ionic crosslinking in hydrogels composing alginate or calcium-dependent transglutaminase activation in matrices composing fibrin.30 US’s usefulness and safety are required for clinical drug release. While using the low-intensity US simplifies setup and minimizes harm to nearby tissues, it unintentionally prolongs treatment time and lowers release efficiency.31
 |
Figure 1 A description of the physicochemical effects of US that result in biological effects at the cell/tissue level. Notes: Reproduced from Entzian K, Aigner A. Drug delivery by ultrasound-responsive nanocarriers for cancer treatment. Pharmaceutics. 2021;13:1135. Copyright © 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).32 |
Acoustic and Attenuation Properties of Materials
Acoustics is the study of energy transfer through a material by mechanical waves. In general, an acoustic wave is produced by utilizing a transducer in a medium, which translates electrical impulses into vibrations transmitted across the medium. These vibrations travel through the medium as compression and expansion mechanical waves.33 It is essential to know the interaction of these waves with any objects or boundaries that can be present to implement several applications. The acoustic and geometric features of a medium determine the efficiency with which energy can be transferred between two places. This also enables the appropriate structuring of materials to govern wave and energy propagation. Typically, materials’ acoustic impedance (Z = ρc) determine their acoustic properties, which quantifies the medium’s resistance to movement when subjected to excitation with a fixed pressure. Where c denotes the material’s sound speed and ρ is the equilibrium density of the fluid. For a given excitation pressure, a high impedance material will have a lower velocity of the particle.34 In a low impedance material, on the other hand, a surface with an amplitude having fixed vibration produces lower pressures. The interface’s wave behavior between different media is determined by acoustic impedance. Acoustic waves pair more efficiently with mediums with similar impedances. As a result, the acoustic impedance of an interface impacts how intensely ultrasonic waves are reflected or scattered. The acoustic impedance has significant implications when developing acoustic systems, for example, resonators or attempting to design materials triggered through the use of sound. The table shows the values of Z, c, and ρ, for various common materials as a reference.
The coefficient of material attenuation is another significant material attribute to consider when developing acoustic systems. Attenuation is the irreversible acoustic energy loss to heat caused by several factors, including molecular relaxation or viscosity.35 When an acoustic wave propagates through a material, the pressure’s amplitude after a distance (L) is determined by p = p0e−αL, where p0 denotes the wave’s initial pressure and α is the coefficient of attenuation (neper per centimeter). The coefficient of attenuation is more frequently represented as a = 8.7α, which is expressed in decibels per centimeter. Attenuation is mostly frequency-dependent, with higher frequencies exhibiting greater attenuation than lower frequencies. Typically, empirical models depict this dependency as a power law: a(f) = a0 fγ, where 0 < γ ≤ 2. To characterize a material’s attenuation across all frequencies, all that is required is knowledge of the value of “a” at a single frequency, the frequency of detection of a, and the power γ. In the case of liquids such as water, γ = 2, however in the case of certain polymers such as PMMA, the relationship is nearly linear (γ ≈ 1).36,37 Table 1 summarizes the attenuation coefficients and measuring frequencies for a few selected materials.
 |
Table 1 Acoustic Properties of Selected Materials33 |
Factors Affecting Acoustic Properties of Sound-Absorbing Materials
Sound absorbers are materials that can absorb a significant amount of sound energy. Sound absorption occurs when sound waves pass through a porous material, resulting in a reduction in sound energy owing to pore wall friction and thermal exchange. Certain factors, including size, porosity, temperature, flow resistivity, thickness, density, compression, and design, significantly affect the acoustic properties of such materials and their nanosystems. Hydrogel polymers are a promising candidate for sound absorption in the transportation and construction industries due to their low cost and biocompatibility. The frequency of sound absorption is determined by the pore size of a sound absorber.38 Compared to the wavelength of sound (which ranges from 5 cm to 15 m), the average pore size of sound-absorbing material is much smaller, at less than 1 mm. Gas molecules present in the porous material oscillate in response to the sound pressure, causing a loss of momentum due to friction. This reduces the momentum of the sound wave of high frequency as it propagates through the porous structure.39 Generally, it has been asserted that tortuosity, porosity, and airflow resistivity are the most important properties for effective sound absorption.40 Recent research suggests that introducing an additional porosity level, for example, dual porosity, may increase the acoustic absorption of such materials.41 Introducing slits into samples of aerated autoclaved concrete improved acoustic absorption by a factor of 1.5–2.42 Recently, studies have continued to include various particles, including hemp,43 reed,44 and polymer foam particles,45 into the materials of composites to introduce varying levels of porosity and enhance the material’s acoustic impedance. The average pore size and overall porosity have a significant impact on acoustic absorption, but sample thickness has a significant effect as well.46 A group of researchers developed the hydrogel–cement composite via blending into millimeter or sub-millimeter sized gel beads.47 Composites with high porosity and a free pore structure had improved sound absorption compared to nonporous control samples. When compared to sound absorption at frequencies ranging from 400 to 1000 Hz, porous PDMS and porous cement exhibit better absorption at refequencies ranging from 200 to 400 Hz and 1200 to 1800 Hz. These findings led to the development of a highly effective US-responsive hydrogel.
In terms of acoustic impedance, hydrogels are almost perfectly matched to water and soft tissues (1.45–1.70 MPa s m−1) owing to their high water content.48 Impedance matching ensures optimal acoustic wave transmission and minimal acoustic reflection at the interfaces. On the basis of hydrogels’ unique acoustic properties, various acoustic machines and devices can be established, such as US coupling agents between skin and probe,49 acoustic camouflages that are undetectable by sonar systems,50,51 and underwater acoustic microphones.48 Acoustic coupling medium of hydrogel was studied as an alternative to water in clinical application of US therapy applications by Adrian et al.49 From 10% to 20% weight in volume, it was found that polyacrylamide had good acoustic features that increased linearly with the concentration of acrylamide. At 1 MHz, the attenuation coefficient, sound velocity, and impedance varied between 0.08 and 0.14 dB/cm, 1546 and 1595 m/s, and 1.58 and 1.60 Mrayl, respectively. A sheep model (in vivo) intraoperative hemostasis experiment revealed that the gel-coupled transducer could induce hemostasis in splenic and hepatic incisions with active bleeding. This study indicates the potential of polyacrylamide to be used as an effective coupling material for FUS therapy. Tough hydrogels filled with water, air, or liquid metal were used by Zhang et al to achieve acoustic properties that are comparable to water, air, and soft solids over a wide range of frequencies.51 In addition to providing unprecedented broadband configurable acoustic propagation, the proposed metagel is cost-effective, ecologically friendly, and human-body compatible. Opportunities for the design and application of future acoustic materials are opened up by these metagel systems. Even though the current metagel sheet uses millimeter-sized parallel channels, more modern 2D or 3D networks with finer characteristics could lead to improved tuning and acoustic properties. For future metagels, more filler media including silicone oil or glycerol may be investigated. The Metagel’s tunable impedance match with various soft solid materials and water over a wide frequency range will aid in the design of novel Medical imaging and underwater acoustic devices.
Tissue-mimicking materials with distinct soft tissue characteristics are necessary for testing therapeutic efficacy. Braunstein et al developed a hydrogel based on poly(vinyl alcohol) (PVA) utilizing various concentrations (5–20% w/w) and molecular weights of PVA ± cellulose scatterers (2.5–10% w/w).52 The acoustic (ie, attenuation, sound speed) and thermal (ie, thermal conductivity, specific heat capacity) properties of hydrogels, as well as their cavitation thresholds, have all been investigated. Analyses of fresh sheep tissue were used to compare the results (kidney, liver, spleen). PVA concentration and attenuation, as well as the content of cellulose, had the greatest impact on sound speed. For the investigated formulations, the acoustic properties of PVA gel (sound speed: 1532 ± 17 to 1559 ± 9 m/s, attenuation coefficient: 0.08 0.01 to 0.37 0.01 dB/cm) were comparable with those of fresh tissue. Cavitation thresholds for 10% PVA hydrogels (50% occurrence: 4.1–5.4 MPa, 75% occurrence: 5.4–8.2 MPa) decreased as cellulose content increased. The bottom line is that PVA cellulose composite hydrogels might be appropriate for a variety of therapeutic US applications because they mimic the cavitation, acoustic, and thermal properties of soft tissue well. For example, Zell et al examined the acoustic characteristics of four different materials for use in the development of tissues (especially breast tissue) phantoms for photoacoustic and US imaging,53 which include agar, polyvinyl alcohol gel (PVA), silicone, and polyacrylamide gel (PAA). Measurements of sound wave transmission under controlled circumstances are used to determine the acoustic properties, ie, impedance, sound speed, and acoustic attenuation. To determine the acoustic attenuation over the 4 to 14 MHz frequency range, two US sources at 5 and 10 MHz were employed. Agar, PVA, and PAA have comparable acoustic properties to water. In silicone polymer, the sound velocity and acoustical attenuation were significantly lower than in human tissue and water. Tissue-mimicking phantoms can be made from any of these materials, which can be cast in any shape. As a result of its lower sound speed, silicone is generally less appropriate than the other materials presented.
Mechanism of Ultrasound-Induced Drug Delivery
Despite extensive studies on drug delivery assisted by US and its uses in therapeutics, the process through which drugs are delivered using US remains a mystery. However, most likely, the mechanism is related to the non-thermal and thermal effects of US radiation energy.54 This section emphasizes the importance of these two consequences. US has an immediate hyperthermia effect due to the absorption of sound vibrations. Because of their high ultrasonic absorption capacity, dense tissues such as bones experience comparatively higher temperature increases than muscle tissues.55 Methods for inducing hyperthermia include high-intensity focused ultrasound (HIFU) and FUS.
Focused US: In order to generate hyperthermia, FUS is preferable to other methods because of its extreme precision and capability to deposit energy, allowing targeted therapy of deep tissues noninvasively via focusing US and the resulting localized heating.
HIFU is used to thermally ablate a tumour or any diseased tissue, and the increased temperature of the tissue can be monitored using MRI, interstitial thermocouples, or US imaging, allowing us to control the temperature to treat the tumour accurately. The local hyperthermia effects are transient, specific, and have the potential to induce FUS as an adjunct drug therapy. The hyperthermia induced by FUS can deeply penetrate the body, resulting in diverse clinical effects, including tumor shrinkage, and is also reported to stimulate the immune system against some tumors.56 The thermal effect results in a cell membrane disruption and an increase in the blood vessel’s permeability.57,58 With the targeted triggered release from polymeric micelles or microbubbles and thermosensitive liposomes, this hyperthermia technique has demonstrated considerable improvements in tumor therapy.59–61 Utilizing the thermal effects of US in drug delivery systems (DDSs) that respond to heat can increase drug concentrations at target sites and improve the effectiveness of therapy.
The non-thermal effect is predominantly related to cavitation as a second possible method of delivery for drugs using US. Cavitation can exist in cavitation nuclei or native microbubbles such as nanobubbles or microbubbles.62 Cavitation occurs in two distinct forms (inertial and stable cavitation, Figure 2), reliant on various parameters, including surface tension, pressure, and US frequency. Stable cavitation results via bubble oscillation in a similar manner to the frequency of applied US.63 To break particles or penetrate tissue cells, this oscillation produces microstreaming (an extremely fast-moving and shearing fluid flow).64 Ultrasound-responsive compounds can be used to enhance this non-inertial cavitation (such as microbubbles, nanodroplets, nanobubbles, etc.) utilized for therapeutic purposes.65 On the other hand, bubbles expand rapidly and eventually collapse when negative pressures hit a certain threshold. Inertial cavitation is the term used to describe the phenomenon. The release of drugs from drug-loaded block copolymer vesicles or micelles triggered by US was most likely facilitated by shear stress and shockwaves generated by bubble collapse.66 The UTMD (ultrasound-targeted microbubble destruction) practice uses this effect to improve drug delivery.67 The disintegration of bubbles filled with gas generates shockwaves, resulting in extremely high pressures and a temperature rise of up to 5000 K at the cavitation spot, representing the extreme local environmental conditions. These unique conditions can result in light burst emission (sonoluminescence), the disruption of tissue (employed for cellular sonication and sonophoresis for improved drug delivery), and the formation of reactive oxygen species (ROS) owing water pyrolysis, which causes chemical reactions.68 Therapeutic agents can enter cells via the increased permeability of the cell membrane caused by the US-induced intracellular production of ROS. The high toxicity of ROS and their role as apoptosis signalling molecules in cancer imply that they can cause immediate cell death in tumors.69
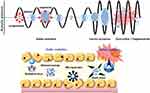 |
Figure 2 The mechanism of ultrasound-induced cavitation effects for drug delivery. Notes: Reproduced from Liu H-L, Fan C-H, Ting C-Y, Yeh C-K. Combining microbubbles and ultrasound for drug delivery to brain tumors: current progress and overview. Theranostics. 2014;4:432. Creative Commons Attribution (CC BY-NC)License.73 |
Cavitation activity can have a variety of biological effects, such as sonoporation and increased vascular permeability.70 The former, sonoporation, is a process that improves intracellular drug uptake due to enhanced irradiated cell membrane porosity and permeability.71 In transdermal drug delivery, this permeabilizing effect is referred to as phonophoresis or sonophoresis. The latter, increased Vascular permeability, is the topic of clinical and preclinical studies aimed at non-destructively opening the blood-brain barrier (BBB).72 The targeted disruption of the BBB through US has been proved to be an effective method of delivering therapeutics to the brain.
Influencing Factors in US-Mediated Drug Delivery
Frequency
Depending on the type of tissue being treated and the intended disease, US waves can penetrate at frequencies from the kilohertz range to the megahertz range. Both high and low frequencies have their advantages and disadvantages when it comes to tissue penetration. Low frequency has less resolution but more tissue penetration, whereas high frequency has excellent resolution but limited tissue penetration [for example, transdermal drug delivery (TDD) requires 55 KHz US waves for increased skin permeabilization]. A frequency of 2.2 MHz is necessary for intravascular thrombus dissolution when using 13 MHz for hyperthermia-based cancer therapy. The therapeutic US frequency is lower than the diagnostic US frequency.74
Lower ultrasonic frequencies reduce attenuation, allowing for deeper tissue penetration and, ultimately, a superior therapeutic effect.75 For microbubble cavitation generation, it is necessary for the ultrasonic frequency to be close to the microbubbles’ resonance frequency. Utilized microbubbles determine the US frequency to be applied. Higher acoustic pressures induce inertial cavitation, which results in the collapse of microbubbles.76 Low frequencies and high acoustic pressures tend to increase the intensity of inertial cavitation.
Intensity
US of high intensity induces hyperthermia and has a potential for tissue modification. The US Food and Drug Administration (FDA) has authorized the use of intensities producing a temperature increase of 1 °C or less. The therapeutic US frequency range is 0.33 W/cm2. But one can also lower the higher intensity by reducing the average intensity over time (duty cycle 3 US intensity) by shortening the pulse duration.75
Duration of Treatment
To prevent overheating, the duration of treatment is governed by producing ultrasonic cavitation and sonoporation based on stable or inertial cavitation. In addition, the time of US for drug administration relies on factors including the type of carriers employed, the number of carriers, the location of the tissues, and the US intensity administered during the therapy. Prolonged treatment periods, inertial cavitation, high acoustic pressure, and constant doses of microbubbles can improve the drug delivery potential; nevertheless, high pressure can also cause unintended tissue damage. While lower pressure necessitates longer treatment durations, which, in turn, generates a large amount of heat. Therefore, the treatment frequency and duration must be optimized for each application.75
Key Factors of Implantable Hydrogels Applied in Drug Delivery
To meet the bioactivity and biocompatibility requirements of practical applications, hydrogel polymers can be produced from endogenous components found in organisms, such as macromolecular proteins, glycosaminoglycans, and DNA nucleotides. Additionally, a variety of synthetic polymers, such as HPMC, PEG, and PVP, can be used as hydrogel materials for bone tissue engineering.77 While these biocompatible materials are nontoxic and have a negligible effect on cellular activities, they are unable to significantly stimulate cell migration, differentiation, and growth. Thus, developing additional variables that enhance cell viability and mechanical properties would be a significant step toward bone tissue repair in the case of osteoarthritis, fracture disease, or bone defects.
Since implantable hydrogels possess the necessary physicochemical characteristics for implantation in the body (in situ), they have gained considerable interest in tissue engineering, drug administration, as well as dermal fillers.78,79 Therefore, a critical component to consider is the solution’s viscosity of the polymer, as this property is helpful for minimally invasive surgical techniques. Certain hydrogels can cause a range of immune-induced, inflammatory, local, and systemic adverse effects that manifest early or late, demonstrating the critical importance of biocompatibility and non-toxicity in an implantable hydrogel system. The porosity of the hydrogel is another critical factor, with densely linked networks being favored for improved nutrition transport and adaptation to adjacent tissues. It also applies to the hydrogel’s mechanical characteristics (modulus and compressive stress, tensile strength, and modulus, storage, the stress of shear, loss moduli, stiffness, density, mesh size, fragility, and many more), as the hydrogel must endure the repeated deformation that appears in the body’s mechanical dynamic environment.80 Multiscale properties of hydrogels have been provided in Figure 3.
Implantable hydrogels are typically mm to cm in size. They are typically surgically implanted into the body or put into direct connection with it to enable drug administration via transepithelial pathways.81 The clinical application of hydrogels implanted surgically for drug delivery applications has been successful, as demonstrated by INFUSE, a collagen gel of type I that induces the release of recombinant human bone morphogenetic protein 2 (BMP2) when implanted into the body for the therapies of long bone fractures and spinal fusion.82
The skin, mucosa, and intestinal epithelium have all been used as epithelial barriers for drug delivery. While these are impermeable to macrohydrogels, they may be permeable to hydrogel-released drugs. Hydrogels, which include those made of synthetic polymers, for example, poly(hydroxyalkyl methacrylate) and poly(vinyl alcohol) as well as biopolymers like collagen, chitosan, and alginate, are commonly utilized as wound dressings.83–86 The potential for adapting these materials for transdermal delivery of the drug is enormous, and hydrogels have already been employed to carry proteins like calcitonin and insulin.81 Hydrogels of alginate, similar to those used to treat wounds for decades, have been proven to deliver potential drugs in a controlled manner to enhance wound healing.87 Wound dressing materials based on fabricated alginate hydrogel (Alg) containing H2S have been found to be potent wound dressings which induce wound closure up to 98 ±1.22%, hence could be applied as the bioactive wound dressing.88 Clinical trials are now being conducted on several of these materials. A multicenter clinical trial demonstrated the safety and efficacy of a hydrogel-based delivery system for recombinant human granulocyte macrophage colony stimulating factor (rhGM-CSF) in the treatment of deep and second-degree burn wounds.89
The hydrogel needs to be injected into the body if the drug delivery target location is found deep inside the tissue or if the biological barriers are impermeable to the selected drug. Using hydrogels, drugs can be delivered directly to where they are needed, bypassing epithelial barriers on their way to the site of action.90,91 Surgical implantation, on the other hand, is an invasive procedure that may result in patient discomfort and surgical risks.92 To solve this challenge, substantial attention is being made for the preparation of injectable hydrogels. These are mostly based on the concepts of body gelation and outside the body gelation occurs but transitions to a flow able condition upon the introduction of appropriate shear stress to permit injection (shear-thinning hydrogels) or external gelation to a collapsible physical form suitable for minimally invasive delivery, followed by in vivo shape recovery (shape-memory hydrogels).
The conventional crosslinking approaches used to synthesize ordinary hydrogels are also used to develop implantable hydrogels. Until now, implantable hydrogels have been cross-linked via a variety of distinct synthesis processes, and chemical and physical linkages have been discovered and coexisted within their chemical structure.93,94 Physical interactions include electrostatic interactions including hydrophobic interactions, van der Waals forces, π-interactions, electrostatic forces, hydrogen, and ionic bonds. Hydrogels with only or primarily physical interactions may be prepared easily without the addition of reactive chemicals, although they are slightly too weak mechanically to withstand long-term exposure to ambient influences such as temperature and pH when injected into the body. Diels–Alder reactions, Schiff base reactions, Michael-type additions, enzyme-mediated reaction, disulfide crosslinking/ thiol exchange, and click chemistry, on the other hand, can be included in injectable hydrogels where chemical bonding is the dominating force. External stimuli (pH, light, temperature, US, enzymes, electric and magnetic fields) can activate these hydrogels, and they have been reported to have significantly improved mechanical stability as well as physicochemical characteristics, as well as increased endurance over time, due to the developed strong covalent bonding.
Generally, chemical crosslinking agents are used to prepare implantable hydrogels based on HA, such as 1,4-butanediol diglycidyl ether (BDDE) and divinyl sulfone (DVS) to overcome HA’s very short half-life in the body, which is several hours because of its rapid enzymatic breakdown by hyaluronidase.95 Furthermore, despite the utilization of chemical cross-linkers, the longevity, and stability of these hydrogels are typically just 6 months in vivo, necessitating frequent implantation to maintain efficacy.96 As a result, there is an unmet demand for the development of new implantable materials that are both safer and longer-lasting.
Advantages of Ultrasound-Responsive Implantable Hydrogel-Based Drug Delivery Systems
Implantable drug delivery systems (IDDSs) are very promising for a variety of drug classes, especially those that cannot be administered orally, have irregular gastrointestinal absorption, or benefit from site-specific dosage. Examples include chemotherapeutic agents, steroids, analgesics, antibiotics, contraceptives, and biologics such as heparin or insulin.97–99 Typically, implants are placed in intramuscular or subcutaneous tissue using specialized insertion equipment, needles, or surgery. Intramuscular or subcutaneous tissue are excellent locations for the implantation of drug-depot devices owing to the presence of higher fat content, allowing for limited innervation, slow drug absorption, adequate hemoperfusion, and a lower risk of localized inflammation (minimal reactivity to foreign material insertion).100 Apart from subcutaneous implantation, numerous other body regions, mainly for delivery to targeted tissue, such as intravascular, intravaginal, intraocular, intracranial, intrathecal, and peritoneal, have also been effectively used as implantation sites.101 Perhaps the most often used clinical use to date is the use of the carotid or cardiac arteries as delivery sites for drug-eluting stents, which deliver therapy to intravascular regions.
US has been employed for drug release and imaging due to its excellent biocompatibility and non-invasive nature. US can exert non-thermal (mechanical) effects as well as thermal effects. The thermal method is often used for tumor ablation and hyperthermia. Mild hyperthermia increases vascular permeability and blood flow. Drugs can enter solid tumors more easily when blood pressure rises because nanocarriers move more easily toward the tumour locations.102 In tumor ablation, focused high-intensity US raises the temperature locally and promptly above 60 °C, providing energy for the tumor cells’ necrosis on the local level. Often, the mechanical action is mediated by microbubbles containing drug carriers attached to their shells. The therapeutic payloads are shed from the shells of the microbubbles as their volumes change continuously.103 Additionally, improved fluid convection, caused by mechanical stress and micro streaming, promotes drug delivery to cells. In most cases, the US-sensitive substance enters the bloodstream during drug delivery by US. Focused ultrasonic waves on tumor tissue not only increase blood vessel permeability for drug-loaded nanocarrier infiltration but also promote drug delivery via thermal or mechanical effects.104 Unlike the previously indicated technique, we have emphasized local implantable systems that incorporate sonosensitive materials in this review paper. As a result, tumor suppression and therapeutic effects are exerted in distinct ways, as discussed more below.
In localized tumor therapy, US modifies the implanted platform structurally to allow drug release or increases cargo release directly through diffusion.105,106 Meng et al developed a hydrogel system that responds to US for the delivery of nanovaccines against breast cancers in a study.105 In the suggested personalized immunotherapy strategy, ultrasonic irradiation caused a gel-to-sol change, resulting in the release of the vaccine. Without US irradiation, the self-healing hydrogel was able to return to its previous state. As a result, the controlled remote US stimulation allowed repetitive vaccination administration from the implantable hydrogel. Therefore, an anticancer immune response was induced. The loading efficiencies of ovalbumin, as a model antigen for tumors, and imiquimod, as an adjuvant for the immune system, into the nanovaccines were 80% and 42%, respectively. The average tumour volume of mice treated with a single injection of a nano vaccine containing hydrogel and multiple rounds of US treatment was considerably less than that of mice treated with the conventional vaccination method (several free antigen injections). After 240 days, 42.8% of the former group’s mice survived. They experimented with synergic anticancer therapy (a combination of tumor vaccine and checkpoint blockade therapy). They demonstrated that the combined therapy significantly prevented tumor recurrence, with 60% of mice surviving 160 days after treatment. In vivo investigations revealed that the suggested hydrogel with self-healing properties could inhibit tumor recurrence and metastasis. Additionally, the proposed approach improved patient compliance, as compared to numerous injection vaccinations.
Another advantage of ultrasound-responsive implanted systems is their use in sonodynamic therapy (SDT). In this treatment approach, the production of reactive oxygen species is generated locally as a result of US irradiation using sonosensitizers. According to She et al’s design, hydrogel development was induced in situ at the temperature of the human body.106 The precursor solution contained catalase coupled with the sonosensitizer meso-tetra (4-carboxyphenyl) porphine (TCPP), beta-glycerol phosphate disodium (GP), and chitosan. Catalase’s presence relieved the solid tumor’s hypoxic status and improved SDT efficacy. US stimulation of TCPP-catalase release during the first round did not influence subsequent turns. Mice with tumors treated with a single dosage of TCPP-catalase-chitosan/GP and three rounds of US irradiation recovered in two weeks and survived for four months. Injection of free TCPP or free TCPP/catalase into tumors and irradiation with US in numerous rounds demonstrated rapid growth. The hydrogel kept the sonosensitizer around the tumor site, but free TCPP or TCPP-catalase was rapidly removed from the solid tumor. Other examples of US-activated platforms for drug release are shown in Figure 4.107,108
 |
Figure 4 Representation of the US mediated release by vibration enhancer. The microgels contain therapeutic compounds and vibration enhancers. Notes: Reproduced from Kubota T, Kurashina Y, Zhao J, Ando K, Onoe H. Ultrasound-triggered on-demand drug delivery using hydrogel microbeads with release enhancer. Materials & design. 2021;203:109580. Under a Creative Commons Licence.107 |
Current Therapeutic Applications
US, as a non-invasive, real-time diagnostic approach, has enabled the development of a novel therapeutic strategy for targeted delivery. With the rapid progress of US technology and US contrast agents (UCAs), spatiotemporally easily controlled US applications with or without UCAs enable site-specific therapeutic agent delivery and targeted modulation with minimal adverse effects, indicating a promising therapy in clinical use. US alone, such as HIFU, could be utilized as a noninvasive therapy approach for tumor thermal ablation, and low frequency focused ultrasound (LIFU) could stimulate bone fracture repair.109,110 In comparison to current disease treatments, such as chemotherapy, cell or gene therapy, and US-triggered therapy has the potential to raise the concentration of therapeutic agents in targeted tissues while minimizing biotoxicity.111 Ultrasonography is one of the most extensively utilized techniques because of its advantages in real-time, portability, noninvasiveness, and low cost. Another outstanding feature of US is its ability to penetrate deeper into tissue than light.112 Furthermore, US may be used in conjunction with a variety of conventional approaches to improve treatment.
Eng et al describe the accidental discovery of an initiator-free photohydrogelation method for linear poly(ethylene glycols) (PEGs) containing methacrylate and coumarin end groups.113 Additionally, they demonstrated the critical involvement of PEGs in this UV-induced gelation that occurs without the use of an initiator. Coumarin groups have been found to have a rheological effect on the hydrogel flexibility and network strength via elongation due to cycloaddition spacing. This technology was used to prepare a variety of hydrogels with varying molecular weights. The same chemistry was used to react with a PEG-containing dicoumarin with various weight ratios of PEG dimethacrylate. Various forms of US triggers could cause the gels to partially degrade. Coumarin photodimers (CPDs) partially reversed to the coumarin form in the polymer solution when 30 kHz US was applied. Breakdowns induced by sonication at 30 kHz and focused US at 1.1 MHz with high intensity were detected at PEG chains in gel states, with no conversion of CPDs to coumarin. They concluded that this strategy may be used to design a controllable delivery vehicle for hydrophobic compounds where traditional photohydrogelation using a photoinitiator and the following presence of its byproduct might result in migration and toxicity issues in the surrounding environment.
Jiang and Kobayashi described an ultrasound-induced drug release from a chitin hydrogel matrix containing gallic acid (GA), a compound utilized in anticancer and wound healing treatments.114 Hydrogels of GA-chitin were formed by phase inversion from a chitin-dimethylacetamide (DMAc)/lithium chloride (LiCl) solution containing GA and exposed to water vapor for 24 h. The GA release from the GA-chitin based hydrogel was investigated using US with a power range of 0–30 W at a 43 kHz frequency. The effect of the amount of GA loaded into hydrogels of 0.25, 0.43, and 0.54 mg/cm3 and concentrations of chitin of 1, 0.5, and 0.1 wt% on the releasing pattern was measured under US exposure (43 kHz) at 30 W. The findings indicate that US enhanced the efficiency of all samples’ release. Additionally, the efficiency of release improved linearly with the increasing power of US, the amount of GA loading, and the reduction in chitin concentration. The maximum rate of release was 0.74 μg/mL·min from a hydrogel loaded with GA at a concentration of 0.54 mg/cm3 formed using a chitin 0.1 wt% mixed solution at 30 W, under US exposure (43 kHz): It is nine times higher than the sample that was not exposed to US. The viscoelasticity of the hydrogel revealed that the material was rigidified by the US irradiation. US was found to be capable of breaking the hydrogen bonds in the hydrogels of GA and chitin using FT-IR. These findings suggest that US can be employed to promote the delivery of GA by chitin hydrogels.
Huang et al described the production and disintegration of stimuli-responsive hydrogels based on PEG and telechelic Dopa via US.23 Fe3+-[PEG-Dopa]4 hydrogels are prepared by Fe3+ stimulated cross-linking of precursors of PEG-dopamine with four arms to produce networks as shown in Figure 5. The [Fe3+]: [Dopa] molar ratio can be used to control the amount of H-bonds, covalent bonds, and coordination bonds in precursor solutions. Networks produced from precursors with a high ratio of [Fe3+]:[Dopa] form mechanically resilient networks (G′ = 6880 ± 240 Pa) that are substantially impervious to disintegration by the US at a ≤43 W/cm2 intensity. On the other hand, networks established at molar ratios of [Fe3+]:[Dopa] of <0.73 are prone to fast disintegration when exposed to the US. Pulsatile exposure of US enables temporal control of hydrogel disintegration and self-healing that is programmable. Sustaining US energy also has the potential to stabilize hydrogels by forming additional cross-links through pendant catechol coupling mediated by free radicals. Taken as a whole, the various ranges of mechanical properties, the capability of self-healing, and differential susceptibility to disintegration by US demonstrate that Fe3+-[PEG-Dopa]4 hydrogels produce class polymers that respond to stimulus with application-specific properties as smart materials for uses ranging from transient medical implants to drug delivery matrices.
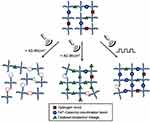 |
Figure 5 Designed US-induced responses of Fe3+-[PEG-Dopa]4 Hydrogels. Notes: Reproduced with permission from Huang W-C, Ali F, Zhao J, Rhee K, Mou C, Bettinger CJ. Ultrasound-mediated self-healing hydrogels based on tunable metal–organic bonding. Biomacromolecules. 2017;18:1162–1171. Copyright © 2017, American Chemical Society23. |
An et al designed a depot based on hyaluronic acid (HA) hydrogel that provides thermal elevation induced by US and cargo release on demand.115 The HA-based hydrogel was produced by using the gold cluster as a sonothermal crosslinker that was formed on the cargo to keep it from leaking until it was dissociated by US. The results indicated that when US at 30 W was used, the HA-based hydrogel considerably elevated the temperature to 53.7 °C, resulting in gold cluster dissociation and subsequently cargo release. Additionally, the produced hydrogel displayed adequate mechanical properties and enhanced biostability for in vivo applications as an implanted hydrogel. On the other hand, Gerayeli et al revealed that ultrasonic stimulation at a low frequency directly applied to a hydrogel at energy less than the threshold for cavitation could potentially control a therapeutic molecule’s release.108 The hydrogel containing the molecules was encapsulated inside a hollow acoustic horn. The acoustic horn’s harmonic modes, in conjunction with having a physical gel framework, trigger a flashing ratchet, releasing all of the trapped molecules in less than 90s at 1.5 W cm−2 an intensity (135 J cm−2 applied energy, 27.9 ± 1.5 kHz US Center frequency). In comparison, US is being used as a distant stimulus for drug-delivery systems, at energy levels over the threshold for cavitation. They offer a flashing ratchet technique at low energy for driving molecule diffusion in a variety of gels that are common in biomedical systems, such as identification of molecules, separation, and DDSs. As a result, US-triggered therapy is currently being explored extensively in the central nervous system, cancer, cardiovascular disease, tissue repair, disease of the skeletal muscles, and other disorders, with promising therapeutic applications.
Cancer Therapy Applications
The efficacy of chemotherapeutics in cancer is based on the capacity to overcome barriers, including the vessel wall or the membranes of tumor cells. Targeted drug delivery by US is a useful strategy that has the potential to produce a variety of beneficial effects, including assisting chemotherapeutic drugs in penetrating these barriers, selectively targeting tumors, and minimizing the possibility of negative side effects in various cancers.116 New magnetic DDS (DDS) using natural Tragacanth gum (TG) has been developed by Jahanban et al for cancer treatment using targeted chemo/hyperthermia.117 Small drug release values were observed in the formulated Dox at physiological conditions (pH 7.4 and T = 37 °C). In contrast, the MH/Dox showed a higher drug release value in cancerous conditions (lower pH (eg, 5.3) and higher GSH (eg, 10 mM)). The cytotoxicity of MH/Dox against MCF7 cells revealed that the combination of chemotherapy and hyperthermia therapy is superior to chemotherapy alone in terms of anti-cancer efficacy, suggesting the potential of developed DDS for chemo/hyperthermia therapy for cancer. Zhu et al developed a hydrogel system (in situ) triggered by ultrasonic (US) (Figure 6). It is designed to treat tumor hypoxia by combining photodynamic therapy (PDT) and chemotherapy.118 After US treatment, the 2.2′-azobis[2-(2-imidazolin-2-yl) propane] dihydrochloride (AIPH) decomposes and releases a significant quantity of alkyl free radicals, which can stimulate the crosslinking reaction of PEG double acrylate (PEGDA) via the amide bond, resulting in the hydrogel structure. This system is capable of immobilizing CAT-Ce6 and doxorubicin (DOX) for a long period at the site of the tumor. Due to the hydrogel’s prolonged retention, tumor hypoxia can be consistently relieved by CAT, enhancing the effect of PDT. Meanwhile, prolonged chemotherapy can be achieved through the sustained release of DOX from the hydrogel. In vivo experiments suggest that combining repeatable PDT with prolonged chemotherapy based on a hydrogel system activated by the US has a synergic effect in anticancer therapy with no apparent side effects.
 |
Figure 6 The development of an ultrasound-triggered hydrogel is depicted schematically and its anticancer chemo-synergistic/photodynamic treatment with long-term hypoxia relief. Notes: Reproduced with permission from Zhu J, Wang X, Yang D, Song X, Li B, Wang W, et al. Ultrasound‐triggered in situ gelation to overcome tumor hypoxia for enhanced photodynamic and sustained chemotherapy. Advanced therapeutics. 2021;4:2100052. © 2021 Wiley‐VCH GmbH.118 |
Wu et al designed a biocompatible and thermosensitive hydrogel system that triggered the release of the loaded drugs.119 The hydrogel was investigated for baseline and in vitro US-responsive release profiles of a DOX (a small drug) and a FITC-dextran (a large molecule). The release rate increased nearly 70 times when induced by US (1 MHz, continuous, 0.4 W/cm2). Without the presence of the US, the release rate reverted to baseline. The DOX release profile in vivo at baseline and in response to the US was determined using subcutaneous injections in the backs of mice and rats. Following a subcutaneous layer injection, in vivo results indicated that the hydrogels remained in situ and delivered a consistent release for at least 7 d; When US-trigger was applied, the hydrogel’s in vivo release increased by almost tenfold. As a result, the mPEG-PLGA-BOX block copolymer hydrogel may be used as a biocompatible, thermosensitive, and implantable hydrogel system for triggering drug release via the US.
In addition, multifunctional theranostic nanoprobes with stimuli-responsive imaging and therapeutic capabilities have demonstrated considerable potential to improve early cancer diagnostic and treatment efficacy. Considered the fundamental characteristic of the tumour microenvironment, elevated amounts of lactate and hydrogen peroxide can be utilized to build potential theranostic strategies.120 Nanogels are versatile enough to serve as both a contrast agent and a cargo carrier. According to Wang et al, nanogels containing two enzymes (catalase and SOD) were used as probes for the dual-modality US and T2-weighted MRI imaging.121 The findings revealed the interaction of NG probes with pathological ROS to increase the concentration of molecular oxygen in an acidic environment, allowing for improved US imaging and T2-weighted MRI. In another study, a responsive enzyme-based nanogel probe with significant biocompatibility was developed as a promising theranostic tool for US imaging and cancer treatment by targeting high lactate and hydrogen peroxide levels.122 They encapsulated lactate oxidase (LOD) and catalase (CAT) in self-assembled nanogels to develop responsive nanoprobe LOD/CAT-loaded nanogels (LCNGs), which were capable of responding to lactate and H2O2 rich tumour microenvironments by generating a large amount of oxygen, which then accumulates into microbubbles for improved US imaging. Besides, LCNGs@DOX was also developed by integrating the nanoprobes with doxorubicin (DOX) for cancer therapy. Both in vitro and in vivo results show that LCNGs@DOX have improved US imaging and effective cell proliferation inhibition, allowing the development of safe and efficient theranostic nanoprobes capable of responsive US imaging and tumor treatment.
Biological systems are extremely sensitive to the location and timing of physiologic stimuli and drugs. This spatiotemporal sensitivity enables the development of new therapeutic strategies. Over time, most devices deliver a steady, unchanging drug dose. However, it is revealed that cancer cells are more susceptible to short-term, high-dosage “bursts” of the chemotherapeutic mitoxantrone than to steady doses over longer periods, implying a potential benefit for implanted devices that provide external dose and timing control.25 Huebsch et al proposed that by disrupting ionically cross-linked hydrogels with US, it would be feasible to produce digital drug delivery that could be activated and then turned off on demand.25 They found that the US does not destroy these materials but rather promotes the release of small molecules, oligonucleotides that have been condensed, and proteins in near-digital form. Simultaneous in vitro investigations indicated that temporally brief, high-dose “bursts” of drug exposure might be used to improve mitoxantrone’s toxicity toward cells of breast cancer. In vitro and in vivo, biocompatible, implantable alginate hydrogels demonstrated the capability to self-heal damage caused by US pulses, allowing on-demand mitoxantrone delivery, and mitoxantrone-loaded gels implanted near tumors were more useful in removing tumor growth when a daily US pulse was applied.
Bone Repair Applications
The bone may regenerate itself through complex physiological processes. However, regeneration is inadequate, too sluggish, or does not occur in the case of non-union fractures as well as certain pathological diseases. Current research has emphasized the formation of biomaterial-based solutions as an alternate strategy. Therapeutic compounds, such as growth factors, are incorporated onto a scaffold or hydrogel that acts as a template for bone repair while delivering the therapeutic agent to promote bone regeneration processes. A novel approach in this field is the use of hydrogels for therapeutic delivery in response to stimuli, which enables precise control of therapeutic drug delivery directly to the site of injury “on-demand”. US-responsive systems have recently been developed, and their capacity to be triggered externally makes them particularly useful in the domain of on-demand drug delivery.123 Levingstone et al developed a hydrogel that is thermally responsive for bone regeneration that uses US to deliver therapeutic therapeutics on-demand.124 Additionally, it was anticipated that the addition of hydroxyapatite (HA) into the hydrogel would enhance sonosensitization, hence increasing ultrasonic sensitivity and enabling regulated therapeutic delivery to the target region. The study indicated the release of sodium fluorescein (NaF), bovine serum albumin (BSA), and bone morphogenetic protein 2 (BMP-2) from hydrogels upon application of US. These findings together reveal the possibility for on-demand therapeutic drug delivery for regeneration of bone using these hydrogels that are thermally responsive when triggered by US.
Biological systems are extremely well controlled both spatially and temporally, which has prompted an interest in DDS that can be changed on demand to flexibly acquire the release profile. However, payload release systems that are triggered by extracorporeal or external stimuli frequently demonstrate significant leakiness during drug release. Kearney et al speculated that a more comprehensive on/off switch might be created by physical encapsulation of nanoparticles in hydrogels, controlling baseline release using steric hindrance, and utilizing the US to change the system’s microarchitecture reversibly to enable switchable release.123 A diagrammatic representation of the strategy is given in Figure 7. To examine this, we initially explored the release of gold nanoparticles coated with PEG from alginate hydrogels that are ionically crosslinked, and it revealed a substantial increase in release rate in response to the US. Additionally, gold nanoparticles conjugated to BMP-2 could be released from hydrogels through the US and retain their bioactivity after alginate encapsulation and ultrasonic release. Furthermore, a non-significant increase in osteogenesis by PEG-AuNPs was observed. Researchers and physicians should be able to mimic and drive natural temporal reactions using this method to enhance control over local bioagent delivery.
 |
Figure 7 BMP-2-AuNPs are bioactive after they have been released by alginate gels in response to the application of US. (A) A schematic representation of the strategy: Under sterile conditions, US is employed to facilitate the BMP-2-AuNP release from alginate microbeads and these are then added to cultures of D1 cells; the medium is also evaluated for diffusion-only release. The activity of alkaline phosphatase on day 7 is utilized to determine the persistence of bioactivity. (B) Dynamic light scattering studies of bare gold nanoparticle hydrodynamic diameters (“Seed AuNPs”) after production and conjugation with BMP-2 (“0 US, BMP-2-AuNPs”). These particles were then exposed to 9.6 mW cm−2 US for 1×2.5 min (one) or 2×2.5 min (two) rounds to determine if the BMP-2 was removed from the AuNPs. (C) Quantification of alkaline phosphatase (ALP) staining for diffusion-only and US-released AuNPs from alginate beads with 9.6 mW cm−2 US, 2.5 min h−1 for 10 h. Notes: Reproduced with permission from Kearney CJ, Skaat H, Kennedy SM, Hu J, Darnell M, Raimondo TM, et al. Switchable release of entrapped nanoparticles from alginate hydrogels. Advanced healthcare materials. 2015;4:1634–1639. © 2015 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.123 |
Central Nervous System Regeneration Applications
The major central nervous system (CNS) disorders include Parkinson’s disease, brain stroke, epilepsy, Alzheimer’s disease, ischemia, glioblastoma, and multiple sclerosis. Their effects are primarily debilitating, and in certain circumstances, patients are permanently disabled. Glioblastoma multiforme (GBM), a type of malignant brain tumor, for example, is difficult to treat because of its rapid proliferation and poor prognosis.125 There are currently no potential therapeutic medicines available to improve CNS regeneration following traumatic diseases or injuries. Indeed, drug penetration throughout the blood-brain barrier (BBB), inadequate targeting of the drug, weak neural progenitor cells, and limited growth of matured neural cells all significantly impair CNS regeneration. To address these limitations, bioengineered implantable hydrogels in combination with therapeutic and cell treatments have been proposed as a means of mimicking the complexities of the CNS micro-environment and architecture. Recent research has focused on developing stimuli-responsive hydrogels as innovative carrier systems capable of guiding the regeneration of neural tissue.126 During hydrogel gelation, various biologically active molecules and pharmacological substances can be enclosed inside the matrix, while the high water content facilitates the uptake and distribution of payload. In general, the kinetics of cargo release is determined by the hydrogel’s properties, such as chemical composition, pore size, and electrical charge.127
The BBB prevents the majority of systemically delivered drugs from entering the parenchymal space, posing a barrier to the successful treatment of CNS diseases. In this regard, one developing method for opening the BBB in a targeted region of the brain employs focused US. Importantly, focused US has the advantage of being less invasive when compared to other techniques used to treat CNS problems, suggesting that this unique method has great potential for application.128 US-responsive hydrogels can deliver bioactive therapies spatially controlled and over extended periods using unidirectional or pulsatile burst release characteristics. Cheng et al described a potential technique for micropatterning nerve cells in three-dimensional (3D) tissue scaffolds for peripheral nerve regeneration.129 They exhibited the 3D micropatterning of PC12 cells in a gelatin-hydroxyphenyl propionic acid (Gtn-HPA) hydrogel using ultrasound standing waves (USWs). The USWs were used to align PC12 cells in 3D along nodal planes in the Gtn-HPA hydrogel precursor solution. Crosslinking the precursor with horseradish peroxidase (HRP) and diluted hydrogen peroxide (H2O2) immobilized the aligned cells for 90–120 s. This procedure of micropatterning is inexpensive and simple to replicate without the use of complex and costly specialized equipment. PC12 cells aligned with USW had no negative effect on viability or proliferative abilities. To our knowledge, this is the first study to examine the effect of USW alignment on the differentiation of the neural cell. After 20 d, PC12 cells that have been differentiated and aligned with the USW demonstrated directional consistency, indicating that this strategy may be a viable alternative for guiding the nerve regeneration process.
Anti-Inflammation Applications
Hydrogels can deform or even disintegrate as a result of forces produced by a variety of physiological processes such as cartilage compression, muscular contraction, and blood circulation in the cardiovascular system. Forces also have an impact on a variety of clinical disorders, including inflammation and the formation of scars.130 Similar to the development of hydrogels that respond to internal mechanical forces, extensive research has been conducted on hydrogels that can control drug release in response to diverse external mechanical stimuli, such as US and magnetic forces.131 These technologies offer control of therapeutic release on a temporal, spatial, and on-demand basis. Taking advantage of these unique mechanical forces, hydrogels have been designed as “smart” DDS capable of releasing encapsulated therapeutics in response to mechanical stimuli. Sun et al reported the development of DDS based on a hydrogel that responds to US with a unique nanoscale network with a dual crosslink design.29 The covalent crosslinks impart mechanical stability to the hydrogel and significantly reduce drug release driven by deformation. Meanwhile, the dynamic covalent boronate ester connections between the hydrogel backbone and the anti-inflammatory chemical tannic acid (TA) enable the effective pulsatile release of TA when triggered by US. As a result, the hydrogel exhibits unique drug release characteristics when compressed and when subjected to US. Proof-of-principle evidence of reduced macrophage inflammatory activity following US-triggered TA release was also reported. The findings suggest that this innovative hydrogel-based DDS could be employed to treat inflammatory diseases affecting load-bearing tissues such as cartilage and muscle.
Anti-Diabetic Applications
Glucose, which is the primary source of energy for the majority of tissues, is normally present in the blood at physiological levels due to a strong feedback regulatory mechanism. The human pancreas functions intrinsically as a glucose-responsive platform, with β-cells monitoring changes in blood glucose levels and secreting endogenous insulin in response. However, such an intricate balance could become significantly dysregulated in certain disorders, such as diabetes mellitus, which is characterized by a significant increase in blood glucose levels (more than 4-fold) due to a lack of insulin secretion or the development of insulin resistance. In the case of Type 1 diabetes, patients are extremely reliant on the administration of supraphysiological insulin doses regularly to reduce and maintain blood glucose levels.132 Traditional diabetes therapy frequently necessitates many injections of daily insulin, which are related to pain and poor glycemic control. Di et al have designed an insulin delivery system that can release insulin in a pulsatile way that is activated by US. This system is capable of providing both long-term sustained and rapid on-demand responses.133 In this system, nanocapsules based on poly(lactic-co-glycolic acid) (PLGA) loaded with insulin are encapsulated in microgels made of chitosan. Insulin nanocapsules can diffuse from nanoparticles passively but must remain encapsulated in the microgel. US treatment enables the quick release of insulin contained in microgels to help regulate levels of blood glucose. When triggered by 30 seconds of US therapy, a chemically generated diabetic mouse model (type 1) demonstrated noninvasive, localized, and pulsatile glycemic control for up to one week.
Cardiovascular Therapeutic Applications
The most prevalent cause of cardiac dysfunction is acute myocardial infarction (MI) induced by ischemia. While cell therapy or growth factors appear promising, bioactive chemicals are poorly retained in the highly vascularized myocardium, preventing the sustained activation required for optimal cellular responses.134 Numerous biomaterials with varying physical and chemical properties have been designed to improve the targeted delivery of cells and/or growth factors for therapeutic angiogenesis in ischemic tissues. Hydrogels are particularly desirable as carrier systems since they are physically similar to the extracellular matrix (ECM) of tissues, they can be designed under relatively mild environments, and they may be delivered with minimal invasiveness.135 Additionally, hydrogels can be developed to degrade in a manner that corresponds to the angiogenic process. As a result, hydrogels have demonstrated significant potential as pro-angiogenic matrices. While restoring blood flow to the site of the infarction and pre-existing ECM degradation attenuation remains a feasible treatment strategy for MI, local delivery of drugs has been constrained by poor accumulation (inefficacy) and needless diffusion (toxicity). Fan et al developed a dual-purpose hydrogel with MI responsivity for the delivery of drugs on-demand to induce angiogenesis and prevent cardiac remodeling after MI by targeting upregulated matrix metalloproteinase-2/9 (MMP-2/9).134 A collagen hydrogel that has been modified with glutathione (GSH) (collagen-GSH) is synthesized by covalently linking amine groups of collagen to sulfhydryl groups of GSH and GST-TIMP-bFGF recombinant protein (bFGF: basic growth factor of fibroblast) by the combination of bFGF with glutathione-S-transferase (GST) and the MMP-2/9 cleavable peptide PLGLAG (TIMP). The GST-TIMP-bFGF loading in the collagen-GSH hydrogel is considerably improved by GST and GSH specific binding. During MI, the TIMP peptide, which is situated between GST and bFGF, is released on-demand in response to MMPs. Furthermore, the TIMP peptide is an MMP competitive substrate, preventing MMPs from degrading the cardiac matrix excessively after MI. Hydrogels containing GST-TIMP-bFGF/collagen-GSH help in the recovery process for MI rats through increasing myocardial vascularization and improving myocardial remodeling. The findings imply that delivery of growth factors on demand via synchronizing controlled binding and release in order to induce angiogenesis and inhibit cardiac remodelling may be a promising strategy for treating ischemic heart disease.
Table 2 illustrates the summary of various types of US mediated hydrogel used in certain biomedical field. We will go through the some of the most promising hydrogel providing US triggered drug release upon irradiation with specific US intensity for a particular time duration according to the application.
 |
Table 2 US Triggered Drug Release from Various Types of Hydrogel |
A summary of various type of US mediated hydrogel used in various biomedical field is provided in Table 2 along with their US intensity and irradiation time based on their application.
Clinical Status of Hydrogel in Drug Delivery Systems
US-responsive formulations have demonstrated potential in imaging and targeted therapy for a diverse array of pathological conditions, including cancer, neurological disorders, tissue engineering, cardiovascular diseases, and wound healing.138 For instance, ThermoDox® (Celsion Corporation, Lawrenceville, NJ, USA) is a clinically tested heat-activated DDS that enables targeted delivery of a cytotoxic drug (DOX) to tumours at temperatures above 40 °C.139 TraceIT® synthetic hydrogels are extensively studied for imaging cancerous cells and preventing radiation-induced damage to healthy cells. Microparticles of polyethylene glycol (PEG) hydrogel containing covalently bound iodine are the basis of the TraceIT® hydrogel tissue marker, which allows MRI, CT, and US imaging of cancerous tissue for future surgical procedures for 3 months.140 Subcutaneous hormonal therapy, Endo’s Vantas®, has been approved by the FDA to prevent the growth of testosterone-dependent prostate cancer cells. When using Vantas®, gonadotropin-releasing hormone (GnRH) or luteinizing hormone (LH-RH) is released continuously from a cylindrical diffusion-controlled reservoir. It contains Histrelin acetate, which is an analogue of GnRH that is more potent than leuprolide acetate-poly(lactic-co-glycolic acid)-based nonhydrogel products (Lupron depot® and Eligard®) in terms of potency. Collectively, these hydrogel depots provide considerable benefits in terms of patient acceptance and convenience through providing long-lasting benefits with a single injection. A hydrogel developed by Augmenix, SpaceOAR®, has been approved by the FDA as a radiation shield for prostate cancer patients. Natural hydrogels have not been approved for use in cancer products, which may be due to the advantages of using synthetic gels as long-term delivery platforms.140
Toxicological Aspects of Smart Nanosystems
Despite the fact that (smart) nanoparticle DDSs offer several advantages over conventional chemotherapy, there are significant concerns about their toxicity.141 Thus, before promising technologies can be translated into therapeutic clinical applications, these potential toxicity issues for human health must be addressed. Because of the unique properties of nanomaterials, conventional drug toxicity assays may be insufficient or inadequate for assessing nanoparticle toxicity completely. Therefore, evaluating the toxicity of nanomaterials and developing validated advanced complementary assays remains difficult, indicating the need for standard toxicity assessment criteria.142 The immune system responds to nanoparticles and other foreign stimuli and thus serves as the primary defence against foreign invasion.143 Exposure of the immune system to nanoparticles can result in inflammation and allergic/autoimmune responses. The extent and type of immunological reactions that nanoparticles can induce, as well as their ability to activate the complement system, are determined by their inflammatory effects, antigenic properties, and immune response.144 Therefore, nano-immuno interactions must be considered when developing such DDSs. Special attention must be delivered to the testing of nanomaterials for the presence of bacterial endotoxin contamination.144 Endotoxins can confound the results of toxicity and efficacy studies because they are potent immune stimulants that can provoke a cytokine storm.145
The use of US is an additional important biosafety issue in the case of US-triggered DDSs. The extent and severity of thermal and mechanical US effects depend on several US parameters such as focusing, frequency, pulse duration, pulse repetition frequency, intensity, and exposure time as well as on the attenuation coefficient and acoustic impedance of biological tissues. This is relevant in this context because thermal and mechanical effects that are advantageous for cancer treatment may also have adverse biological effects on healthy tissues.146 During HIFU treatment, for instance, the extremely high level of US energy around the focal point inevitably damages healthy tissues, resulting in severe side effects such as transient skin burns, pain, and nerve injury. Additionally, if there are any residual air bubbles, US application can have undesirable cavitation effects. Organs that contain gas, like the lungs and digestive tract, are not suitable for treatment involving US. To limit the risks of thermal or mechanical injuries caused by US, appropriate indices (thermal index, mechanical index) have been introduced. Under consideration of the biological effects of US, these indices are useful for the development of safe and effective tumor-specific imaging and treatment modalities.28
Developing efficient US-triggered drug delivery micro-/nanoplatforms with tolerable side effects is a complex process involving multiple parameters that must be balanced against one another. A characteristic considered advantageous in one therapeutic setting may have adverse effects in another. Consequently, developing nanoscale DDSs requires an in-depth knowledge of the biological interactions that occur inside an animal or human system, starting with the administration of nanoparticles and ending with their final fate.147
Challenges and Limitations
Immune response minimization, biodegradability, controlled release, and minimal biofilm formation are all desirable characteristics of an implanted ultrasonically responsive hydrogel. Several examples were extensively discussed in this review paper, along with their efficacy in cancer, CNS disorders, diseases of the skeletal muscles, cardiovascular, and diabetes diseases, as well as their comparison to conventional systemic therapy. There are indeed several challenges and unaddressed technological concerns that have limited the potential application of hydrogels. Here, we mentioned the following unaddressed challenges and potential development directions to help hydrogel-based research move forward toward biomedical application in the future.
- Potential adverse effects and long-term effectiveness following hydrogel injection/implant (eg, calcification, fibrosis, inflammation, joint effusion and joint pain). For implantation in the rabbit cornea, Sarojini et al developed a PHEMA-based hydrogel. Unfortunately, approximately 12 weeks after implantation, calcification was found at the injection site of the hydrogel, indicating poor biocompatibility for long-term use.148 In addition, most of the materials used in hydrogel fabrication would activate immune-mediated foreign body responses (FBR), lead to fibrosis enveloping the hydrogel, and prevent the infiltration of additional cells into the hydrogel, ultimately leading to treatment failure. Anderson et al synthesized a library of alginate analogues containing 77 members, among them three triazole-containing alginate analogues demonstrated negligible fibrosis in primates, suggesting that future in vivo applications of these synthetic materials could effectively avoid FBR.149 Therefore, a comprehensive and methodical understanding of these materials is required to use them for biomedical applications.
- Incorrect administration of hydrogels for aesthetics would cause patients to experience pain, nodules, swelling, and other complications.150 It is also necessary to pay attention to therapies that rely on stem cells. To establish a cell-free therapy, researchers have grown and harvested allogeneic secretomes and loaded them into hydrogels. It has also been demonstrated that cell-encapsulated hydrogels can act a secretome depository constantly secreting and releasing disease-fighting factors when placed on the disease site. As an implantable device, the efficacy and adverse effects of secretome were dose-dependent,151 which must be taken into consideration when designing hydrogels for specific diseases.
- Numerous studies have demonstrated that the biomechanical and biochemical properties of the stroma matrix are constantly and subtly changing, which may spatiotemporally regulate the biological functions of cells. Although numerous dynamic implantable systems have been engineered and implemented, they have typically been implemented for drug delivery. In addition, cell behaviours and associated signalling communications within these dynamic implantable platforms have not yet been comprehensively investigated. Therefore, there is still a considerable amount of work to be done to develop rational implantable hydrogel systems for improving our understanding and knowledge of cell behaviors in vivo, which is significant and profound for the advancement of precise therapy.
- The fate of the implantable US responsive hydrogel system is a problem with the usage of biodegradable implant platforms and devices. The literature does not properly discuss the implanted material’s catabolism. There is a gap in the literature regarding the long-term toxicity of US responsive responsive hydrogel in vivo used generally, in the domain of drug delivery, and particularly targeted drug delivery. The majority of currently available stimuli-sensitive technologies are unsuitable for clinical usage due to insufficient in vivo toxicity results.
- Internal or external stimuli’s efficacy in inducing drug release must be explored in deep-seated tissues and in large animals, which is not a focus of many studies at the moment. It is necessary to resolve the issues associated with the penetration depth of US applied externally.
- The advancement of biocompatible 3D printing materials is a step toward producing hydrogels or polymers with customizable forms. This is particularly useful in the case of customized implantation. Additionally, the reduction in implant size at a given therapeutic dose improves patient compliance. Protocols for drug loading must be improved to achieve this goal.
- The administration of therapeutic agents to particular sites using implantable US responsive hydrogels minimizes the unwanted effects associated with systemic drug delivery and enhances efficiency. This is extremely important in immunotherapy, a rapidly emerging field of research for cancer treatment. Local administration of immunomodulators has a longer-lasting systemic effect than conventional chemotherapy and radiotherapy. The hydrogel systems developed for this objective must have advanced topologies, as simple compression moulding does not allow for the embedding of live antibodies and cells in the framework. Thus, advancements in the design of novel US responsive hydrogels have the potential to improve immunotherapy, as local delivery is essential in this treatment approach.
- Although responsiveness to internal stimuli has demonstrated efficient treatment efficacy, drug administration via external stimulation gives more accuracy in controlling the dose and timing of the release. Furthermore, variations in disease progression necessitate a change in the physician’s therapeutic strategy. Wireless drug reservoirs, in this aspect, appear to represent the next generation of implanted systems. However, the incorporation of electrical circuits in the development of such platforms impedes the implant’s biodegradability. Covering such systems with materials that are biocompatible may enable the DDS to remain in the body for an extended period.
Conclusion
Over the last several decades, advancements in the chemistry of materials, fabrication approaches, polymer physics, and our comprehensive analysis of cell and tissue biology have contributed to the design of hydrogel DDS. On-demand drug release can also be difficult to control at a high level. When it comes to on-demand release, a perfect responsive hydrogel system be able to respond precisely to stimuli in terms of drug release. With the rapid advancement of US technology and UCAs, spatiotemporally controlled US applications with or without UCAs enable site-specific therapeutic agent delivery and targeted modulation with minimum side effects. Despite significant advances in ultrasound-responsive hydrogel, the clinical application of hydrogel products is currently limited owing to unanticipated complications and adverse effects, and improper administration. After constructing a library of materials and conducting a screening, these issues can be addressed through material optimization. Even though hydrogels loaded with cells have been reported to possess promising therapeutic effects for disease treatment and tissue regeneration in vitro, their long-term effectiveness in vivo is still unspecified because of the complicated cellular microenvironment. In order to assist their clinical applications in vivo, a systematic and exhaustive understanding of the interactions between matrix and cells is necessary. In addition, despite the fact that numerous types of potent hydrogels have been produced, their drug delivery applications are primarily limited. Smart hydrogels could be used for a wider range of applications in the future if a better understanding of how they affect cell biological responses and how they send and receive signals when signalling transductions are incorporated within them.
Consent for Publication
All authors have read and approved the manuscript for submission.
Acknowledgment
This work was supported by the Zhejiang Provincial Natural Science Foundation of China (No. LGF19H160026 to XMF), and Zhejiang Provincial Medical Science and Technology Planning Project (No. 2019RC120 to LGC).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Zhejiang Provincial Natural Science Foundation of China (No. LGF19H160026 to XMF), and Zhejiang Provincial Medical Science and Technology Planning Project (No. 2019RC120 to LGC).
Disclosure
Authors declared no competing interest.
References
1. Liu D, Yang F, Xiong F, Gu N. The smart drug delivery system and its clinical potential. Theranostics. 2016;6:1306. doi:10.7150/thno.14858
2. Homayun B, Lin X, Choi H-J. Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics. 2019;11:129. doi:10.3390/pharmaceutics11030129
3. Davoodi P, Lee LY, Xu Q, et al. Drug delivery systems for programmed and on-demand release. Adv Drug Deliv Rev. 2018;132:104–138. doi:10.1016/j.addr.2018.07.002
4. Fenton OS, Olafson KN, Pillai PS, Mitchell MJ, Langer R. Advances in biomaterials for drug delivery. Adv Mater. 2018;30:1705328.
5. Gao S, Tang G, Hua D, et al. Stimuli-responsive bio-based polymeric systems and their applications. J Mater Chem B. 2019;7:709–729. doi:10.1039/C8TB02491J
6. Jahanban-Esfahlan R, Massoumi B, Abbasian M, et al. Dual stimuli-responsive polymeric hollow nanocapsules as “smart” drug delivery system against cancer. Polymer Plastics Technol Mater. 2020;59:1492–1504. doi:10.1080/25740881.2020.1750652
7. Murphy NP, Lampe KJ. Mimicking biological phenomena in hydrogel-based biomaterials to promote dynamic cellular responses. J Mater Chem B. 2015;3:7867–7880. doi:10.1039/C5TB01045D
8. Tang S, Richardson BM, Anseth KS. Dynamic covalent hydrogels as biomaterials to mimic the viscoelasticity of soft tissues. Prog Mater Sci. 2021;120:100738. doi:10.1016/j.pmatsci.2020.100738
9. Skardal A, Devarasetty M, Kang H-W, et al. A hydrogel bioink toolkit for mimicking native tissue biochemical and mechanical properties in bioprinted tissue constructs. Acta Biomaterialia. 2015;25:24–34. doi:10.1016/j.actbio.2015.07.030
10. Sikdar P, Uddin MM, Dip TM, et al. Recent advances in the synthesis of smart hydrogels. Mater Adv. 2021;2:4532–4573.
11. Zhang Y, Huang Y. Rational design of smart hydrogels for biomedical applications. Front Chem. 2021;8:615665. doi:10.3389/fchem.2020.615665
12. Cimen Z, Babadag S, Odabas S, Altuntas S, Demirel G, Demirel GB. Injectable and self-healable pH-responsive gelatin–PEG/laponite hybrid hydrogels as long-acting implants for local cancer treatment. ACS Appl Polymer Mater. 2021;3:3504–3518. doi:10.1021/acsapm.1c00419
13. Heffernan JM, Overstreet DJ, Vernon BL, et al. In vivo evaluation of temperature‐responsive antimicrobial‐loaded PNIPAAm hydrogels for prevention of surgical site infection. J Biomed Mater Res B Appl Biomater. 2022;110:103–114. doi:10.1002/jbm.b.34894
14. Krüger-Genge A, Tondera C, Hauser S, et al. Immunocompatibility and non-thrombogenicity of gelatin-based hydrogels. Clin Hemorheol Microcirc. 2021;77:335–350. doi:10.3233/CH-201028
15. Xu Y, Patino Gaillez M, Rothe R, et al. Conductive hydrogels with dynamic reversible networks for biomedical applications. Adv Healthcare Mater. 2021;10:2100012. doi:10.1002/adhm.202100012
16. Cao H, Duan L, Zhang Y, Cao J, Zhang K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct Target Ther. 2021;6:1–31. doi:10.1038/s41392-020-00451-w
17. Miller DL, Smith NB, Bailey MR, et al. Overview of therapeutic ultrasound applications and safety considerations. J Ultrasound Med. 2012;31:623–634. doi:10.7863/jum.2012.31.4.623
18. Soleimani K, Derakhshankhah H, Jaymand M, Samadian H. Stimuli-responsive natural gums-based drug delivery systems for cancer treatment. Carbohydr Polym. 2021;254:117422. doi:10.1016/j.carbpol.2020.117422
19. Ahmadi A, Hosseini-Nami S, Abed Z, et al. Recent advances in ultrasound-triggered drug delivery through lipid-based nanomaterials. Drug Discov Today. 2020;25:2182–2200. doi:10.1016/j.drudis.2020.09.026
20. Delaney LJ, Isguven S, Eisenbrey JR, Hickok NJ, Forsberg F. Making waves: how ultrasound-targeted drug delivery is changing pharmaceutical approaches. Mater Adv. 2022;3:3023–3040. doi:10.1039/d1ma01197a
21. Mishra B, Upadhyay M, Reddy Adena S, Vasant B, Muthu M. Hydrogels: an introduction to a controlled drug delivery device, synthesis and application in drug delivery and tissue engineering. Austin J Biomed Eng. 2017;4:1037–1049.
22. Zhang Y, Jiang R, Fang A, et al. A highly transparent, elastic, injectable sericin hydrogel induced by ultrasound. Polym Test. 2019;77:105890. doi:10.1016/j.polymertesting.2019.05.006
23. Huang W-C, Ali F, Zhao J, Rhee K, Mou C, Bettinger CJ. Ultrasound-mediated self-healing hydrogels based on tunable metal–organic bonding. Biomacromolecules. 2017;18:1162–1171. doi:10.1021/acs.biomac.6b01841
24. Sun Z, Song C, Wang C, Hu Y, Wu J. Hydrogel-based controlled drug delivery for cancer treatment: a review. Mol Pharm. 2019;17:373–391.
25. Huebsch N, Kearney CJ, Zhao X, et al. Ultrasound-triggered disruption and self-healing of reversibly cross-linked hydrogels for drug delivery and enhanced chemotherapy. Proc Natl Acad Sci. 2014;111:9762–9767. doi:10.1073/pnas.1405469111
26. Brudno Y, Mooney DJ. On-demand drug delivery from local depots. J Control Release. 2015;219:8–17. doi:10.1016/j.jconrel.2015.09.011
27. Kooiman K, Roovers S, Langeveld SA, et al. Ultrasound-responsive cavitation nuclei for therapy and drug delivery. Ultrasound Med Biol. 2020;46:1296–1325.
28. Boissenot T, Bordat A, Fattal E, Tsapis N. Ultrasound-triggered drug delivery for cancer treatment using drug delivery systems: from theoretical considerations to practical applications. J Control Release. 2016;241:144–163. doi:10.1016/j.jconrel.2016.09.026
29. Sun W, Jiang H, Wu X, et al. Strong dual-crosslinked hydrogels for ultrasound-triggered drug delivery. Nano Res. 2019;12:115–119. doi:10.1007/s12274-018-2188-4
30. Nele V, Wojciechowski JP, Armstrong JP, Stevens MM. Tailoring gelation mechanisms for advanced hydrogel applications. Adv Funct Mater. 2020;30:2002759. doi:10.1002/adfm.202002759
31. Wood AK, Sehgal CM. A review of low-intensity ultrasound for cancer therapy. Ultrasound Med Biol. 2015;41:905–928. doi:10.1016/j.ultrasmedbio.2014.11.019
32. Entzian K, Aigner A. Drug delivery by ultrasound-responsive nanocarriers for cancer treatment. Pharmaceutics. 2021;13:1135. doi:10.3390/pharmaceutics13081135
33. Athanassiadis AG, Ma Z, Moreno-Gomez N, et al. Ultrasound-responsive systems as components for smart materials. Chem Rev. 2021;122:5165–5208. doi:10.1021/acs.chemrev.1c00622
34. Sailesh R, Yuvaraj L, Pitchaimani J, Doddamani M, Chinnapandi LBM. Acoustic behaviour of 3D printed bio-degradable micro-perforated panels with varying perforation cross-sections. Appl Acoust. 2021;174:107769. doi:10.1016/j.apacoust.2020.107769
35. Kinsler LE, Frey AR, Coppens AB, Sanders JV. Fundamentals of Acoustics.
36. Szabo TL, Wu J. A model for longitudinal and shear wave propagation in viscoelastic media. J Acoust Soc Am. 2000;107:2437–2446. doi:10.1121/1.428630
37. Hartmann B, Jarzynski J. Ultrasonic hysteresis absorption in polymers. J Appl Phys. 1972;43:4304–4312. doi:10.1063/1.1660920
38. Gao N, Wu J, Lu K, Zhong H. Hybrid composite meta-porous structure for improving and broadening sound absorption. Mech Syst Signal Process. 2021;154:107504. doi:10.1016/j.ymssp.2020.107504
39. Vér IL, Beranek LL. Noise and Vibration Control Engineering: Principles and Applications. John Wiley & Sons; 2005.
40. Cox T, d’Antonio P. Acoustic Absorbers and Diffusers: Theory, Design and Application. CRC Press; 2016.
41. Olny X, Boutin C. Acoustic wave propagation in double porosity media. J Acoust Soc Am. 2003;114:73–89. doi:10.1121/1.1534607
42. Laukaitis A, Fiks B. Acoustical properties of aerated autoclaved concrete. Appl Acoust. 2006;67:284–296. doi:10.1016/j.apacoust.2005.07.003
43. Glé P, Gourdon E, Arnaud L. Acoustical properties of materials made of vegetable particles with several scales of porosity. Appl Acoust. 2011;72:249–259. doi:10.1016/j.apacoust.2010.11.003
44. Karabulut S, Caliskan M. Development of an ecological, smooth, unperforated sound absorptive material.
45. Cuiyun D, Guang C, Xinbang X, Peisheng L. Sound absorption characteristics of a high-temperature sintering porous ceramic material. Appl Acoust. 2012;73:865–871. doi:10.1016/j.apacoust.2012.01.004
46. Yilmaz ND, Banks‐Lee P, Powell NB, Michielsen S. Effects of porosity, fiber size, and layering sequence on sound absorption performance of needle‐punched nonwovens. J Appl Polym Sci. 2011;121:3056–3069. doi:10.1002/app.33312
47. Rutkevičius M, Mehl GH, Paunov VN, et al. Sound absorption properties of porous composites fabricated by a hydrogel templating technique. J Mater Res. 2013;28:2409–2414. doi:10.1557/jmr.2013.194
48. Gao Y, Song J, Li S, et al. Hydrogel microphones for stealthy underwater listening. Nat Commun. 2016;7:1–11.
49. Prokop AF, Vaezy S, Noble ML, Kaczkowski PJ, Martin RW, Crum LA. Polyacrylamide gel as an acoustic coupling medium for focused ultrasound therapy. Ultrasound Med Biol. 2003;29:1351–1358. doi:10.1016/S0301-5629(03)00979-7
50. Yuk H, Lin S, Ma C, Takaffoli M, Fang NX, Zhao X. Hydraulic hydrogel actuators and robots optically and sonically camouflaged in water. Nat Commun. 2017;8:1–12. doi:10.1038/ncomms14230
51. Zhang K, Ma C, He Q, et al. Metagel with broadband tunable acoustic properties over air–water–solid ranges. Adv Funct Mater. 2019;29:1903699. doi:10.1002/adfm.201903699
52. Braunstein L, Brüningk SC, Rivens I, Civale J, Haar G. Characterization of acoustic, cavitation, and thermal properties of poly(vinyl alcohol) hydrogels for use as therapeutic ultrasound tissue mimics. Ultrasound Med Biol. 2022;48:1095–1109. doi:10.1016/j.ultrasmedbio.2022.02.007
53. Zell K, Sperl JI, Vogel MW, Niessner R, Haisch C. Acoustical properties of selected tissue phantom materials for ultrasound imaging. Phys Med Biol. 2007;52:N475. doi:10.1088/0031-9155/52/20/N02
54. Wei P, Cornel EJ, Du J. Ultrasound-responsive polymer-based drug delivery systems. Drug Deliv Transl Res. 2021;11:1323–1339. doi:10.1007/s13346-021-00963-0
55. Joshi A, Chaudhari R, Jayant RD. On-demand controlled drug delivery. Adv Personal Nanotherap. 2017;2017:131–156.
56. Escoffre J-M, Novell A, de Smet M, Bouakaz A. Focused ultrasound mediated drug delivery from temperature-sensitive liposomes: in-vitro characterization and validation. Phys Med Biol. 2013;58:8135. doi:10.1088/0031-9155/58/22/8135
57. Kamaly N, Yameen B, Wu J, Farokhzad OC. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem Rev. 2016;116:2602–2663. doi:10.1021/acs.chemrev.5b00346
58. Karimi M, Ghasemi A, Zangabad PS, et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem Soc Rev. 2016;45:1457–1501. doi:10.1039/c5cs00798d
59. Cressey P, Amrahli M, So P-W, Gedroyc W, Wright M, Thanou M. Image-guided thermosensitive liposomes for focused ultrasound enhanced co-delivery of carboplatin and SN-38 against triple negative breast cancer in mice. Biomaterials. 2021;271:120758. doi:10.1016/j.biomaterials.2021.120758
60. Tang H, Guo Y, Peng L, et al. In vivo targeted, responsive, and synergistic cancer nanotheranostics by magnetic resonance imaging-guided synergistic high-intensity focused ultrasound ablation and chemotherapy. ACS Appl Mater Interfaces. 2018;10:15428–15441. doi:10.1021/acsami.8b01967
61. Mukhopadhyay D, Sano C, AlSawaftah N, El-Awady R, Husseini GA, Paul V. Ultrasound-mediated cancer therapeutics delivery using micelles and liposomes: a review. Recent Pat Anticancer Drug Discov. 2021;16:498–520. doi:10.2174/1574892816666210706155110
62. Bhatnagar S, Kwan JJ, Shah AR, Coussios -C-C, Carlisle RC. Exploitation of sub-micron cavitation nuclei to enhance ultrasound-mediated transdermal transport and penetration of vaccines. J Control Release. 2016;238:22–30. doi:10.1016/j.jconrel.2016.07.016
63. Ahmadi F, McLoughlin IV, Chauhan S, Ter-haar G. Bio-effects and safety of low-intensity, low-frequency ultrasonic exposure. Prog Biophys Mol Biol. 2012;108:119–138. doi:10.1016/j.pbiomolbio.2012.01.004
64. Mitragotri S. Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nat Rev Drug Discov. 2005;4:255–260. doi:10.1038/nrd1662
65. Averkiou MA, Bruce MF, Powers JE, Sheeran PS, Burns PN. Imaging methods for ultrasound contrast agents. Ultrasound Med Biol. 2020;46:498–517. doi:10.1016/j.ultrasmedbio.2019.11.004
66. Zhou Q-L, Chen Z-Y, Wang Y-X, Yang F, Lin Y, Liao -Y-Y. Ultrasound-mediated local drug and gene delivery using nanocarriers. Biomed Res Int. 2014;2014. doi:10.1155/2014/963891
67. Mayer CR, Geis NA, Katus HA, Bekeredjian R. Ultrasound targeted microbubble destruction for drug and gene delivery. Expert Opin Drug Deliv. 2008;5:1121–1138. doi:10.1517/17425247.5.10.1121
68. Cintas P, Tagliapietra S, Caporaso M, Tabasso S, Cravotto G. Enabling technologies built on a sonochemical platform: challenges and opportunities. Ultrason Sonochem. 2015;25:8–16. doi:10.1016/j.ultsonch.2014.12.004
69. Hervouet E, Simonnet H, Godinot C. Mitochondria and reactive oxygen species in renal cancer. Biochimie. 2007;89:1080–1088. doi:10.1016/j.biochi.2007.03.010
70. Zhang L, Lin Z, Zeng L, et al. Ultrasound-induced biophysical effects in controlled drug delivery. Sci China Life Sci. 2021;2021:1–13.
71. Yang Y, Li Q, Guo X, Tu J, Zhang D. Mechanisms underlying sonoporation: interaction between microbubbles and cells. Ultrason Sonochem. 2020;67:105096. doi:10.1016/j.ultsonch.2020.105096
72. Sun T, Zhang Y, Power C, et al. Closed-loop control of targeted ultrasound drug delivery across the blood–brain/tumor barriers in a rat glioma model. Proc Natl Acad Sci. 2017;114:E10281–E90. doi:10.1073/pnas.1713328114
73. Liu H-L, Fan C-H, Ting C-Y, Yeh C-K. Combining microbubbles and ultrasound for drug delivery to brain tumors: current progress and overview. Theranostics. 2014;4:432. doi:10.7150/thno.8074
74. Joshi B, Joshi A. Ultrasound-based drug delivery systems. In: Bioelectronics and Medical Devices. Elsevier; 2019:241–260.
75. Schroeder A, Kost J, Barenholz Y. Ultrasound, liposomes, and drug delivery: principles for using ultrasound to control the release of drugs from liposomes. Chem Phys Lipids. 2009;162:1–16. doi:10.1016/j.chemphyslip.2009.08.003
76. Ma J, Du LF, Chen M, et al. Drug‑loaded nano‑microcapsules delivery system mediated by ultrasound‑targeted microbubble destruction: a promising therapy method. Biomed Rep. 2013;1:506–510. doi:10.3892/br.2013.110
77. Zhao W, Jin X, Cong Y, Liu Y, Fu J. Degradable natural polymer hydrogels for articular cartilage tissue engineering. J Chem Technol Biotechnol. 2013;88:327–339. doi:10.1002/jctb.3970
78. Hyun H, Park MH, Lim W, et al. Injectable visible light-cured glycol chitosan hydrogels with controlled release of anticancer drugs for local cancer therapy in vivo: a feasible study. Artif Cells, Nanomed Biotechnol. 2018;46:874–882. doi:10.1080/21691401.2018.1470529
79. Pertici V, Pin-Barre C, Rivera C, et al. Degradable and injectable hydrogel for drug delivery in soft tissues. Biomacromolecules. 2018;20:149–163. doi:10.1021/acs.biomac.8b01242
80. Collins MN, Birkinshaw C. Hyaluronic acid based scaffolds for tissue engineering—a review. Carbohydr Polym. 2013;92:1262–1279. doi:10.1016/j.carbpol.2012.10.028
81. Tiwari G, Tiwari R, Sriwastawa B, et al. Drug delivery systems: an updated review. Int J Pharm Investig. 2012;2:2. doi:10.4103/2230-973X.96920
82. Bessa PC, Casal M, Reis R. Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery). J Tissue Eng Regen Med. 2008;2:81–96. doi:10.1002/term.74
83. Jayakumar R, Prabaharan M, Kumar PS, Nair S, Tamura H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv. 2011;29:322–337. doi:10.1016/j.biotechadv.2011.01.005
84. Pandit A, Ashar R, Feldman D. The effect of TGF-beta delivered through a collagen scaffold on wound healing. J Investig Surg. 1999;12:89–100. doi:10.1080/089419399272647
85. Momoh FU, Boateng JS, Richardson SC, Chowdhry BZ, Mitchell JC. Development and functional characterization of alginate dressing as potential protein delivery system for wound healing. Int J Biol Macromol. 2015;81:137–150. doi:10.1016/j.ijbiomac.2015.07.037
86. Thorn R, Greenman J, Austin A. An in vitro study of antimicrobial activity and efficacy of iodine-generating hydrogel dressings. J Wound Care. 2006;15:305–310. doi:10.12968/jowc.2006.15.7.26929
87. Hariyadi DM, Islam N. Current status of alginate in drug delivery. Adv Pharmacol Pharm Sci. 2020;2020:1–16. doi:10.1155/2020/8886095
88. Nazarnezhada S, Abbaszadeh-Goudarzi G, Samadian H, et al. Alginate hydrogel containing hydrogen sulfide as the functional wound dressing material: in vitro and in vivo study. Int J Biol Macromol. 2020;164:3323–3331. doi:10.1016/j.ijbiomac.2020.08.233
89. Zhang L, Chen J, Han C. A multicenter clinical trial of recombinant human GM‐CSF hydrogel for the treatment of deep second‐degree burns. Wound Repair Regener. 2009;17:685–689. doi:10.1111/j.1524-475X.2009.00526.x
90. Lynch CR, Kondiah PP, Choonara YE, Du Toit LC, Ally N, Pillay V. Hydrogel biomaterials for application in ocular drug delivery. Front Bioengine Biotechnol. 2020;8:228. doi:10.3389/fbioe.2020.00228
91. Kumar A, Pillai J. Implantable drug delivery systems: an overview. In: Nanostructures for the Engineering of Cells, Tissues and Organs. Elsevier; 2018:473–511.
92. Yu L, Ding J. Injectable hydrogels as unique biomedical materials. Chem Soc Rev. 2008;37:1473–1481. doi:10.1039/b713009k
93. Le TMD, Jung B-K, Li Y, et al. Physically crosslinked injectable hydrogels for long-term delivery of oncolytic adenoviruses for cancer treatment. Biomater Sci. 2019;7:4195–4207. doi:10.1039/C9BM00992B
94. Lee JH. Injectable hydrogels delivering therapeutic agents for disease treatment and tissue engineering. Biomater Res. 2018;22:1–14. doi:10.1186/s40824-018-0138-6
95. La Gatta A, Schiraldi C, Papa A, De Rosa M. Comparative analysis of commercial dermal fillers based on crosslinked hyaluronan: physical characterization and in vitro enzymatic degradation. Polym Degrad Stab. 2011;96:630–636. doi:10.1016/j.polymdegradstab.2010.12.025
96. Kim Z-H, Lee Y, Kim S-M, Kim H, Yun C-K, Choi Y-S. A composite dermal filler comprising cross-linked hyaluronic acid and human collagen for tissue reconstruction. J Microbiol Biotechnol. 2015;25:399–406. doi:10.4014/jmb.1411.11029
97. Forwith KD, Chandra RK, Yun PT, Miller SK, Jampel HD. ADVANCE: a multisite trial of bioabsorbable steroid‐eluting sinus implants. Laryngoscope. 2011;121:2473–2480. doi:10.1002/lary.22228
98. Matheny KE, Carter KB, Tseng EY, Fong KJ. Safety, feasibility, and efficacy of placement of steroid‐eluting bioabsorbable sinus implants in the office setting: a prospective case series. In: International Forum of Allergy & Rhinology. Wiley Online Library; 2014:808–815.
99. Mohamed M, Borchard G, Jordan O. In situ forming implants for local chemotherapy and hyperthermia of bone tumors. J Drug Deliv Sci Technol. 2012;22:393–408. doi:10.1016/S1773-2247(12)50066-3
100. Fumimoto Y, Matsuyama A, Komoda H, et al. Creation of a rich subcutaneous vascular network with implanted adipose tissue–derived stromal cells and adipose tissue enhances subcutaneous grafting of islets in diabetic mice. Tissue Eng Part C Methods. 2009;15:437–444. doi:10.1089/ten.tec.2008.0555
101. Grabowska-Derlatka L, Derlatka P, Szeszkowski W, Cieszanowski A. Diffusion-weighted imaging of small peritoneal implants in “potentially” early-stage ovarian cancer. Biomed Res Int. 2016;2016. doi:10.1155/2016/9254742
102. Seynhaeve A, Amin M, Haemmerich D, Van Rhoon G, Ten Hagen T. Hyperthermia and smart drug delivery systems for solid tumor therapy. Adv Drug Deliv Rev. 2020;163:125–144. doi:10.1016/j.addr.2020.02.004
103. Roovers S, Segers T, Lajoinie G, et al. The role of ultrasound-driven microbubble dynamics in drug delivery: from microbubble fundamentals to clinical translation. Langmuir. 2019;35:10173–10191. doi:10.1021/acs.langmuir.8b03779
104. Wang X, Yan F, Liu X, et al. Enhanced drug delivery using sonoactivatable liposomes with membrane-embedded porphyrins. J Control Release. 2018;286:358–368. doi:10.1016/j.jconrel.2018.07.048
105. Meng Z, Zhang Y, She J, et al. Ultrasound-mediated remotely controlled nanovaccine delivery for tumor vaccination and individualized cancer immunotherapy. Nano Lett. 2021;21:1228–1237. doi:10.1021/acs.nanolett.0c03646
106. She J, Zhou X, Zhang Y, et al. Thermo‐triggered in situ chitosan‐based gelation system for repeated and enhanced sonodynamic therapy post a single injection. Adv Healthcare Mater. 2021;10:2001208. doi:10.1002/adhm.202001208
107. Kubota T, Kurashina Y, Zhao J, Ando K, Onoe H. Ultrasound-triggered on-demand drug delivery using hydrogel microbeads with release enhancer. Mater Des. 2021;203:109580. doi:10.1016/j.matdes.2021.109580
108. Gerayeli F, Khalef N, Bakri A, Benech P, Martin DK. Ultrasound-stimulated Brownian ratchet enhances diffusion of molecules retained in hydrogels. Nanomed Nanotechnol Biol Med. 2021;31:102308. doi:10.1016/j.nano.2020.102308
109. Devarakonda SB, Myers MR, Lanier M, Dumoulin C, Banerjee RK. Assessment of gold nanoparticle-mediated-enhanced hyperthermia using MR-guided high-intensity focused ultrasound ablation procedure. Nano Lett. 2017;17:2532–2538. doi:10.1021/acs.nanolett.7b00272
110. Bez M, Sheyn D, Tawackoli W, et al. In situ bone tissue engineering via ultrasound-mediated gene delivery to endogenous progenitor cells in mini-pigs. Sci Transl Med. 2017;9:eaal3128. doi:10.1126/scitranslmed.aal3128
111. Sitta J, Howard CM. Applications of ultrasound-mediated drug delivery and gene therapy. Int J Mol Sci. 2021;22:11491. doi:10.3390/ijms222111491
112. Yang F, Li S, Jiao M, et al. Advances of light/ultrasound/magnetic-responsive nanoprobes for visualized theranostics of urinary tumors. ACS Appl Bio Mater. 2022;5:438–450.
113. Eng YJ, Xu J, Sugiarto S, et al. Initiator-free photohydrogelation of linear coumarin-containing poly(ethylene glycol) methacrylates and study of ultrasound-triggered gel breakdown. ACS Appl Polymer Mater. 2021;3:4264–4274. doi:10.1021/acsapm.1c00752
114. Jiang H, Kobayashi T. Ultrasound stimulated release of gallic acid from chitin hydrogel matrix. Mater Sci Engine C. 2017;75:478–486. doi:10.1016/j.msec.2017.02.082
115. An JY, Um W, You DG, et al. Gold-installed hyaluronic acid hydrogel for ultrasound-triggered thermal elevation and on-demand cargo release. Int J Biol Macromol. 2021;193:553–561. doi:10.1016/j.ijbiomac.2021.10.071
116. Luo M-H, Yeh C-K, Situ B, Yu J-S, Li B-C, Chen Z-Y. Microbubbles: a novel strategy for chemotherapy. Curr Pharm Des. 2017;23:3383–3390. doi:10.2174/1381612823666170113092148
117. Jahanban-Esfahlan R, Soleimani K, Derakhshankhah H, et al. Multi-stimuli-responsive magnetic hydrogel based on Tragacanth gum as a de novo nanosystem for targeted chemo/hyperthermia treatment of cancer. J Mater Res. 2021;36:858–869. doi:10.1557/s43578-021-00137-1
118. Zhu J, Wang X, Yang D, et al. Ultrasound‐triggered in situ gelation to overcome tumor hypoxia for enhanced photodynamic and sustained chemotherapy. Adv Therap. 2021;4:2100052. doi:10.1002/adtp.202100052
119. Wu C-H, Sun M-K, Kung Y, et al. One injection for one-week controlled release: in vitro and in vivo assessment of ultrasound-triggered drug release from injectable thermoresponsive biocompatible hydrogels. Ultrason Sonochem. 2020;62:104875. doi:10.1016/j.ultsonch.2019.104875
120. Lee J, Min H-S, You DG, et al. Theranostic gas-generating nanoparticles for targeted ultrasound imaging and treatment of neuroblastoma. J Control Release. 2016;223:197–206. doi:10.1016/j.jconrel.2015.12.051
121. Wang X, Niu D, Li P, et al. Dual-enzyme-loaded multifunctional hybrid nanogel system for pathological responsive ultrasound imaging and T 2-weighted magnetic resonance imaging. ACS Nano. 2015;9:5646–5656. doi:10.1021/nn5068094
122. Wu Q, Zhang Q, Yu T, et al. Self-assembled hybrid nanogel as a multifunctional theranostic probe for enzyme-regulated ultrasound imaging and tumor therapy. ACS Appl Bio Mater. 2021;4:4244–4253. doi:10.1021/acsabm.1c00079
123. Kearney CJ, Skaat H, Kennedy SM, et al. Switchable release of entrapped nanoparticles from alginate hydrogels. Adv Healthcare Mater. 2015;4:1634–1639. doi:10.1002/adhm.201500254
124. Levingstone T, Ali B, Kearney C, Dunne N. Hydroxyapatite sonosensitization of ultrasound‐triggered, thermally responsive hydrogels: an on‐demand delivery system for bone repair applications. J Biomed Mater Res B Appl Biomater. 2021;109:1622–1633. doi:10.1002/jbm.b.34820
125. Khan AR, Yang X, Fu M, Zhai G. Recent progress of drug nanoformulations targeting to brain. J Control Release. 2018;291:37–64. doi:10.1016/j.jconrel.2018.10.004
126. Grimaudo MA, Krishnakumar GS, Giusto E, et al. Bioactive injectable hydrogels for on demand molecule/cell delivery and for tissue regeneration in the central nervous system. Acta Biomaterialia. 2022;140:88–101. doi:10.1016/j.actbio.2021.11.038
127. Koetting MC, Peters JT, Steichen SD, Peppas NA. Stimulus-responsive hydrogels: theory, modern advances, and applications. Mater Sci Engine. 2015;93:1–49.
128. Fisher DG, Price RJ. Recent advances in the use of focused ultrasound for magnetic resonance image-guided therapeutic nanoparticle delivery to the central nervous system. Front Pharmacol. 2019;1348. doi:10.3389/fphar.2019.01348
129. Cheng KW, Alhasan L, Rezk AR, et al. Fast three-dimensional micropatterning of PC12 cells in rapidly crosslinked hydrogel scaffolds using ultrasonic standing waves. Biofabrication. 2019;12:015013. doi:10.1088/1758-5090/ab4cca
130. Barnes LA, Marshall CD, Leavitt T, et al. Mechanical forces in cutaneous wound healing: emerging therapies to minimize scar formation. Adv Wound Care. 2018;7:47–56. doi:10.1089/wound.2016.0709
131. Wang J, Kaplan JA, Colson YL, Grinstaff MW. Mechanoresponsive materials for drug delivery: harnessing forces for controlled release. Adv Drug Deliv Rev. 2017;108:68–82. doi:10.1016/j.addr.2016.11.001
132. Katsarou A, Gudbjörnsdottir S, Rawshani A, et al. Type 1 diabetes mellitus. Nat Rev Dis Primers. 2017;3:1–17. doi:10.1038/nrdp.2017.16
133. Di J, Yu J, Wang Q, et al. Ultrasound-triggered noninvasive regulation of blood glucose levels using microgels integrated with insulin nanocapsules. Nano Res. 2017;10:1393–1402. doi:10.1007/s12274-017-1500-z
134. Fan C, Shi J, Zhuang Y, et al. Myocardial‐infarction‐responsive smart hydrogels targeting matrix metalloproteinase for on‐demand growth factor delivery. Adv Mater. 2019;31:1902900. doi:10.1002/adma.201902900
135. Raucci MG, D’Amora U, Ronca A, Ambrosio L. Injectable functional biomaterials for minimally invasive surgery. Adv Healthcare Mater. 2020;9:2000349. doi:10.1002/adhm.202000349
136. Wu C-H, Sun M-K, Shieh J, et al. Ultrasound-responsive NIPAM-based hydrogels with tunable profile of controlled release of large molecules. Ultrasonics. 2018;83:157–163. doi:10.1016/j.ultras.2017.03.019
137. Chen P, Wang Q, Wan X, et al. Wireless electrical stimulation of the vagus nerves by ultrasound-responsive programmable hydrogel nanogenerators for anti-inflammatory therapy in sepsis. Nano Energy. 2021;89:106327. doi:10.1016/j.nanoen.2021.106327
138. Upadhyay A, Dalvi SV. Microbubble formulations: synthesis, stability, modeling and biomedical applications. Ultrasound Med Biol. 2019;45:301–343.
139. Nardecchia S, Sánchez-Moreno P, de Vicente J, Marchal JA, Boulaiz H. Clinical trials of thermosensitive nanomaterials: an overview. Nanomaterials. 2019;9:191. doi:10.3390/nano9020191
140. Mandal A, Clegg JR, Anselmo AC, Mitragotri S. Hydrogels in the clinic. Bioengine Transl Med. 2020;5:e10158. doi:10.1002/btm2.10158
141. Hossen S, Hossain MK, Basher M, Mia M, Rahman M, Uddin MJ. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: a review. J Adv Res. 2019;15:1–18. doi:10.1016/j.jare.2018.06.005
142. Sayes CM, Reed KL, Warheit DB. Assessing toxicity of fine and nanoparticles: comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicol Sci. 2007;97:163–180. doi:10.1093/toxsci/kfm018
143. Kunzmann A, Andersson B, Thurnherr T, Krug H, Scheynius A, Fadeel B. Toxicology of engineered nanomaterials: focus on biocompatibility, biodistribution and biodegradation. Biochimica et Biophysica Acta. 2011;1810:361–373. doi:10.1016/j.bbagen.2010.04.007
144. Standardization IOf. ISO/TR 10993-22 Biological Evaluation of Medical Devices–Part 22: Guidance on Nanomaterials. ISO Geneva; 2017.
145. Anchordoquy TJ, Barenholz Y, Boraschi D, et al. Mechanisms and Barriers in Cancer Nanomedicine: Addressing Challenges, Looking for Solutions. ACS Publications; 2017.
146. Shankar H, Pagel PS, Warner DS. Potential adverse ultrasound-related biological effects: a critical review. J Am Soc Anesthesiolog. 2011;115:1109–1124.
147. Rajabi M, Srinivasan M, Mousa SA. Nanobiomaterials in drug delivery. In: Nanobiomaterials in Drug Delivery. Elsevier; 2016:1–37.
148. Vijayasekaran S, Chirila T, Robertson TA, et al. Calcification of poly (2-hydroxyethyl methacrylate) hydrogel sponges implanted in the rabbit cornea: a 3-month study. J Biomater Sci. 2000;11:599–615. doi:10.1163/156856200743896
149. Vegas AJ, Veiseh O, Doloff JC, et al. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nat Biotechnol. 2016;34:345–352. doi:10.1038/nbt.3462
150. Yin Y, Jiang X, Sun L, et al. Continuous inertial cavitation evokes massive ROS for reinforcing sonodynamic therapy and immunogenic cell death against breast carcinoma. Nano Today. 2021;36:101009. doi:10.1016/j.nantod.2020.101009
151. Wuschko S, Gugerell A, Chabicovsky M, et al. Toxicological testing of allogeneic secretome derived from peripheral mononuclear cells (APOSEC): a novel cell-free therapeutic agent in skin disease. Sci Rep. 2019;9:1–14. doi:10.1038/s41598-019-42057-5
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.