Back to Journals » Drug Design, Development and Therapy » Volume 15
Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry for Simultaneous Determination of Antipsychotic Drugs in Human Plasma and Its Application in Therapeutic Drug Monitoring
Received 3 December 2020
Accepted for publication 21 January 2021
Published 12 February 2021 Volume 2021:15 Pages 463—479
DOI https://doi.org/10.2147/DDDT.S290963
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Tuo Deng
Yingjie Qi, Guangxuan Liu
Department of Pharmacy, Cancer Hospital of China Medical University, Liaoning Cancer Hospital & Institute, Shenyang, 110042, Liaoning Province, People’s Republic of China
Correspondence: Yingjie Qi
Department of Pharmacy, Cancer Hospital of China Medical University, Liaoning Cancer Hospital & Institute, Number 44 Xiaoheyan Road, Dadong District, Shenyang, 110042, Liaoning Province, People’s Republic of China
Tel +8618909811762
Email [email protected]
Purpose: We developed and validated an ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method for simultaneous therapeutic drug monitoring (TDM) and clinical pharmacokinetic antipsychotic drugs: clozapine (CLP), chlorpromazine (CPZ), risperidone (RPD), paliperidone (PLD), quetiapine (QTP;), aripiprazole (APZ), dehydroaripiprazole (DAP), olanzapine (OZP), ziprasidone (ZRD), and amisulpride (ASP).
Methods: Analytes and internal standards (ISs) were separated using a Phenomenex phenyl-hexyl column (50.0 × 2.1 mm, 1.7 μm) with water containing 0.1% formic acid and 2 mM ammonium acetate, and methanol containing 0.1% formic acid and 2 mM ammonium acetate as the mobile phase. The antipsychotic drugs and ISs were extracted from 50 μL of plasma using acetonitrile.
Results: The calibration ranges were 25.0– 1500.0 ng/mL for CLP, CPZ, DAP, and QTP, 10.0– 600.0 ng/mL for CPZ and ZRD, 2.5– 150.0 ng/mL for RPD, 5.0– 300.0 ng/mL for PLD and OZP, and 20.0– 1200.0 ng/mL for ASP. Validation was carried out according to the guidelines for bioanalytical validation, including assessment of calibration curves, specificity, accuracy, precision, recovery, stability, dilution integrity, and matrix effect. All the results satisfied the requirements.
Conclusion: The results provided significant information to support future clinical TDM and rational drug use research. The proposed method also provided a simple, reliable, specific, and suitable technique for TDM and pharmacokinetic studies.
Keywords: UPLC, tandem mass spectrometry, antipsychotic drug, therapeutic drug monitoring, individualized therapy
Introduction
Mental illness can seriously endanger human health and life. More than 400 million people worldwide are currently suffering from psychosocial, neurological, or mental disorders according to a World Health Organization report, and the number of patients with mental disorders is growing rapidly.1–3 There are currently approximately 16 million people with mental illnesses in China, including 9 million patients with schizophrenia.4 The main treatment methods for mental illnesses include drug treatment supplemented by psychological support.5–9 However, mental illnesses such as Schizophrenia have a chronic relapsing course, despite the intake of anti psychotic therapy as well as the specific characteristics of the drug. The drug concentration may have an important impact, with similar doses having different effects because of individual differences in pharmacokinetics.10,11 In addition, clinical judgments regarding the use of antipsychotic drugs are hampered by a lack of clear indicators, and diagnoses may thus be influenced by clinical training, experience, and other subjective factors.12 It is also difficult to distinguish side effects and toxic reactions from aggravated disease symptoms.13,14 Combination therapies involving different classes of drugs are commonly used to maximize therapeutic benefit while minimizing toxic side effects in patients with mental illness, in light of the reported side effects associated with the use of psychotropic drugs. However, drug–drug interactions may also affect the concentration and efficacy of antipsychotic drugs used in combination. Previous clinical studies showed that the efficacy and toxicity of antipsychotic drugs were both closely related to their serum concentrations.15,16 Drug dosages should thus be individualized for different patients to maintain plasma drug concentrations within a safe and effective range, to ensure the efficacy and control the side effects of antipsychotic drugs in clinical practice.
Therapeutic drug monitoring (TDM) is a clinical technology developed in the 1970s. It uses modern analytical techniques to determine the concentrations of drugs in body fluids based on pharmacokinetic and pharmacodynamic principles, to study the relationships between drug concentration and clinical efficacy and toxicity, and to provide a theoretical basis for individualized clinical dosing regimens. TDM is currently more commonly used and standardized in developed regions such as Europe, America, and Japan. TDM has become a routine aspect of clinical rational drug use for some drugs, including immunosuppressants (cyclosporine, tacrolimus), psychotropic drugs (anti-epileptic drugs), antibiotics (voriconazole, linezolid, vancomycin), anti-tumor drugs (methotrexate, imatinib, paclitaxel), and cardiovascular drugs (digoxin). TDM has become an important role for modern hospital pharmacies. Regarding the application status of antipsychotic drugs, inter-individual pharmacokinetic differences are an important factor affecting the poor clinical efficacy of psychotropic drugs.17–19 Numerous factors can cause fluctuations in blood concentration of psychotropic drugs, including genetic polymorphisms of metabolic enzymes and transporters, differences in drug preparations, changes in liver and kidney functions, and drug–drug interactions.20–22 TDM is therefore important for guiding the individualized clinical application of antipsychotic drugs.
TDM of antipsychotic drug levels in plasma requires a rapid, simple, reproducible, and highly sensitive analytical method. Various methods, including fluorescent polarization immunoassay (FPIA), high-performance liquid chromatography (HPLC), HPLC-tandem mass spectrometry (MS/MS), and ultra-performance liquid chromatography (UPLC)-MS/MS are currently used for TDM of antipsychotic drugs.23,24 Among these methods, UPLC-MS/MS is the most common and promising method. UPLC-MS/MS has several obvious advantages over other methods,25–28 including: 1) higher selectivity and sensitivity than HPLC and FPIA; 2) lower operating costs than FPIA with no need for specific immunoassay kits; 3) high specificity and unaffected by metabolites and other drugs, making it especially suitable for analyzing combinations of drugs; and 4) the ability to detect multiple drugs at the same time. UPLC-MS/MS has thus become the most common method for the in vivo determination of drugs and TDM.
The current study thus aimed to develop and validate a new UPLC-MS/MS method for the simultaneous determination of 10 antipsychotic drugs in human plasma using the corresponding isotopic-labeled internal standards (ISs) and to investigate its application in clinical TDM. The study also aimed to examine the relationship between the blood concentrations of antipsychotic drugs and their efficacy and toxicity. The results showed that the established method could simultaneously determine the 10 tested antipsychotic drugs, with fast detection speed, high accuracy, high sensitivity, and low cost. These results will support the use of clinical TDM and rational drug use research.
Methods
Chemicals and Reagents
Clozapine (CLP), chlorpromazine (CPZ), risperidone (RPD), paliperidone (PLD), quetiapine (QTP), aripiprazole (APZ), dehydroaripiprazole (DAP), olanzapine (OZP), ziprasidone (ZRD), amisulpride (ASP) and internal standards (CLP-d8, RPD-d4, APZ-d8, OZP-d8, ASP-d5) were obtained from the National Institutes for Food and Drug Control (Beijing, China) and TRC (Toronto, Canada). The chemical structure diagram of analytes and internal standards were shown in Figure 2. Plasma sample-processing kits were purchased from Calibra Medical Equipment Co., Ltd. (Hangzhou, China). Methanol and acetonitrile were purchased from Fisher Scientific, chromatography-grade ammonium acetate was purchased from J&K Scientific Ltd., and formic acid was purchased from Sigma-Aldrich (St. Louis, MO, USA). Pure water was prepared using a Milli-Q water purification system (Millipore Corp., USA).
Equipment and Conditions
UPLC
Chromatography was performed using an Agilent 1290 UPLC system. Compounds were separated on a Phenomenex phenyl-hexyl C18 column (50.0 × 2.1 mm, 1.7 μm; Phenomenex Inc., USA). The mobile phase was composed of water containing 0.1% (v/v) formic acid and 2 mM/L ammonium acetate, and methanol containing 0.1% (v/v) formic acid and 2 mM/L ammonium acetate. The gradient method is shown in Table 1. The column temperature was set to 35°C and the sample injection volume was 2.0 μL.
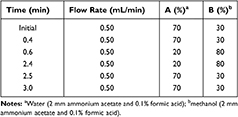 |
Table 1 Gradient Condition of HPLC |
MS
MS was carried out using a QTRAP 4500 MS system (AB Sciex) with electrospray ionization as the detector. Data were processed using Analyst v1.6.2 software was used as data processing system. High-purity nitrogen was produced by the nitrogen generator SCIWAY ZABN (Shanghai, China). Critical MS parameters including ion spray voltage, collision energy, and declustering potential were optimized to maximize sensitivity. The results are presented in Table 2 and Figure 3. Other optimized MS parameters included source temperature 400°C, entrance potential 20, curtain gas 30, GS1 25, and GS2 20.
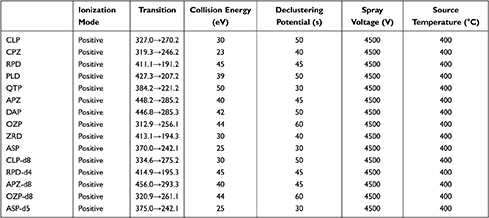 |
Table 2 MS Parameters of Analytes and IS |
Preparation of Calibrators and Quality Control (QC) Samples
Analyses and IS stock solutions were prepared by dissolving the standards in HPLC-grade methanol to a final concentration of 100.0 μg/mL. The calibration and QC solutions were prepared by diluting the stock solutions with HPLC-grade methanol. Calibration curve and QC samples were then prepared by adding different concentrations of the standard solutions to 50.0 μL blank plasma to produce the following calibration concentrations: 25.0, 50.0, 100.0, 300.0, 750.0, and 1500.0 ng/mL for CLP, APZ, DAP, and QTP; 10.0, 20.0, 40.0, 120.0, 300.0, and 600.0 ng/mL for CPZ and ZRD; 2.5, 5.0, 10.0, 30.0, 75.0, and 150.0 ng/mL for RPD; 5.0, 10.0, 20.0, 60.0, 150.0, and 300.0 ng/mL for PLD and OZP; and 20.0, 40.0, 80.0240.0, 600.0, and 1200.0 ng/mL for ASP. The concentration selection of the calibration curve and QC samples is mainly based on the effective concentration range of these ten antipsychotic drugs. Therefore, the wide linear range and low LLOQ did not have much application significance in terms of TDM.
Four QC samples to determine the lower limit of quantification (LLOQ), low concentration quality control (LQC), medium concentration quality control (MQC), and high concentration quality control (HQC), respectively, were freshly prepared, and the following concentrations were used: 25.0, 50.0, 500.0, and 1200.0 ng/mL for CLP, APZ, DAP, and QTP; 10.0, 20.0, 50.0, and 480.0 ng/mL for CPZ and ZRD; 2.5, 5.0, 25.0, and 120.0 ng/mL for RPD; 5.0, 10.0, 50.0, and 240.0 ng/mL for PLD and OZP; and 20.0, 40.0, 200.0, and 960.0 ng/mL for ASP.
The LLOQs were 25.0 ng/mL for CLP, APZ, DAP, and QTP, 10.0 ng/mL for CPZ and ZRD, 2.5 ng/mL for RPD, 5.0 ng/mL for PLD, and 20.0 ng/mL for ASP.
IS working solutions of 100 ng/mL for CLP-d8, APZ-d8, and ASP-d5, and 20 ng/mL for RPD-d4 and OZP-d8 were prepared by dilution of the stock solution of each IS in methanol. APZ-d8 was used as a common IS for APZ, DAP, CLP, QTP, DAP, and ZRD, and RPD-d4 was chosen as a common IS for RPD and PLD.
Plasma Samples
Plasma samples were prepared by protein precipitation using acetonitrile. The plasma samples were processed as follows: 50.0 μL plasma sample, 20.0 μL IS solution, and 150.0 μL protein precipitant were transferred to an Eppendorf tube and vortexed for 5.0 min. The samples were then centrifuged at 15,000.0 rpm for 8.0 min at 4°C and 200 μL of purified water was added to 40 μL supernatant. After thorough vortexing for 1.0 min, the analyte solutions were injected into the UPLC-MS/MS system for analysis.
Method Validation
The method was fully validated using healthy human plasma according to the European Medicines Agency29 and US Food and Drug Administration guidelines for bioanalytical method validation.30
Specificity
Specificity was determined by comparing chromatograms of blank plasma (eight different human plasma samples) with corresponding spiked plasma samples to eliminate the effects of interference from endogenous compounds. Specificity was also investigated by analyzing 30 potential co-medications, including nine antiepileptics (levetiracetam, oxcarbazepine, topiramate, phenytoin, phenobarbital, lamotrigine, mono-hydroxy-carbazepine, carbamazepine, valproic acid), eight anti-insomnia drugs (diazepam, nitrazepam, flunitrazepam, triazolam, estazolam, oxazepam, zolpidem, zopiclone), 10 antidepressants (clomipramine, amitriptyline, doxepin, venlafaxine, maprotiline, amoxapine, trazodone, mianserin, fluoxetine, moclobemide), and three anxiolytics (buspirone, gepirone, ipsapirone) at therapeutic plasma levels. The MRM signal of interference for each analyte should be <20% of the LLOQ for the corresponding analytes and <5% for the corresponding IS. We also examined crosstalk between the analytes and the IS. We evaluated the effect of the IS on the response of each analyte by analyzing extracted blank plasma spiked with only IS in five replicates. The MRM signal in the transition window of each analyte should be ≤20% of the LLOQ. The effect of the analytes on the response of each IS was evaluated by analyzing the highest standard samples without IS in five replicates. The MRM signals in the transition window of each IS should be ≤5% of the IS in the LLOQ.
Linearity and LLOQ
We determined linearity by analyzing three complete standard curves on three different days. The calibration curve concentration ranges were 25–1500 ng/mL for CLP, CPZ, DAP, and QTP, 10.0–600.0 ng/mL for CPZ and ZRD, 2.5–150.0 ng/mL for RPD, 5.0–300.0 ng/mL for PLD and OZP, and 20.0–1200.0 ng/mL for ASP. The LLOQ of each molecule was determined based on the signal-to-noise ratio, which was at least 10:1.
Accuracy and Precision
Repeatability and reproducibility were investigated by analyzing the intra- and inter-day precision (RSD%) and accuracy (RE%) of the four levels of QCs. Six replicates of each level of QC were assayed in one run for the intra-day study and in three runs on three different days for the inter-day study. The RSD and RE should be within ±15%, except LLOQ which should not exceed 20%. The carryover was evaluated by injecting a blank sample after the ULOQ sample of the calibration standard, which should not exceed 20% of LLOQ.
Recovery and Matrix Effect
The absolute extraction recoveries of the analytes and IS were calculated by comparing the peak area of blank plasma spiked with analytes before extraction with those of the extracted blank plasma spiked with analytes. The matrix effect was obtained by comparing the mean peak areas of the analytes directly diluted in extracted blank plasma with the peak areas of the analytes diluted in water at the same concentration. For the recovery and matrix effect tests, four concentrations at LLOQ and three QCs were analyzed with six replicates in a single run. According to the guidelines, recovery and matrix effects ≤15% were considered acceptable.
Stability
Stability tests were carried out at three QC levels under different conditions to ensure the stability and correctness of the method, including room temperature (12 h), autosampler (24 h in the autosampler at 4°C), freeze-thaw (three freeze-thaw cycles (−20.0°C to room temperature)), and long-term stabilities (at −80°C for 30 days).
Dilution Integrity
Dilution integrity was investigated to ensure the dilution reliability of the plasma samples, because the concentrations of some clinical samples were higher than the ULOQ. This was determined by diluting the samples with a 15-fold concentration of ULOQ for each antipsychotic drug to the respective QC level for the standard plasma using a blank samples matrix. The criteria were deemed acceptable when the precision and the accuracy were within ±15%.
Application
We chose steady-state trough concentrations of antipsychotic drugs as the TDM index because these concentrations are highly correlated with efficacy and side effects. Meanwhile, we determined the therapeutic ranges of the antipsychotic drugs based on the Consensus Guidelines for TDM in Neuropsychopharmacology: Update 2017.16 Inclusion criteria were established because of the many different factors affecting drug concentration, including severe liver and kidney damage, drug-drug interactions, and patient compliance. The inclusion criteria were as follows: all patients with a chief psychiatric complaint who were administered one antipsychotic medication; drugs administered according to the manufacturer’s instructions; normal liver and kidney functions or liver and kidney function damage should not affect the in vivo processes of the antipsychotic drugs; and patients should show good compliance with the medication regimen, given that patients with severe mental illness may have medication-compliance issues resulting in exacerbations.
In this study, we collected clinical samples from patients with mental illnesses who were taking antipsychotic drugs (CLP, CPZ, DAP, QTP, CPZ, ZRD, RPD, PLD, OZP, and/or ASP) in the period between April 2018 and May 2020. Consent for the use of the patients’ clinical data for clinical study was signed by the patients’ guardians. Blood samples obtained from the clinic were centrifuged immediately at 5000 rpm for 10 min at 4°C and the plasma layer was transferred to Eppendorf tubes. Blank plasma was obtained from the Hematology Department of Shengjing Hospital for method development and validation.
Results and Discussion
Equipment Optimization
In this study, we chose the Phenomenex phenyl-hexyl column (50.0 × 2.1 mm, 1.7 μm) as the analytical chromatographic column to produce symmetric sharp peaks, appropriate retention times, and good separation for all the tested antipsychotic drugs. It terms of the mobile phase, we used methanol as the organic phase because acetonitrile interacts with the phenyl-hexyl column. We added 2 mM/L ammonium acetate and 0.1% formic acid (v/v) in the mobile phase (water and methanol) to optimize ionization of the analytes. The gradient elution method was applied to improve the peak shapes of all the analytes (Table 1). Under these chromatographic conditions, the retention times were as follows: 1.26 min for OZP, 1.29 min for ASP, 1.36 min for PLD, 1.38 min for RPD, 1.40 min for CLP and DAP, 1.41 min for QTP, 1.44 min for ZRD, 1.46 min for APZ, and 1.48 min for CPZ. In addition, we found that a stronger and more stable signal could be obtained in the positive compared with the negative ion mode. The detected ion pairs for all analytes and the main MS parameters are shown in Table 2. The LLOQs of the 10 antipsychotic drugs were all ≥3-fold lower than the minimum concentration required for TDM according to the consensus guidelines (LLOQ/recommendation: 25/350 ng/mL for CLP, 10/30 ng/mL for CPZ, 2.5/20 ng/mL for RPD, 5/20 ng/mL for PLD, 25/100 ng/mL for QTP, 25/100 ng/mL for APZ, 25/150 ng/mL for DAP, 5/20 ng/mL for OZP, 10/50 ng/mL for ZRD, and 20/100 ng/mL for ASP).16
Method Validation
The method was fully validated using healthy human plasma according to the European Medicines Agency29 and US Food and Drug Administration guidelines for bioanalytical method validation.30
Selectivity and Crosstalk
Typical UPLC-MS/MS chromatograms of analytes are presented in Figure 1. The retention times of CLP, CPZ, RPD, PLD, QTP, APZ, DAP, OZP, ZRD, and ASP were 1.40, 1.48, 1.38, 1.36, 1.41, 1.46, 1.44, 1.26, 1.40, and 1.29 min, respectively. The endogenous substances in the blank plasma samples did not interfere with the determination of the analytes and ISs based on the chromatograms. These results showed that it was feasible to process plasma samples by protein precipitation. In the selectivity analysis, we tested 39 drugs and eight different blank human plasma samples. No significant interference from exogenous or endogenous compounds was detected in any of the tests, and there was no obvious crosstalk between the analytes and IS.
 |
Figure 1 Continue. |
 |
Figure 1 Continue. |
 |
Figure 1 Continue. |
 |
Figure 1 Continue. |
 |
Figure 2 Chemical structure diagram of analytes and internal standards. |
 |
Figure 3 The chromatograms of daughter scan of analytes and internal standards. |
Linearity and LLOQ
Good linearity was obtained from the calibration curves at concentration ranges of 25–1500 ng/mL for CLP, CPZ, DAP, and QTP, 10.0–600.0 ng/mL for CPZ and ZRD, 2.5–150.0 ng/mL for RPD, 5.0–300.0 ng/mL for PLD and OZP, and 20.0–1200.0 ng/mL for ASP. Typical equations of the calibration curves were as follows: y=0.0676x+0.225 (r=0.9976, CLP), y=0.0023x+0.0026 (r=0.9992, CPZ), y=0.0302x+0.0226 (r=0.9993, RPD), y=0226x+0.0205 (r=0.9981, PLD), y=0.0169x-0.0173 (r=0.9983, QTP), y=0.0170x+0.0119 (r=0.9988, APZ), y=0.0036x+0.0124 (r=0.9978, DAP), y=0.0140x+0.0259 (r=0.9943, OZP), y=0.0018x+0.0210 (r=0.9976, ZRP), and y=0.0065x-0.0030 (r=0.9986, ASP). The LLOQs of CLP, CPZ, DAP, QTP, CPZ, ZRD, RPD, PLD, OZP, and ASP were 25.0, 25.0, 25.0, 25.0, 10.0, 10.0, 2.5, 5.0, 5.0, and 20.0 ng/mL respectively.
Precision and Accuracy
The current method exhibited satisfactory precision and accuracy. The intra- and inter-day precisions and accuracies for CLP, CPZ, DAP, QTP, APZ, ZRD, RPD, PLD, OZP, and ASP are summarized in Table 3. All the accuracy and precision values were <10% for the intra- and inter-day studies, respectively.
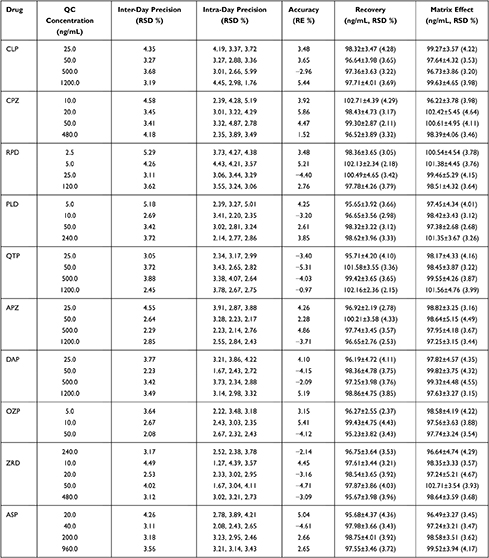 |
Table 3 Methodology Verification Results of Precision, Accuracy, Extraction Recovery and Matrix Effect |
Recovery, Matrix Effect, and Carry Over
The recovery and matrix effects were evaluated using six replicates of QC samples at three concentrations and LLOQ. The extraction recoveries of CLP, CPZ, DAP, QTP, APZ, ZRD, RPD, PLD, OZP, and ASP ranged from 96.64–98.32%, 96.52–102.71%, 96.19–98.86%, 95.71–101.58%, 96.65–100.21%, 95.67–98.54%, 97.78–102.13%, 96.65–98.62%, 95.23–99.43%, and 95.68%-98.75%, respectively, and the extraction recovery of IS ranged from 98.6%-101.32%. The absolute matrix effects were 96.73%–99.63% for CLP, 99.22%-102.42% for CPZ, 98.51%-101.38% for RPD, 97.38–101.35% for PLD, 98.17–101.56% for QTP, 97.25%-98.82% for APZ, 97.63%-99.82% for DAP, 96.64%-98.58% for OZP, 97.24%-102.71% for ZRD, and 96.49%-99.52% for ASP, suggesting no ionization enhancement or suppression. The matrix effect of IS ranged from 97.35%-102.20%. The results are shown in Table 3. In addition, there was no significant carry-over effect for all analytes and IS.
Stability
The stability of the analytes in human plasma is summarized in Table 4. The simulated human plasma samples showed good room temperature, freeze/thaw, long-term, and autosampler stabilities. The stability tests thus ensured the controllability of the sample processing process and the accuracy of the results.
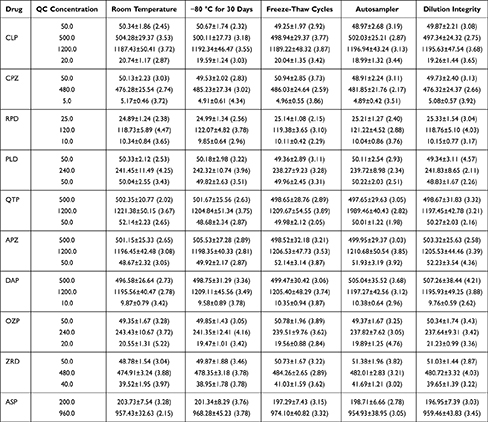 |
Table 4 Stability of Ten Antipsychotic Drugs Under Various Storage Conditions (Data are Mean ± SD, n=4) |
Dilution Integrity
The dilution integrities of the 10 analytes were investigated by diluting the samples with the 15-fold concentration of ULOQ for each antipsychotic drug to the respective QC level (Table 4). The dilution integrity was qualified, indicating that diluting high-concentration clinical plasma samples with blank plasma would not affect the accuracy of the analysis results.
Application
In this study, we carried out TDM for 105 cases of CLP, 170 cases of CPZ, 108 cases of QTP, 156 cases of APZ, 129 cases of ZRD, 181 cases of RPD, 127 cases of PLD, 164 cases of OZP, and 94 cases of ASP using the UPLC/MS-MS method (Table 5). Plasma drug concentrations varied among individuals, even if they were administered the same dose. To improve treatment effects while avoiding side effects, we hoped that the plasma drug concentrations would be maintained between the lowest effective concentration and the lowest toxic concentration. However, TDM results demonstrated marked inter-individual variability in plasma concentrations, producing trough concentrations lower than the MEC in 346 of 1234 patients (28.0%) and trough concentrations higher than the MTC in 194 of 1234 patients (15.7%). These results demonstrated the need to monitor the blood concentrations of antipsychotic drugs. There are several possible reasons for these findings and differences. Diversity and genetic polymorphisms of metabolic enzymes might explain the large individual differences. For example, CLP may be metabolized by CYP1A2, CYP2C19, and CYP3A4 and OZP may be metabolized by UGT1A4, CYP1A2, and CYP2D6, while RPD, QTP, and APZ are predominantly metabolized by CYP2D6 and CYP3A4.16,31,32 In addition to the effects of metabolic enzymes, we previously found that all 10 drugs were substrates of P-gp, which might further aggravate fluctuations in drug concentrations. In contrast, PLD and ASP showed smaller fluctuations than other drugs because >90% of ASP and >60% of PLD are excreted unchanged via the kidneys.16 In addition, metabolic enzymes and transporter-mediated pharmacokinetic interactions between antipsychotics and other drugs could also lead to significant differences in plasma concentrations. The contribution of DAP should be considered when monitoring the blood concentration of APZ, because DAP is the active metabolite of APZ. Finally, we should also consider drug-drug interactions given that most antipsychotic drugs are substrates of metabolic enzymes and P-gp. In brief, we successfully applied a UPLC/MS-MS method for monitoring antipsychotic drugs, providing reliable theoretical support for the individualized treatment of clinical patients.
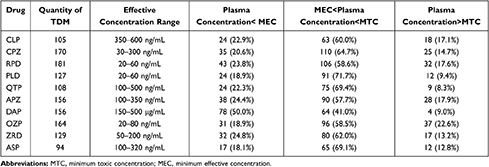 |
Table 5 Determination Results of the Serum Concentration of Antipsychotic Drugs in Patients |
Conclusions
In this study, we developed and validated a UPLC-MS/MS method to allow the simultaneous quantification of CLP, CPZ, RPD, PLD, QTP, APZ, DAP, OZP, ZRD, and ASP in human plasma samples. The proposed method could be used for TDM or pharmacokinetic studies of these molecules. Furthermore, stable isotope-labeled ISs, as the gold standard for absolute quantitation, were chosen as the final ISs in our study, because they can compensate for matrix effects and for variations in extraction recovery. Additionally, the method only required a small volume (50 μL) of plasma, thus making it suitable for TDM and for studying pharmacokinetics in young children. The TDM results demonstrated marked inter-individual variability in plasma concentrations of anti-psychotic drugs, with about 40% of patients having plasma concentrations outside the therapeutic window. In brief, the proposed method provides a precise, accurate, specific, and sensitive technique suitable for TDM in routine clinical practice and for pharmacokinetic studies of CLP, CPZ, RPD, PLD, QTP, APZ, DAP, OZP, ZRD, and ASP.
Acknowledgment
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Disclosure
The authors report no conflicts of interest and are responsible for the content of this article.
References
1. Lafarga T, O’Connor P, Hayes M. In silico methods to identify meat-derived prolyl endopeptidase inhibitors. Food Chem. 2015;175:337–343. doi:10.1016/j.foodchem.2014.11.150
2. Ewertzon M, Hanson E. Support interventions for family members of adults with mental illness: a narrative literature review. Issues Ment Health Nurs. 2019;40(9):768–780. doi:10.1080/01612840.2019.1591547
3. Höhl W, Moll S, Pfeiffer A. Occupational therapy interventions in the treatment of people with severe mental illness. Curr Opin Psychiatry. 2017;30(4):300–305. doi:10.1097/YCO.0000000000000339
4. Xu ZY, Huang FF, Kösters M, Nicolas R. Challenging mental health related stigma in China: systematic review and meta-analysis. II. Interventions among people with mental illness. Psychiatry Res. 2017;255:457–464. doi:10.1016/j.psychres.2017.05.002
5. Pardis P, Remington G, Panda R, Lemez M, Agid O. Clozapine and tardive dyskinesia in patients with schizophrenia: a systematic review. J Psychopharmacol. 2019;33:1187–1198. doi:10.1177/0269881119862535
6. Butler M, Urosevic S, Desai P, et al. Treatment for bipolar disorder in adults: a systematic review. Agency for Healthcare Research and Quality (US). 2018.
7. Nagai G, Mihara K, Nakamura A, et al. Prediction of an optimal dose of aripiprazole in the treatment of schizophrenia from plasma concentrations of aripiprazole plus its active metabolite dehydroaripiprazole at week 1. Ther Drug Monit. 2017;39(1):62–65. doi:10.1097/FTD.0000000000000358
8. Mauri MC, Paletta S, Maffini M, et al. Clinical pharmacology of atypical antipsychotics: an update. Excli J. 2014;13:1163–1191.
9. Zhang L, Li YG, He S, et al. Quantitative efficacy of three antipsychotic drugs for schizophrenia based on a real-world study in China. Acta Pharmacol Sin. 2019;40(12):1611–1620. doi:10.1038/s41401-019-0285-x
10. Han M, Deng C. BDNF as a pharmacogenetic target for antipsychotic treatment of schizophrenia. Neurosci Lett. 2020;726:133870. doi:10.1016/j.neulet.2018.10.015
11. Xu QQ, Wu X, Xion YY, et al. Pharmacogenomics can improve antipsychotic treatment in schizophrenia. Front Med. 2013;7(2):180–190. doi:10.1007/s11684-013-0249-3
12. Silva MA, Key S, Han E, Malloy M. Acute pancreatitis associated with antipsychotic medication: evaluation of clinical features, treatment, and polypharmacy in a series of cases. J Clin Psychopharmacol. 2016;36(2):169–172. doi:10.1097/JCP.0000000000000459
13. Alves MD, Micoulaud-Franchi JA, Simon N, Vion-Dury J. Electroencephalogram modifications associated with atypical strict antipsychotic monotherapies. J Clin Psychopharmacol. 2018;38(6):555–562. doi:10.1097/JCP.0000000000000953
14. Parikh T, Goyal D, Scarff JR, Lippmann S. Antipsychotic drugs and safety concerns for breast-feeding infants. South Med J. 2014;107(11):686–688. doi:10.14423/SMJ.0000000000000190
15. Grundmann M, Kacirova I, Urinovska R. Therapeutic drug monitoring of atypical antipsychotic drugs. Acta Pharm. 2014;64(4):387–401. doi:10.2478/acph-2014-0036
16. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1–2):9–62. doi:10.1055/s-0043-116492
17. Jönsson AK, Spigset O, Reis M. A compilation of serum concentrations of 12 antipsychotic drugs in a therapeutic drug monitoring setting. Ther Drug Monit. 2019;41(3):348–356. doi:10.1097/FTD.0000000000000585
18. Spina E, Hiemke C, de Leon J. Assessing drug-drug interactions through therapeutic drug monitoring when administering oral second-generation antipsychotics. Expert Opin Drug Metab Toxicol. 2016;12(4):407–422. doi:10.1517/17425255.2016.1154043
19. Bustillo M, Zabala A, Querejeta I, et al. Therapeutic drug monitoring of second-generation antipsychotics for the estimation of early drug effect in first-episode psychosis: a cross-sectional assessment. Ther Drug Monit. 2018;40(2):257–267. doi:10.1097/FTD.0000000000000480
20. Bogers JP, Bui H, Herruer M, Cohen D. Capillary compared to venous blood sampling in clozapine treatment: patients and healthcare practitioners experiences with a point‐of‐care device. Eur Neuropsychopharmacol. 2015;25(3):319–324. doi:10.1016/j.euroneuro.2014.11.022
21. Hiemke C, Baumann P, Bergemann N, et al. AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry. 2011;44(6):195–235. doi:10.1055/s-0031-1286287
22. Remmerie B, De Meulder M, Weiner S, Savitz A. Comparison of capillary and venous drug concentrations after administration of a single dose of risperidone, paliperidone, quetiapine, olanzapine, or aripiprazole. Clin Pharmacol Drug Dev. 2016;5(6):528–537. doi:10.1002/cpdd.290
23. Patteet L, Cappelle D, Maudens KE, et al. Advances in detection of antipsychotics in biological matrices. Clin Chim Acta. 2015;441:11–22. doi:10.1016/j.cca.2014.12.008
24. Tron C, Kloosterboer SM, van der Nagel BC, et al. Dried blood spots combined with ultra-high-performance liquid chromatography mass spectrometry for the quantification of the antipsychotics risperidone, aripiprazole, pipamperone, and their major metabolites. Ther Drug Monit. 2017;39(4):429–440. doi:10.1097/FTD.0000000000000411
25. Nahar L, Onder A, Sarker SD. A review on the recent advances in HPLC, UHPLC and UPLC analyses of naturally occurring cannabinoids (2010–2019). Phytochem Anal. 2020;31(4):413–457. doi:10.1002/pca.2906
26. Mei YB, Luo SB, Ye LY, et al. Validated UPLC-MS/MS method for quantification of fruquintinib in rat plasma and its application to pharmacokinetic study. Drug Des Devel Ther. 2019;13:2865–2871. doi:10.2147/DDDT.S199362
27. Zhou W, Li SL, Zhao T, et al. Effects of dexmedetomidine on the pharmacokinetics of dezocine, midazolam and its metabolite 1-hydroxymidazolam in beagles by UPLC-MS/MS. Drug Des Devel Ther. 2020;14:2595–2605. doi:10.2147/DDDT.S254055
28. Moussa BA, Mahrouse MA, Fawzy MG. A validated LC-MS/MS method for simultaneous determination of linagliptin and metformin in spiked human plasma coupled with solid phase extraction: application to a pharmacokinetic study in healthy volunteers. J Pharm Biomed Anal. 2019;163:153–161. doi:10.1016/j.jpba.2018.09.052
29. European Medicines Agency. Guideline on bioanalytical method validation. 2011.
30. U.S. Food and drug administration, bioanalytical method validation. Guidance for Industry; 2018.
31. Zhang XD, Xiang Q, Zhao X, Ma LY, Cui YM. Association between aripiprazole pharmacokinetics and CYP2D6 phenotypes: a systematic review and meta-analysis. J Clin Pharm Ther. 2019;44(2):163–173. doi:10.1111/jcpt.12780
32. Na Takuathung M, Hanprasertpong N, Teekachunhatean S, Koonrungsesomboon N. Impact of CYP1A2 genetic polymorphisms on pharmacokinetics of antipsychotic drugs: a systematic review and meta-analysis. Acta Psychiatr Scand. 2019;139(1):15–25. doi:10.1111/acps.12947
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

