Back to Journals » OncoTargets and Therapy » Volume 13
TRPV4 Overexpression Promotes Metastasis Through Epithelial–Mesenchymal Transition in Gastric Cancer and Correlates with Poor Prognosis
Authors Wang H, Zhang B, Wang X, Mao J, Li W, Sun Y, Yuan Y, Ben Q, Hua L , Qian A
Received 16 April 2020
Accepted for publication 28 July 2020
Published 21 August 2020 Volume 2020:13 Pages 8383—8394
DOI https://doi.org/10.2147/OTT.S256918
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Gaetano Romano
Huafeng Wang,1,* Benyan Zhang,1,* Xue Wang,1,* Jianhua Mao,2 Weiguang Li,3 Yunwei Sun,3 Yaozong Yuan,3 Qiwen Ben,3 Li Hua,4 Aihua Qian3
1Department of Pathology, Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 2State Key Laboratory of Medical Genomics, Shanghai Institute of Hematology, Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 3Department of Gastroenterology, Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 4Faculty of Public Health, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Aihua Qian; Li Hua Email [email protected]; [email protected]
Purpose: Transient receptor potential vanilloid 4 (TRPV4) has been reported to be involved in the progression of several human tumors. Nevertheless, clinical significance and molecular mechanism of TRPV4 in gastric cancer (GC) remain poorly defined.
Patients and Methods: Immunohistochemistry assays were used to investigate the correlation between the expression of TRPV4 and epithelial–mesenchymal transition (EMT) markers in human GC tissues. The correlations between TRPV4 expression and clinicopathological features and between TRPV4 expression and survival rates were also examined. TRPV4 knockdown was performed by using small interfering RNAs. In vitro, Cell Counting Kit-8 (CCK-8) assay, colony formation assay, and transwell assay were employed to further explore the biological functions of TRPV4, and Western blotting was used to evaluate the changes in the expression of TRPV4 protein and EMT-related proteins in HGC-27 and MGC-803 human GC cell lines.
Results: TRPV4 expression was upregulated in GC tissues and cell lines. TRPV4 overexpression was associated with greater depth of tumor invasion, lymph node metastasis, higher TNM stage, poor overall survival, and worse disease-free survival. TRPV4 expression was inversely correlated with E-cadherin expression and positively correlated with vimentin expression. In vitro, knockdown of TRPV4 inhibited GC cell proliferation, colony formation, and invasion. Furthermore, the knockdown of TRPV4 modulated EMT by upregulating E-cadherin expression and downregulating the expression of N-cadherin and vimentin. In addition, the EMT-related transcription factor Snail was downregulated, whereas the expression levels of other transcription factors such as Slug and Twist did not change.
Conclusion: TRPV4 was upregulated in human GC and the overexpression of TRPV4 could promote GC progression, partially through Snail-mediated EMT.
Keywords: TRPV4, gastric cancer, epithelial–mesenchymal transition, metastasis, prognosis
Introduction
Despite the fact that various therapeutic strategies for gastric cancer (GC) have been developed, the 5-year survival rate after diagnosis of late-stage gastric cancer has been considerably low. Therefore, researchers have endeavored to find targeted drugs that are less toxic and more efficient, especially for metastatic gastric cancer. Nowadays, there is an increasing interest in ion channels, especially the mechanosensitive ion channels, as potential drug targets in many types of cancers.1–4
Transient receptor potential vanilloid 4 (TRPV4) is the vanilloid subfamily of the TRP channels, which is involved in mechanotransduction processes like volume regulation and osmosensing.4 Mechanical stress is a critical mediator of cancer progression and mechanosensitive ion channels are functionally up-regulated in cancers that depend on Ca2+ signaling.3,5,6 Several studies demonstrated that TRPV4 was overexpressed in different types of cancers, including human colon cancer, breast cancer, and hepatocellular carcinoma, and was involved in cancer cell proliferation and migration.4,7-9 Thus far, only two studies demonstrated that activation of calcium-sensing receptor (CaSR) and vasoactive intestinal peptide receptor 1 (VPAC1) could promote gastric cancer cell progression through TRPV4-mediated Ca2+ signaling.10,11 In these studies, the expression of TRPV4 protein, as the downstream signal of CaSR and VPAC1, was detected in GC tissues and normal tissues in small biopsy samples. However, little is known about clinical significance, molecular mechanisms, and the role of TRPV4 in human GC.
Clinically, GC with distant metastasis is generally associated with poor prognosis and lacks effective therapies. It is well understood that epithelial–mesenchymal transition (EMT) is associated with tumor metastasis. EMT is a cell differentiation process by which polarized epithelial cells undergo molecular changes, lose their cell polarity and cell-cell adhesion, and gain migratory capacity, invasiveness, and elevated resistance to apoptosis, thereby assuming mesenchymal phenotype.12 During EMT, the expression level of the adhesion molecule E-cadherin decreases, whereas the expression levels of mesenchymal markers (N-cadherin and vimentin) increase.12 Several transcription factors such as Snail, Slug, Twist, ZEB1, and ZEB2 regulate the EMT progress. A recent study showed that TPRV4 antagonist decreased the migration capability by attenuating EMT in vitro in human hepatocellular carcinoma.9 It has not been established yet whether TRPV4 can regulate GC EMT and how TRPV4 affects EMT-related factors.
Therefore, in the present study, we investigated the clinical significance of TRPV4 expression in human gastric adenocarcinoma tissues and its relationship with EMT-related proteins using immunohistochemical staining. Moreover, we examined the effects of TRPV4 protein knockdown on GC cell proliferation, colony formation, cell invasion, and the expression of EMT-related proteins in GC cell lines in vitro.
Patients and Methods
Samples
A total of 107 patients with gastric adenocarcinoma in Ruijin Hospital affiliated to Shanghai Jiaotong University School of Medicine (Shanghai, China), who underwent gastrectomy between January 2010 and December 2011, were included in this study. These patients consisted of 77 males and 30 females; whose ages ranged from 28 to 80 years with the median being 57.35 years. All GC tissues were processed for pathological diagnosis according to the 2010 World Health Organization classification. The clinicopathological characteristics such as age, gender, tumor size, differentiation grade, Lauren classification, depth of tumor invasion, lymph node metastasis, and tumour-node-metastasis (TNM) stage were analyzed. All patients had not received chemotherapy, radiotherapy, or immunotherapy prior to surgery. Patients were followed until death or December 31, 2017. The overall survival time was calculated from the date of surgery until the date of death or last follow-up visit. The disease-free survival time was calculated from surgery to the disease relapse or until the date of last follow-up visit. The study was approved by Ruijin Hospital Research Ethics Committees. All patients were enrolled by signing a consent form.
Tissue Microarray Construction
The tissue cores with a diameter of 1.5 mm taking from each paraffin-embedded (FFPE) tissue block were rearrayed in recipient paraffin masses by using a Tissue Microarray System. Cases containing only stromal tissues or inadequate tissues in the cores were excluded from the analyses. Tissue microarrays (TMAs) including 107 GC samples and 81 adjacent noncancerous tissues (taken at a distance of >3 cm from the tumor margin) were constructed.
Immunohistochemical Staining
The expression levels of TRPV4, E-cadherin, and vimentin were assessed by immunohistochemical staining on tissue microarray slides using the peroxidase technique. Tissue microarray sections were cut at 4 μm thickness, dewaxed in xylene, and rehydrated in graduated alcohol. Endogenous peroxidase activities were blocked by incubating the slides with H2O2 in methanol for 10 min at room temperature. Then, an antigen retrieval process was performed in a water bath at 95–99°C for 15 min in citrate buffer (pH 6.0). After blocking with 20% bovine serum albumin (BSA), these pretreated slides were incubated at 4°C overnight with antibodies against TRPV4 (1:100 dilution, #ACC-034-AO, Alomone labs), E-cadherin (1:50 dilution, clone NCH-38, Dako), and vimentin (1:100 dilution, clone V9, Dako), followed by incubation with the HRP-conjugated secondary antibody (Dako). The reaction products were visualized using diaminobenzidine and counterstained with haematoxylin. Negative controls were included by replacing the primary antibodies with PBS.
Evaluation of Immunohistochemical Staining
The expression levels of TRPV4, E-cadherin, and vimentin were scored using the semi-quantitative immunoreactivity score. Staining intensity index and staining proportion for each section were scored by two independent researchers who were blinded regarding clinicopathological characteristics. Staining intensity indices were 0 (no staining), 1 (weak staining), 2 (moderate staining), and 3 (strong staining). Staining proportion scores ranged between 0 and 100. The intensity indices and proportion scores were then multiplied to obtain the final immunohistochemical (IHC) scores that ranged from 0 to 300. The scores were categorized into low expression and high expression based on a cutoff value determined by the X-tile software (The Rimm Laboratory at Yale University; http://www.tissuearray.org/rimmlab) as previously described.13 For TRPV4, the expression level was considered low if the IHC score was 89 or less and high if the IHC score was more than 89. For E-cadherin, the expression level was considered low if the IHC score was 180 or less and high if the IHC score was more than 180. For vimentin, the expression level was considered low if the IHC score was 5 or less and high if the IHC score was more than 5.
Cell Culture
Two gastric cancer cell lines, HGC-27 and MGC-803, and a normal gastric mucosa cell line GES-1 were obtained from American Type Culture Collection (ATCC), and cultured in DMEM media supplemented with 10% fetal-bovine serum (FBS) in 37°C and 5% CO2 under saturated humidity.
Recombinant Lentivirus for Cell Infection
TRPV4 shRNA recombinant lentivirus was constructed, packaged, amplified, and titrated by Shanghai Genechem Co Ltd. The TRPV4 shRNA construct was made using the target sequence (5′-AGAACTTGGGCATCATCAA-3′) oligonucleotides. The corresponding control shRNA with a scrambled sequence was made using the sequence (5′-TTCTCCGAACGTGTCACGT-3′) oligonucleotides. HGC-27 and MGC-803 were infected with lentivirus packed TRPV4 shRNA (shTRPV4) or control shRNA (shCtrl). After 48 h, the cells were harvested for further experimental studies.
Western Blot
The total cell proteins were extracted using RIPA lysis buffer and the protein concentrations were measured with BCA Protein Assay Kit (Songong BioTech, China). Proteins were separated by SDS-PAGE and transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, MA, USA). The membranes were probed with primary antibodies specific to TRPV4 (Abcam, ab191580, 1:1000), E-Cadherin (Cell Signaling Technology, #3195, 1:1000), N-Cadherin (Cell Signaling Technology, #4061, 1:1000), vimentin (Cell Signaling Technology, #5741, 1:1000), Slug (Cell Signaling Technology, #9585, 1:1000), Snail (Cell Signaling Technology, #3879, 1:1000), Twist (Cell Signaling Technology, #46,702, 1:1000), and GAPDH (Cell Signaling Technology, #2118, 1:1000). HRP-labeled secondary antibodies (Beyotime, China) were incubated with the PVDF membranes at a dilution of 1:1000 for 1 h. Finally, the membranes were visualized using highly sensitive ECL luminescence reagent (Beyotime, China).
Cell Counting Kit-8 (CCK-8) Assay
LV-TRPV4 shRNA or LV-control shRNA transfected cells in a 96-well plate were incubated in 10 μL Cell Counting Kit-8 solution (Tojindo, Japan) at 37°C for 1 h. Then, absorbance at 450 nm was measured at 24, 48, and 72 h according to the protocol of CCK-8, then proliferation curves were plotted.
Colony Formation Assay
LV-TRPV4 shRNA or LV-control shRNA transfected cells (about 0.5 × 102) were placed in 60 mm culture dishes and the complete media were supplemented every 2–3 days. After 14 days, the cells were fixed with methanol and stained with 0.5% crystal violet. The numbers of clones were manually counted. All tests were done in triplicate.
Invasion Capacity Evaluation
Invasive capacity was evaluated via the transwell assay. A transwell membrane with 8-µm pore size pre-coated with matrigel (1:6) was used. LV-TRPV4 shRNA or LV-control shRNA transfected cells (1 × 105) were placed on the upper chambers supplemented with 100 μL DMEM containing 1% FBS, and lower chambers were supplemented with DMEM containing 10% FBS. After incubation at 37°C for 48 h, the penetrated cells were fixed in 3.7% formaldehyde for 15 min and stained with 0.1% crystal violet at room temperature for 30 min. Then, the numbers of cells were counted under a microscope. All experiments were conducted in triplicate.
Statistical Analysis
Data were expressed as means ± standard deviations. Student’s t-test or Mann–Whitney test was used to analyze the differences between two groups. The relationships between the TRPV4 expression levels and clinicopathological parameters were analyzed using chi-square test. The correlations between TRPV4 expression and EMT-related proteins (E-cadherin or Vimentin) were determined by the Spearman’s rank correlation test. Survival curves were plotted with the Kaplan–Meier method and compared using the Log rank test. The factors that were shown to be of prognostic significance in the univariate models were evaluated with a multivariable cox regression model. The differences with P < 0.05 in a two-sided test were considered to be statistically significant. Statistical analyses were performed with SPSS version 22.0.
Results
TRPV4 Was Upregulated in Gastric Cancer Tissues
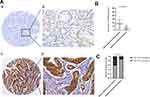
To explore the role of TRPV4 in gastric adenocarcinoma tissues, immunohistochemical analyses of TMAs were used to detect the TRPV4 expression levels in 107 samples of gastric adenocarcinoma tissues and 81 samples of adjacent noncancerous tissues. TRPV4-positive staining was mainly localized in the cytoplasms of gastric cancer cells and adjacent noncancerous gastric epithelia at different levels (Figure 1A). Gastric cancer tissues showed a significant rise in the IHC scores for the expression level of TRPV4 protein compared with adjacent noncancerous tissues (P < 0.001) (Figure 1B). For the IHC analyses, the expression levels of TRPV4 protein were categorized into high or low expression based on the cut-off value obtained by using X-tile software program. The gastric cancer tissues with high TRPV4 expression levels accounted for 40.19% of the total samples (43/107), which was significantly higher than the proportion observed in adjacent noncancerous tissues (12.35% (10/81), χ2 =17.65; P < 0.001) (Figure 1C).
High Expression of TRPV4 Correlates with Aggressive Phenotypes of Gastric Cancer
We analyzed relationships between the expression levels of TRPV4 protein and clinicopathological parameters. As shown in Table 1, high TRPV4 expression levels were positively correlated with aggressive phenotypes of gastric cancer, including TNM stage (P < 0.001), depth of tumor invasion (P = 0.001), and lymph node metastasis (P < 0.001). However, the TRPV4 expression levels were not significantly associated with age, gender, tumor size, tumor differentiation, and Lauren classification.
 |
Table 1 Association of TRPV4 Expression with Clinicopathological Characteristics in Patients with Gastric Cancer |
Association of the Expression Levels of TRPV4 Protein with EMT-Related Protein in Gastric Cancer Tissues
Our results showed that high TRPV4 expression levels were closely correlated with invasion and metastasis, such as depth of tumor invasion and lymph node metastasis. It is well known that EMT plays a critical role in invasion and metastasis of tumors. Thus, we investigated the relationship between the expression levels of the TRPV4 and those of EMT-related proteins in gastric cancer tissues by immunohistochemical analyses. As shown in Figure 2 and Table 2, the levels of TRPV4 expression were positively correlated with those of vimentin expression (r = 0.206, P < 0.05) and were inversely correlated with those of E-cadherin expression in gastric cancer tissues (r = −0.336, P < 0.001).
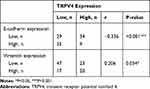 |
Table 2 Correlation Between Expression of TRPV4, E-Cadherin and Vimentin in Gastric Cancer |
High Expression Levels of TRPV4 Protein Were Associated with Poor Prognosis
Subsequently, the correlation between the levels of TRPV4 expression and survival time of gastric cancer patients was evaluated using Log rank test. We found that gastric cancer patients with high levels of TRPV4 expression had markedly reduced overall survival and disease-free survival (Figure 3A and B). This result was consistent with the prognostic analysis of TRPV4 gene in gastric cancer obtained online from Kaplan–Meier Plotter (http://kmplot.com/analysis/) (Figure 3G). In addition, low levels of E-cadherin expression (Figure 3C and D) and high levels of vimentin expression l (Figure 3E and F) were associated with poor overall survival and disease-free survival. In univariate analyses, some factors, including the levels of TRPV4 expression, E-cadherin expression, and vimentin expression, tumor size, depth of tumor invasion, lymph node metastasis, and TNM stage, were also found to be related to overall survival and disease-free survival. However, in multivariable cox regression analyses, only TNM stage was an independent prognostic factor for patients with gastric cancer, while no prognostic significance was observed with respect to other factors (Tables 3 and 4). Therefore, TRPV4 may not be an independent prognostic factor for overall survival and disease-free survival in patients with gastric cancer.
 |
Table 3 Univariate and Multivariate Cox Regression Analyses of Overall Survival in Patients with Gastric Cancer |
 |
Table 4 Univariate and Multivariate Cox Regression Analyses of Disease-Free Survival in Patients with Gastric Cancer |
 |
Figure 3 Survival curves using the Kaplan–Meier method by Log rank test. (A and B) The patients with high TRPV4 expression had shorter overall survival and disease-free survival than those with low TRPV4 expression. (C and D) The patients with low E-cadherin expression had shorter overall survival and disease-free survival than those with high E-cadherin expression. (E and F) The patients with high vimentin expression had shorter overall survival and disease-free survival than those with low vimentin expression. (G) Overall survival analysis of TRPV4 gene in gastric cancer obtained from Kaplan–Meier Plotter online (http://kmplot.com/analysis/). *P<0.05, **P<0.01, ***P<0.001. Abbreviations: Cum, cumulative; TRPV4, transient receptor potential vanilloid 4. |
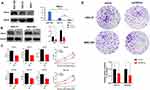
Down-Regulation of TPRV4 Inhibited Proliferation, Colony Formation, and Invasion of Gastric Cancer Cells
The results obtained with Western blot showed that, in the cellular level, the expression levels of TRPV4 protein were relatively higher in human gastric cancer cell lines (HGC-27 and MGC-803) than that in normal gastric mucosa cell (GES-1) (Figure 4A, MGC-803 vs GES-1, P < 0.001; HGC-27 vs GES-1, P < 0.01). To further investigate the effects of TPRV4 on the proliferation and invasion of gastric cancer cells, TPRV4 knockdown was carried out and the results showed that the expression levels of TRPV4 protein were reduced in HGC-27 and MGC-803 cell lines (Figure 4B, P < 0.001). Down-regulation of TRPV4 by shRNA significantly hindered the proliferation capacities of gastric cancer cell lines at 24, 48, and 72 h by CCK-8 assay (Figure 4C, P < 0.001). Colony formation assay also showed that the numbers of colonies in TRPV4 shRNA groups were fewer than those in control groups (Figure 4D, P < 0.05). Moreover, transwell assay demonstrated that TPRV4 knockdown greatly inhibited invasion capability of gastric cancer cell lines at 48 h after shRNA transfection (Figure.5A and B). The numbers of the invasive cells decreased following transfection with TRPV4 shRNA compared to control shRNA. (Figure 5C, HGC-27: 13.7 ± 2.3 vs 36.7 ± 1.5, P = 0.0001; Figure 5D, MGC-803: 23.7 ± 2.3 vs 43.3 ± 3.8, P = 0.015).
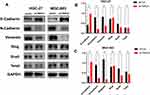
Down-Regulation of TPRV4 Regulated EMT-related Proteins in Gastric Cancer Cells
Tissue microarray showed that the levels of TRPV4 expression positively correlated with those of vimentin expression and inversely correlated with those of E-cadherin expression in gastric cancer tissues. In vitro, we also found that the expression levels of N-Cadherin and vimentin proteins decreased with down-regulation of the levels of TRPV4 expression in both HGC-27 and MGC-803 cell lines, while the levels of E-cadherin expression increased (Figure 6). To investigate the potential mechanisms that TRPV4 induced EMT, the protein expression levels of EMT-associated transcription factors, including Snail, Slug, and Twist, were measured. The results revealed that the expression level of Snail decreased, while the expression levels of Slug and Twist did not significantly change (Figure 6).
Discussion
TRPV4 belongs to the TRP family, which plays diverse roles in biological and pathological processes. Several studies showed that TPRV4 contributed to tumor development. However, the functions and mechanisms of TRPV4 in human GC remain largely unclear. Although two previous studies indicated that TRPV4 as the downstream signals of calcium-sensing receptor (CaSR) and vasoactive intestinal peptide receptor 1 (VPAC1) was involved in GC progression, only 10 GC biopsy samples were used to investigate TRPV4 expression in GC.10,11 Larger numbers of samples are needed to demonstrate the differences in the expression levels of TRPV4 between gastric cancer tissues and normal tissues, and to explore the association of TRPV4 expression with clinicopathological parameters and prognosis. In the present study, our results showed that the expression levels of TRPV4 protein were upregulated in gastric adenocarcinoma tissues compared with adjacent noncancerous tissues through tissue microarray by immunohistochemical staining. Furthermore, we demonstrated that higher expression levels of TRPV4 were associated with more aggressively clinicopathological features, such as greater depth of tumor invasion, lymph node metastasis, and higher TNM stage. Tumor progression and metastasis are the main factors influencing the prognosis. We found that patients with higher expression levels of TRPV4 had markedly reduced overall survival and disease-free survival, which was consistent with prognostic analyses of TRPV4 mRNA expression in gastric cancer obtained from Kaplan–Meier Plotter. These results indicated that TRPV4 overexpression had a clinical implication on the aggression of gastric cancer and poor prognosis in the patients with gastric cancer. In vitro, the expression levels of TRPV4 protein in gastric cancer cell lines HGC-27 and MGC-803 were observed to be upregulated than that in normal gastric mucosa cell line GES-1. Functionally, in vitro assays revealed that down-regulation of TPRV4 in gastric cell lines inhibited cell proliferation and invasion. However, the multivariate analysis using the cox proportional hazards model indicated that only TNM stage, rather than the expression level of TRPV4, was an independent prognostic factor for survival in GC patients. Therefore, to some extent, TRPV4 may not be considered as a potential prognostic biomarker for GC patients.
Metastasis is a major cause of cancer-related mortality. Thus, the mechanisms of gastric cancer metastasis must be clarified in order to develop new therapeutic methods and improve prognosis. Transient receptor potential (TRP) channels transduce mechanical forces by facilitating the transport of second-messenger ions such as calcium ion, an ion that has been implicated in cancer metastasis.14–17 EMT is one of the critical steps in the early stages of cancer metastasis.18 A hallmark of EMT is the down-regulation of E-cadherin.19 EMT is modulated by diverse micro-environmental, membrane, and intracellular cues, and can be triggered by various overexpressed transcription factors in gastric cancer.20 Recently, the relationship between the plasma membrane ion channels and EMT has been reported.21 The link between TRPV4 and E-cadherin has been identified in several studies. Janssen et al provided evidence for colocalization between TRPV4 and E-cadherin in human bladder tissues by immunofluorescence experiments and revealed the molecular connection between TRPV4 and α-catenin that catenates E-cadherin to the actin-microfilament network.22 Sokabe et al demonstrated that TRPV4 was associated with E-cadherin complex via β-catenin in skin keratinocytes.23 In breast cancer, TRPV4 activation induced E-cadherin down-regulation and TRPV4 was required for the secretion of proteins involved in remodeling of the extracellular matrix.7 A recent study showed that TRPV4-specific antagonist HC-067047 decreased the migration capability by attenuating the EMT process in hepatocellular carcinoma cells.9 Collectively, these data support the role of TRPV4 in regulating the EMT process. To determine whether TRPV4 could promote GC progression through the induction of EMT, relationship between TRPV4 and EMT-related proteins was investigated in gastric cancer tissues and cell lines. By immunohistochemical staining, we found that the levels of TRPV4 expression were positively correlated with the expression levels of vimentin and inversely correlated with those of E-cadherin in GC tissues. In vitro experiments also revealed that the expression level of E-cadherin was up-regulated while the expression levels of mesenchymal markers (vimentin and N-Cadherin) were down-regulated upon TRPV4 silencing, which suggested that TRPV4 was capable of inducing EMT in human GC. The EMT event is induced by E-cadherin loss that can be carried out by many transcription factors, including Snail, Slug, ZEB1, ZEB2, KLF8, and Twist.18 Transcription factors can decrease the expression of epithelial genes by binding to E-box DNA sequences and activate mesenchymal genes.24 As these transcription factors have distinct expression profiles, their contributions to EMT depend on the cell or tissue types involved.19 In the present study, silencing TRPV4 in gastric cell lines led to down-regulation of the EMT transcription factor Snail, while the expression levels of Slug and Twist did not change. We postulated that TRPV4 could induce EMT in human GC partly through Snail pathway. In GC stroma, the diverse extracellular stimulus signals from the surrounding microenvironment, which can activate TRPV4, may increase the expression levels of the EMT transcription factors in the nuclei and render cells to obtain an EMT-related morphology. The downstream signaling cascades of TRPV4 may contain many signaling pathways, including ERK, AKT, β-catenin, GSK, etc.20 Further studies are required to elucidate the detailed mechanisms about how TRPV4 activates Snail in GC EMT.
Conclusion
In this study, we demonstrated for the first time that overexpression of TRPV4 in gastric adenocarcinoma tissues was associated with aggressive clinicopathological characteristics and poor outcome of GC patients. Our observations have further characterized the molecular mechanisms of TRPV4 in human GC and indicated that TRPV4 could promote GC EMT via Snail. It is likely that TRPV4 can be exploited as a potential marker of GC progression and as a target for therapeutic interventions.
Acknowledgments
The present study was supported by the National Natural Science Foundation of China (No. 81000151 and No.81000174) and the Shanghai Health Commission in China (No. 201940342).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhu G, Wang X, Yang Z, et al. Effects of TRPM8 on the proliferation and angiogenesis of prostate cancer PC-3 cells in vivo. Oncol Lett. 2011;2:1213–1217. doi:10.3892/ol.2011.410
2. Peng M, Wang Z, Yang Z, et al. Overexpression of short TRPM8 variant alpha promotes cell migration and invasion, and decreases starvation-induced apoptosis in prostate cancer LNCaP cells. Oncol Lett. 2015;10:1378–1384. doi:10.3892/ol.2015.3373
3. Zhang J, Zhou Y, Huang T, Wu F, Kang W. PIEZO1 functions as a potential oncogene by promoting cell proliferation and migration in gastric carcinogenesis. Mol Carcinog. 2017;57:1144–1155.
4. Yu S, Huang S, Ding Y, Wang W, Wang A, Lu Y. Transient receptor potential ion-channel subfamily V member 4: a potential target for cancer treatment. Cell Death Dis. 2019;10:497. doi:10.1038/s41419-019-1708-9
5. Fiorio Pla A, Gkika D. Emerging role of TRP channels in cell migration: from tumor vascularization to metastasis. Front Physiol. 2013;4:311. doi:10.3389/fphys.2013.00311
6. Petho Z, Najder K, Bulk E, Schwab A. Mechanosensitive ion channels push cancer progression. Cell Calcium. 2019;80:79–90. doi:10.1016/j.ceca.2019.03.007
7. Lee WH, Choong LY, Jin TH, et al. TRPV4 plays a role in breast cancer cell migration via Ca(2+)-dependent activation of AKT and downregulation of E-cadherin cell cortex protein. Oncogenesis. 2017;6:e338. doi:10.1038/oncsis.2017.39
8. Lee WH, Choong LY, Mon NN, et al. TRPV4 regulates breast cancer cell extravasation, stiffness and actin cortex. Sci Rep. 2016;6:27903. doi:10.1038/srep27903
9. Fang Y, Liu G, Xie C, et al. Pharmacological inhibition of TRPV4 channel suppresses malignant biological behavior of hepatocellular carcinoma via modulation of ERK signaling pathway. Biomed Pharmacother. 2018;101:910–919. doi:10.1016/j.biopha.2018.03.014
10. Tang B, Wu J, Zhu MX, et al. VPAC1 couples with TRPV4 channel to promote calcium-dependent gastric cancer progression via a novel autocrine mechanism. Oncogene. 2019;38:3946–3961. doi:10.1038/s41388-019-0709-6
11. Xie R, Xu J, Xiao Y, et al. Calcium promotes human gastric cancer via a novel coupling of calcium-sensing receptor and TRPV4 channel. Cancer Res. 2017;77:6499–6512. doi:10.1158/0008-5472.CAN-17-0360
12. Liao TT, Yang MH. Revisiting epithelial‐mesenchymal transition in cancer metastasis: the connection between epithelial plasticity and stemness. Mol Oncol. 2017;11:792–804. doi:10.1002/1878-0261.12096
13. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–7259. doi:10.1158/1078-0432.CCR-04-0713
14. Yang SL, Cao Q, Zhou KC, Feng YJ, Wang YZ. Transient receptor potential channel C3 contributes to the progression of human ovarian cancer. Oncogene. 2009;28:1320–1328. doi:10.1038/onc.2008.475
15. Yang LL, Liu BC, Lu XY, et al. Inhibition of TRPC6 reduces non-small cell lung cancer cell proliferation and invasion. Oncotarget. 2017;8:5123–5134. doi:10.18632/oncotarget.14034
16. Holzmann C, Kappel S, Kilch T, et al. Transient receptor potential melastatin 4 channel contributes to migration of androgen-insensitive prostate cancer cells. Oncotarget. 2015;6:41783–41793. doi:10.18632/oncotarget.6157
17. Middelbeek J, Kuipers AJ, Henneman L, et al. TRPM7 is required for breast tumor cell metastasis. Cancer Res. 2012;72:4250–4261. doi:10.1158/0008-5472.CAN-11-3863
18. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi:10.1172/JCI39104
19. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi:10.1038/nrm3758
20. Huang L, Wu RL, Xu AM. Epithelial-mesenchymal transition in gastric cancer. Am J Transl Res. 2015;7:2141–2158.
21. Azimi I, Monteith GR. Plasma membrane ion channels and epithelial to mesenchymal transition in cancer cells. Endocr Relat Cancer. 2016;23:R517–R25. doi:10.1530/ERC-16-0334
22. Janssen DAW, Hoenderop JG, Jansen KCFJ, Kemp AW, Heesakkers JPFA, Schalken JA. The mechanoreceptor TRPV4 is localized in adherence junctions of the human bladder urothelium: a morphological study. J Urol. 2011;186:1121–1127. doi:10.1016/j.juro.2011.04.107
23. Sokabe T, Tominaga M. The TRPV4 cation channel: a molecule linking skin temperature and barrier function. Commun Integr Biol. 2010;3:619–621. doi:10.4161/cib.3.6.13461
24. Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi:10.1038/nrc2131
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.