Back to Journals » Infection and Drug Resistance » Volume 13
Tuberculosis Treatment Outcomes and Associated Factors Among Patients Treated at Woldia General Hospital in Northeast Ethiopia: An Institution-Based Cross-Sectional Study
Received 6 August 2020
Accepted for publication 22 September 2020
Published 6 October 2020 Volume 2020:13 Pages 3423—3429
DOI https://doi.org/10.2147/IDR.S275568
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Addisu Getie,1 Birhan Alemnew2
1Department of Nursing, College of Health Sciences, Woldia University, Woldia, Ethiopia; 2Department of Medical Laboratory, College of Health Sciences, Woldia University, Woldia, Ethiopia
Correspondence: Addisu Getie Email [email protected]
Background: Tuberculosis remains a major global health problem. It causes ill-health among millions of people each year and ranks alongside the human immunodeficiency virus (HIV) as a leading cause of death worldwide. For effective tuberculosis control, it is a prerequisite to detect the cases as early as possible and to ensure that the tuberculosis patients complete their treatment and get cured. However, the burden of the problem is still a national issue, and there is a scarcity of research to show treatment outcomes and associated factors of tuberculosis at the North Wollo Zone, specifically Woldia.
Methods: Institution-based, retrospective register-based data were collected from medical records of tuberculosis patients from 2015 up to 2018 at Woldia General Hospital. The data were analyzed using SPSS version 24, and multiple logistic regression methods were used to investigate the association between independent and dependent variables. A P-value of less than 5% was considered statistically significant in the final model.
Results: The prevalence of successful tuberculosis treatment outcomes was 80.7%. Among all patients, 73% were pulmonary tuberculosis cases. This study results show that age less than 24 years old [AOR: 4.7; 95% CI (1.3– 10.1)], male sex [AOR: 2.8; 95% CI (2.1– 4.8)], year of registration in 2018 [AOR: 4.8; 95% CI (3.9– 7.4)], and HIV negative status [AOR: 3.9; 95% CI (1.4– 10.7)] were found to be significantly associated factors with the treatment outcomes of tuberculosis.
Conclusion: The study showed that nearly 20% of tuberculosis patients had an unsuccessful treatment outcome. Older age, female sex, year of registration in 2015, and being HIV positive were found significantly associated with poor tuberculosis treatment outcomes. Therefore, targeted measures should be considered to decrease poor TB treatment outcomes among high-risk patients through careful monitoring, making the DOTs program more accessible, counseling, and linking HIV patients.
Keywords: TB treatment, treatment outcome, successful treatment outcome
Introduction
Mycobacterium tuberculosis is an intracellular bacterium, causing respiratory illness, tuberculosis. The bacilli infect about one-third of the world’s population, leaving the majority with an asymptomatic state of a disease called latency.1 While only a few proportions (10%) of those latently infected develop active TB, the majority (90%) will remain asymptomatic.2 Tuberculosis is a life-threatening disease caused by mycobacterium tuberculosis having an increased prevalence in developing countries.3 It is a major global health problem and ranks alongside HIV as a leading cause of mortality worldwide.4
Although there is the availability of highly effective treatment for decades, tuberculosis remains a challenge especially for low-income countries.5 According to WHO 2019 tuberculosis report, there are an estimated 10 million people infected with TB and 1.2 million tuberculosis deaths among peoples living with HIV/AIDS globally. Of these cases, the highest report was from Africa (24%), next to Asia (44%).6 Ethiopia is one of the high tuberculosis burden countries. The Ethiopian Federal Ministry of Health (FMOH) hospital statistics data have shown that tuberculosis is the leading cause of morbidity, the third cause of hospital admission, and the second cause of death in Ethiopia, after malaria.7 A high proportion of patients died (10.1%) or defaulted (18.3%), which is a serious public health concern that needs to be addressed urgently.8 The Ethiopian tuberculosis prevalence survey 2011 showed that 55% of tuberculosis cases were found among peoples younger than 35 years old.9
The proportion of deaths was higher in smear-negative pulmonary tuberculosis patients than smear-negative tuberculosis patients. The defaulters and treatment failures rate were higher in developing countries including Ethiopia.10 Multiple therapies (the combination of three or more anti-TB drugs for at least 6 months) are adopted for the treatment of active TB as well as a preventative therapy for latent TB.11 A great issue about unsuccessful tuberculosis treatment outcome is due to drug resistance; Multiple Drug Resistance (MDR) and Extensive Drug Resistance (XDR) which was a big mankind problem causing increase burden of tuberculosis.12 The prevalence of unsuccessful tuberculosis treatment outcome was reported in different studies; Afar (18.2%),13 West Ethiopia (17.5%),3 Central Africa (27.6%),14 and India (14%).15
The studies showed that there are different factors for poor TB treatment outcomes. Older age, type of tuberculosis, TB/HIV comorbidity, previous tuberculosis treatment, low income, limited access to transport, distance from home to the treatment center, limited interest in information about the disease and its treatment, limited social support, multidrug resistance, and comorbidity have all been found to be related to unsuccessful treatment outcomes.16–19 The national tuberculosis control program should exert concerted efforts to identify undetected tuberculosis cases in the communities. Although the prevalence of tuberculosis is decreased through time, it is concerning that most tuberculosis patients in the community were younger adults which indicate tuberculosis is circulating in the communities of Ethiopia.9
Despite intensive efforts to control, tuberculosis remains a major public health problem in low-income countries. Even though different researches showed the prevalence of tuberculosis in different parts of Ethiopia, the treatment outcomes were not well studied in Northern Ethiopia, specifically, the North Wollo Zone where there are a large number of tuberculosis patients were found. Therefore, this study intended to assess tuberculosis treatment outcomes and associated factors at Woldia General Hospital.
Methods
Study Design and Setting
A retrospective document review from 2015 to 2018 was conducted on May 10–30/2019 at Woldia General Hospital, which is found in Woldia town, is a capital city of North Wollo Zone. It is located 520 Km away from the capital city Addis Ababa.
Study Population
All tuberculosis patients who got the treatment of tuberculosis at Woldia General Hospital from 2015 to 2018 were included as a study population and patients who had incomplete data were excluded from the study. Patient charts that did not report outcome variables; successful treatment outcome, completed, treatment failure, loss to follow-up, transferred out, and death was considered incomplete data which was handled by case deletion.
Sampling Technique and Sample Size
A total of 270 tuberculosis patients were treated from 2015 to 2018 at Woldia General Hospital. Then, all these patients were included in the study.
Method of Data Collection
The data were collected through medical record reviews of patients using a prepared standard checklist in the tuberculosis clinic. The checklist was pretested and standardized among 5% of the study population in Dessie Referral Hospital for content validity. The contents of the checklist include socio-demographic, HIV status, type of tuberculosis, and treatment outcome. The data were collected by four nurses after giving one-day training on the data collection tool and techniques of data collection. Demographic and clinical data such as age, sex, type of tuberculosis, HIV status, Cotrimoxazole preventive therapy (CPT), and ART status were retrieved from the tuberculosis registry.
Variables
The dependent variable was a tuberculosis treatment outcome which is a successful tuberculosis treatment outcome and unsuccessful treatment outcome. Unsuccessful treatment outcome includes; failure, loss to follow-up, and death. The independent variables were; age, sex, weight, residence, year of registration, HIV status, ART status, CPT initiation, type of tuberculosis, and patient category.
Ethical Clearance
The study was approved by the Institutional Review Board of Woldia University. A patient informed consent was not required because the data were obtained from retrospective chart review. The data were collected after a written informed consent was taken from the hospital manager. All patient data will be kept confidential. The study was also conducted in accordance with the declaration of Helsinki. Any data intended for sharing will be de-identified.
Data Processing and Analysis
After the data collection, the data template format was prepared, coded, and entered into Epi Data version 4.2. Then, the data were exported to SPSS version 24 for analysis. Descriptive analysis was employed to describe the percentages and distributions of the respondent’s socio-demographic characteristics and the factors that influence the tuberculosis treatment outcome. Bivariate and multivariate analyses were used for determining the association of the independent variable with the dependent variable. A crude and adjusted odds ratio with the corresponding 95% confidence intervals was also computed. P-value <0.05 was considered statistically significant in this study. The results were presented in the form of tables, graphs, and charts.
Result
All 270 registered tuberculosis cases were included which gives a 100% response rate. Of these 270 cases, 53.3% were males and 38.5% were found in the age group between 24 and 44 years. The mean age of the respondents was 36.64 years ± 6.758 SD. Among all treated tuberculosis patients, 18.5% were HIV-positive cases, 73% were pulmonary tuberculosis cases, 59.4% were smear-positive results, and 8.5% had presumptive multidrug-resistant tuberculosis (Table 1).
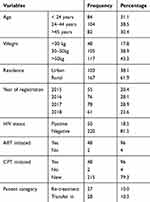 |
Table 1 Socio-Demographic and Clinical Profile of Tuberculosis Patients Registered at Woldia General Hospital, from 2015 to 2018 (2019) |
Tuberculosis Treatment Outcome
Of the total registered 270 cases, 80.7% had a successful treatment outcome and 19.3% had an unsuccessful treatment outcome. Among tuberculosis patients who got treatment, 46.67% were cured, and 81.5% had completed their treatment regimen (Table 2).
 |
Table 2 Treatment Outcomes of Tuberculosis Among Tuberculosis Patients Registered at Woldia General Hospital, from 2015 to 2018 (2019) |
Factors Associated with Tuberculosis Treatment Outcome
As reviled in Table 3, treatment outcomes of tuberculosis were affected by age, sex, year of registration, and HIV status of the patient. Patients whose age was less than 24 years old were 4 0.7 times more likely to had successful treatment outcomes than those whose age was greater than 45 years old [AOR: 4.7; 95% CI (1.3–10.1)]. Similarly, male tuberculosis patients were 2.8 times more likely to have successful treatment outcomes than female patients [AOR: 2.8; 95% CI (2.1–4.8)]. Regarding the year of treatment, patients whose year of treatment was in 2018 were 4.8 times more likely to had successful treatment outcomes than whose year of treatment was in 2015 [AOR: 4.8; 95% CI (3.9 −7.4)]. Human Immunodeficiency Virus negative tuberculosis patients were 3.9 times more likely to had a successful treatment outcome than HIV positive tuberculosis patients [AOR: 3.9; 95% CI (1.4–10.7)] (Table 3).
 |
Table 3 Bivariable and Multivariate Analyses of Tuberculosis Treatment Outcomes Among Tuberculosis Patients Registered at Woldia General Hospital, from 2015 to 2018 (2019) |
Discussion
Although the prevalence of successful tuberculosis treatment outcomes higher than the previous estimates in Ethiopia, it is concerning that some tuberculosis patients had unsuccessful tuberculosis treatment outcomes. The finding of this study had no satisfactory treatment outcome as compared to the national tuberculosis treatment survey which was a 96% success rate. This might be due to the reason that the current study was from a single institution where many tuberculosis patients from rural residences got treatment, whereas the national survey on tuberculosis treatment included a more urbanized population causing an increased success rate. The successful tuberculosis treatment outcome in this study was lower than studies conducted in the Tigray region (89.2%),5 Southern Ethiopia (85.2%),16 Northeastern Ethiopia (89.9%),20 Northern Ethiopia (94%),21 Debretabour hospital (87.1%),22 and Eastern Ethiopia (92.5%).23 The discrepancy might be due to sociodemographic, cultural, and socioeconomic variations. On the other had this finding is higher than a study conducted in India (60%).24 This variation is because of the difference in study subjects; in India, the study subjects were MDR tuberculosis patients whose treatment outcome is surely unsuccessful compared to all other tuberculosis patients. The result of this study also higher than studies conducted in the Oromia region (78.29%),25 North Shoa (63.62%),26 and Ethiopian city administrative hospitals (55.8%).4 This discrepancy might be due to the reason that the previous studies conducted among prisoners, MDR tuberculosis patients, and tuberculosis patients at referral hospitals; where complicated cases were treated causing more unsuccessful treatment outcome, whereas the current study was undergone at the general hospital; where less complicated patients were treated which increases the treatment success rate. The result of this study was almost similar to studies done in Adama city (80.8%),27 West Ethiopia (82.5%),3 Gonder (79%),28 and Afar region (81.8%).13
In this study, a successful tuberculosis treatment outcome was higher among patients treated in 2018 than those who were treated in 2015 and 2016. This might be due to the reason that patients become aware of the treatment regimen and got better treatment over time. This finding is supported by a study conducted at Awi Zone, Northwest Ethiopia; the success rate was 213/100,000 in 2012 and 189/100,000 in 201629 and Harar town, Eastern Ethiopia; the success rate was 53.2% in 2011 and 64.2% in 2015.23
The sex of the patient was significantly associated with successful tuberculosis treatment outcomes. Males were nearly three times more likely to have successful tuberculosis treatment outcomes as compared to females; the finding was in line with a study conducted in Northeast Ethiopia.30 This might be because females have different maternal-related complications and are less immune-competent as compared to males that decrease successful treatment outcomes. Similarly, the age of the patient had a significant association with tuberculosis treatment outcome. Patients whose age was less than 24 years were nearly five times more likely to have a successful treatment outcome than the patients whose age was greater than 45 years old. This finding was supported by studies done in Northern Ethiopia prisons,21 Tigray region,5 and Arba Minch, Southern Ethiopia.18 This might be due to the reason older patients may have the concomitant disease and general psychological deterioration. The present study also showed that HIV-negative tuberculosis patients were nearly five times more likely to have successful treatment outcomes than their counterparts. Similar results were reported from Afar, Eastern Ethiopia,13 Northeastern Ethiopia,20 Southwest Ethiopia,31 and Arba Minch South Ethiopia.18 This might be explained with HIV-negative tuberculosis patients were more immune competent than HIV-positive ones, HIV-positive patients might not take the drug as prescribed due to the fear of drug interaction and the side effects. The odds of having had successful tuberculosis treatment outcomes were 4.8 times high on those patients treated in 2018 as compared to patients treated in 2015. A similar study in Debretabour, Ethiopia, showed that the year of registration had a significant association with tuberculosis treatment outcome.22 This might be explained by enhancement in patient awareness regarding the disease, and the respective drugs might also be modified and changed in the drug regimen through time.
Conclusion
The current study showed that nearly 20% of tuberculosis patients had an unsuccessful treatment outcome. Older age, female sex, year of registration in 2015, and being HIV positive were found significantly associated with poor tuberculosis treatment outcomes. Therefore, targeted measures should be considered to decrease poor tuberculosis treatment outcomes among high-risk patients through; careful monitoring, making the DOTs program more accessible, counseling, and linking HIV patients. Furthermore, patients at high-risk of unsuccessful treatment outcome should be identified early and given additional follow-up as well as given a combination of medical interventions and social support.
Abbreviations
AIDS, acquired immunodeficiency syndrome; AOR, adjusted odds ratio; ART, anti-retro therapy; CI, confidence interval; COR, crude odds ratio; CPT, cotrimoxazole preventive therapy; DOTs, directly observed therapies; HIV, human immunodeficiency virus; MDR, multidrug resistance; SD, standard deviation; SPSS, Statistical Package for Social Science; TB, tuberculosis; WHO, World Health Organization; XDR, extensive drug resistance.
Data Sharing Statement
The datasets used for analysis during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The study was approved by the Institutional Review Board of Woldia University. A patient informed consent was not required because the data were obtained from retrospective chart review. The data were collected after a written informed consent was taken from the hospital manager. All patient data will be kept confidential. The study was also conducted in accordance with the declaration of Helsinki. Any data intended for sharing will be de-identified.
Acknowledgments
We are grateful to thank all the hospital administrators for their willing full participation with a timely fashion.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed on the journal to which the article will be submitted; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests for this work.
References
1. Dorhoi A, Reece ST, Kaufmann SH. For better or for worse: the immune response against Mycobacterium tuberculosis balances pathology and protection. Immunol Rev. 2011;240(1):235–251.
2. Lee S-W, Wu LS-H, Huang G-M, Huang K-Y, Lee T-Y, Weng JT-Y, editors. Gene expression profiling identifies candidate biomarkers for active and latent tuberculosis. BMC Bioinform. 2016;17:S3.
3. Kassa J, Dedefo M, Korsa A, Dibessa T. Factors affecting treatment outcome of tuberculosis among tuberculosis patients in West Ethiopia. J Bioanal Biomed. 2018;10(1):24–29.
4. Taye G, Defar A, Taddele T, et al. Treatment outcome and associated factors among TB patients in Ethiopia: hospital-based retrospective study. Am J Epidemiol Infect Dis. 2018;6(1):14–19.
5. Berhe G, Enquselassie F, Aseffa A. Treatment outcome of smear-positive pulmonary tuberculosis patients in Tigray Region, Northern Ethiopia. BMC Public Health. 2012;12(1):537.
6. World Health Organization. Global tuberculosis report; WHO/CDS/TB/2019:23.
7. MoHoE. Tuberculosis, Leprosy and TB/HIV Prevention and Control Programme Manual.
8. Tessema B, Muche A, Bekele A, Reissig D, Emmrich F, Sack U. Treatment outcome of tuberculosis patients at Gondar University Teaching Hospital, Northwest Ethiopia. A five-year retrospective study. BMC Public Health. 2009;9(1):371.
9. Ethiopian health and nutrition research institute. Ethiopian national population based tuberculosis survey. 2011.
10. Yakob B, Alemseged F, Paulos W, Badacho A. Trends in treatment success rate and associated factors among tuberculosis patients in Ethiopia: a retrospective cohort study. Health Sci J. 2018;12(5):598.
11. Yáñez A, Hassanzadeh‐Kiabi N, Ng MY, et al. Detection of a TLR 2 agonist by hematopoietic stem and progenitor cells impacts the function of the macrophages they produce. Eur J Immunol. 2013;43(8):2114–2125.
12. Nebenzahl-Guimaraes H, Jacobson KR, Farhat MR, Murray MB. Systematic review of allelic exchange experiments aimed at identifying mutations that confer drug resistance in Mycobacterium tuberculosis. J Antimicrob Chemother. 2014;69(2):331–342.
13. Zenebe T, Tefera E. Tuberculosis treatment outcome and associated factors among smear-positive pulmonary tuberculosis patients in Afar, Eastern Ethiopia: a retrospective study. Braz J Infect Dis. 2016;20(6):635–636.
14. Trébucq A, Schwoebel V, Kashongwe Z, et al. Treatment outcome with a short multidrug-resistant tuberculosis regimen in nine African countries. Int J Tuberc Lung Dis. 2018;22(1):17–25.
15. Ramachandran G, Kupparam HKA, Vedhachalam C, et al. Factors influencing tuberculosis treatment outcome in adult patients treated with thrice-weekly regimens in India. Antimicrob Agents Chemother. 2017;61(5).
16. Gebrezgabiher G, Romha G, Ejeta E, Asebe G, Zemene E, Ameni G. Treatment outcome of tuberculosis patients under directly observed treatment short course and factors affecting outcome in southern Ethiopia: a five-year retrospective study. PLoS One. 2016;11(2):e0150560.
17. Ejeta E, Beyene G, Balay G, Bonsa Z, Abebe G. Factors associated with unsuccessful treatment outcome in tuberculosis patients among refugees and their surrounding communities in Gambella Regional State, Ethiopia. PLoS One. 2018;13(10):e0205468.
18. Dale D, Nega D, Yimam B, Ali E. Predictors of poor tuberculosis treatment outcome at Arba Minch General Hospital, Southern Ethiopia: a case-control study. J Tuberc Ther. 2017;2(110):2.
19. Peltzer K, Louw J. Prevalence and associated factors of tuberculosis treatment outcome among hazardous or harmful alcohol users in public primary health care in South Africa. Afr Health Sci. 2014;14(1):157–166.
20. Mekonnen D, Derbie A, Mekonnen H, Zenebe Y. Profile and treatment outcomes of patients with tuberculosis in Northeastern Ethiopia: a cross sectional study. Afr Health Sci. 2016;16(3):663–670.
21. Adane K, Spigt M, Dinant G-J. Tuberculosis treatment outcome and predictors in northern Ethiopian prisons: a five-year retrospective analysis. BMC Pulm Med. 2018;18(1):37.
22. Melese A, Zeleke B. Factors associated with poor treatment outcome of tuberculosis in Debre Tabor, northwest Ethiopia. BMC Res Notes. 2018;11(1):25.
23. Tola A, Minshore KM, Ayele Y, Mekuria AN. Tuberculosis treatment outcomes and associated factors among TB patients attending public hospitals in Harar town, Eastern Ethiopia: a five-year retrospective study. Tuberc Res Treat. 2019;2019.
24. Nair D, Velayutham B, Kannan T, et al. Predictors of unfavourable treatment outcome in patients with multidrug-resistant tuberculosis in India. Public Health Action. 2017;7(1):32–38.
25. Woldeyohannes D, Assefa T, Aman R, Tekalegn Y, Hailemariam Z. Predictors of time to unfavorable treatment outcomes among patients with multidrug resistant tuberculosis in Oromia region, Ethiopia. PLoS One. 2019;14(10):e0224025.
26. Berihun YA, Nguse TM, Gebretekle GB. Prevalence of tuberculosis and treatment outcomes of patients with tuberculosis among inmates in Debrebirhan prison, North Shoa Ethiopia. Ethiop J Health Sci. 2018;28(3):347–354.
27. Tesema T, Seyoum D, Ejeta E, Tsegaye R. Determinants of tuberculosis treatment outcome under directly observed treatment short courses in Adama City, Ethiopia. PLoS One. 2020;15(4):e0232468.
28. Belayneh T, Kassu A, Tigabu D, Asmare G, Tilaye S, Klinkenberg E. Characteristics and treatment outcomes of “transfer-out” pulmonary tuberculosis patients in Gondar, Ethiopia. Tuberc Res Treat. 2016;2016.
29. Alemu T, Gutema H. Trend in magnitude of tuberculosis in Awi Zone, Northwest Ethiopia: a five-year tuberculosis surveillance data analysis. BMC Res Notes. 2019;12:209.
30. Malede A, Shibabaw A, Hailemeskel A, Belay M, Asrade S. Treatment outcome of tuberculosis patients and associated risk factors at Dessie and Woldiya Town Health Institutions, Northeast Ethiopia: a retrospective cross sectional study. J Bacteriol Parasitol. 2015;6:1.
31. Sengul A, Akturk UA, Aydemir Y, Kaya N, Kocak ND, Tasolar FT. Factors affecting successful treatment outcomes in pulmonary tuberculosis: a single-center experience in Turkey, 2005–2011. J Infect Dev Ctries. 2015;9(08):821–828.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
