Back to Journals » International Journal of Nanomedicine » Volume 12
Triclosan resistance reversion by encapsulation in chitosan-coated-nanocapsule containing α-bisabolol as core: development of wound dressing
Authors De Marchi JG , Jornada DS, Silva FK, Freitas AL, Fuentefria AM, Pohlmann AR , Guterres SS
Received 6 June 2017
Accepted for publication 9 August 2017
Published 25 October 2017 Volume 2017:12 Pages 7855—7868
DOI https://doi.org/10.2147/IJN.S143324
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Israel (Rudi) Rubinstein
João Guilherme B De Marchi,1 Denise S Jornada,1 Fernanda K Silva,1 Ana L Freitas,2 Alexandre M Fuentefria,2 Adriana R Pohlmann,1,2 Silvia S Guterres1
1Pharmaceutical Sciences Graduate Program, 2Department of Organic Chemistry, Institute of Chemistry, Federal University of Rio Grande do Sul, Porto Alegre, RS, Brazil
Abstract: The use of nanoparticles may be particularly advantageous in treating bacterial infections due to their multiple simultaneous mechanisms of action. Nanoencapsulation is particularly useful for lipophilic drugs. In this scenario, triclosan is considered a good candidate due to its lipophilicity, broad-spectrum activity, and safety. In the present study, we have developed and characterized an antimicrobial suspension of triclosan and α-bisabolol against pathogenic strains that are resistant (Pseudomonas aeruginosa) and susceptible (Escherichia coli, Staphylococcus aureus, and Candida albicans) to triclosan. We also aimed to determine the minimum inhibitory concentration, using serial microdilution adapted from a CLSI methodology (Clinical and Laboratory Standards Institute). Challenge test was used to confirm the antimicrobial effectiveness of the nanocapsule formulation, as well as after its incorporation into a commercial wound dressing (Veloderm®). The zeta potential of P. aeruginosa before and after contact with cationic nanocapsules and the ratio between the number of nanocapsules per colony forming unit (CFU) were determined to evaluate a possible interaction between nanocapsules and bacteria. The results showed that nanoencapsulation has improved the antimicrobial activity when tested with two different methodologies. The number of nanocapsules per CFU was high even in great dilutions and the zeta potential was reverted after being in contact with the cationic nanocapsules. The nanocapsules were able to improve the activity of triclosan, even when tested within 28 days and when dried in the wound dressing.
Keywords: antimicrobial effect, triclosan, α-bisabolol, chitosan, nanocapsules
Introduction
Nanoparticles have been studied for their antimicrobial properties1–3 and as carriers for antimicrobial drugs, which have shown promising results.4,5 Even when tested against resistant microorganisms, nanoemulsion,6 liposomes,7,8 and nanoparticles9,10 could reverse drug resistance. The many different modes of action of nanocarries make the occurrence of multiple concurrent mutations unlikely to develop resistance to nanoparticles.11
Lipophilic drugs are suitable candidates for encapsulation in organic nanoparticles for presenting positive logarithm values of drug distribution (Log D), which determines their mechanism of encapsulation in polymeric nanocapsules.12 Log D is the lipophilicity of molecules estimated by calculating the logarithm of the octanol–water distribution coefficient of a molecule, considering the pH value of the medium that affects the proportion of unionized and ionized species and their distributions in the organic and aqueous phases.12
Triclosan is a lipophilic antimicrobial drug that has been used for over 30 years in the treatment of infections. It is the most potent and widely used bisphenol with a favorable safety and nontoxic profile.13 Triclosan has a broad spectrum of antimicrobial activity against a variety of microorganisms.14 However, bacteria, such as Pseudomonas aeruginosa, present resistance to triclosan due to efflux pump mechanisms.15 Some characteristics, such as high log D (5.17), thermal stability, and broad spectrum of activity, make triclosan a good candidate for encapsulation into organic nanoparticles, such as nanocapsules. Polymeric nanocapsules contain two domains, ie, an oily core and a polymeric wall, that are dispersed in water with the use of surfactants.
In order to properly carry the drug, the oily core of the nanocapsules needs to be able to disperse triclosan. Keeping that in mind, α-bisabolol, which is a monocyclic sesquiterpene alcohol, was considerd to be a good candidate because of its characteristics. Bisabolol is a viscous oil known to have anti-inflammatory, antimicrobial, and wound-healing properties,16,17 and it is also a lipophilic antibiotic activity enhancer.18 The log D of α-bisabolol is 5.07, demonstrating its lipophilic character, which can guarantee the encapsulation of poorly water-soluble drugs, such as triclosan.
Studies of nanoparticles have demonstrated the importance of the positive charge, which improves interaction with microorganisms.19,20 To take advantage of positively charged nanoparticles, many studies have been reported on the use of chitosan as coating.21,22 In addition, the use of this polycationic biopolymer in an antimicrobial formulation is interesting due to its biodegradability and antimicrobial activity.1,23
An innovative strategy for antimicrobial nanoparticle formulations is to incorporate them into medical products,24 mainly for wound dressings impregnated with silver nanoparticles25,26 to inhibit bacterial growth (BG). A good candidate for this application is Veloderm®, which is a biological wound dressing with good healing properties.27,28 The use of biological wound dressing alone had few problems reported, such as infections, and consequently the interruption of treatment due to its lack of antimicrobial effect.29 This problem could be solved by incorporating an antimicrobial nanocapsule formulation into this occlusive dressing.
Therefore, the aims of this study were to develop and characterize an antimicrobial nanocapsule formulation containing triclosan and α-bisabolol, to evaluate its effect against pathogenic strains that are resistant (Pseudomonas aeruginosa) and susceptible (Escherichia coli, Staphylococcus aureus, and Candida albicans) to triclosan, and to verify its suitability for incorporation into wound dressings.
Materials and methods
Materials
Poly(epsilon-caprolactone) (PCL, Mn 80 kDa), chitosan of low molecular weight (50–190 kDa) and 75%–85% deacetylation degree, Mueller-Hinton broth 2, RPMI-MOPS, and MTT bromite of 3-(4,5-dimetiltiazol-2-il)-2,5-dipheniltetrazolium) were obtained from Sigma-Aldrich (St Louis, MO, USA). Medium-chain triglyceride (MCT) was purchased from Delaware (Porto Alegre, RS, Brazil), α-bisabolol and triclosan were acquired from Fragon (São Paulo, SP, Brazil), isopropanol and acetone were obtained from Vetec (São Paulo, SP, Brazil), and acetonitrile and ethanol HPLC standard were purchased from Tedia (São Paulo, SP, Brazil). Glacial acetic acid, Lipoid S75® (soybean lecithin), and polysorbate 80 were acquired from Fmaia (Belo Horizonte, MG, Brazil), Lipoid (Ludwigshafen, RP, Germany), and Henrifarma (São Paulo, SP, Brazil), respectively.
Production of nanocapsules and controls
Interfacial deposition of preformed polymers was the method used to produce the nanocapsules.30 α-bisabolol was used as oil core and PCL as polymeric wall. The nanocapsules were coated with soybean lecithin, polysorbate 80, and chitosan. An ethanol solution of lecithin was added to an organic phase composed of PCL, α-bisabolol, and triclosan in acetone. The organic phase was injected into an aqueous phase containing polysorbate 80, as stabilizer. Polysorbate 80 and lecithin were used in the same proportion. Reduced volume of aqueous phase (20 mL) compared to the organic phase (25 mL) was used as previously proposed.31 The nanocapsules were coated with the cationic biopolymer using 0.7% of chitosan in 1% acetic acid aqueous solution, based on an adapted technique from Mayer et al.32 Triclosan or α-bisabolol were dispersed in polysorbate 80 aqueous solutions in the same proportion used to obtain the nanocapsule formulations. Blank nanocapsule formulation (NCBL) was prepared using MCT in the place of α-bisabolol. All formulations were adjusted to the same concentration with a final volume of 10 mL, as described in Table 1.
Physicochemical characterization of nanocapsules
The characterization of nanocapsules were carried out to determine the size distribution profiles, mean diameters, polydispersity, zeta potential, pH, drug content, encapsulation efficiency, release profile, transmission electronic microscopy, and particle number density. Laser diffraction analysis (from 40 nm to 2 mm) was performed in a Mastersizer 2000 equipment (Malvern Instruments, Malvern, UK). Each sample was inserted in the wet unit containing distilled water, without any previous treatment, for a laser obscuration of 2%. Refraction indexes of 1.590 (before coating with chitosan) and 1.345 (after coating with chitosan) were used to calculate the volume-weight mean diameters (D[4,3]), the polydispersity (SPAN), and the median diameter by number of particles [d(0.5)n]. Dynamic light scattering analysis (from 0.6 to 1,000 nm) was carried out in a Zetasizer Nano ZS instrument (Malvern Instruments). Each sample was diluted (500 times) in pre-filtered (Millipore®, 0.45 μm) ultrapure water. The particle diameter profiles were determined to calculate the hydrodynamic mean diameter (Dh) and the polydispersity index (PDI) for each batch of formulation. Zeta potential values were determined by electrophoretic mobility using the same instrument (Zetasizer Nano ZS) after diluting each sample (500 times) in 10 mmol/L NaCl aqueous solution. The pH values were determined by direct measurement using potentiometer (B474; Micronal, São Paulo, Brazil) calibrated at 4.00 and 7.00 with phosphate buffer.
Encapsulation efficiency (EE%) was analyzed by ultrafiltration-centrifugation method33 using a ultrafiltration-centrifugation unit (Millipore; Amicon® Ultra, cut-off 10 kDa), centrifuged at 1,844× g (RCF) for 5 minutes. The same methodology was applied in diluted formulations in ultrapure water (1:100 and 1:1,000, v/v) in order to determine the ability of the drug to remain encapsulated, allowing the serial microdilutions to perform the microbiological experiments. All triclosan quantifications were performed by high performance liquid chromatography (HPLC) (HPLC model LC 20A, Shimadzu Co., Tokyo, Japan) using a previously validated method,31 regarding accuracy, linearity, precision, and specificity parameters.34 Nova-Pax RP-18 column, Waters® (Milford, CT, USA), was used as a stationary phase. A mixture of acetonitrile:H2O (60:40, v/v) with apparent pH of 4.5 corrected with acetic acid was used as a mobile phase. The drug was detected at λ=280 nm. The particle number density (dNP) was determined by turbidimetry35 in a spectrophotometer Cary 50 UV-Vis (Varian, Palo Alto, USA) at λ=395 nm.
The drug release profile was determined using cellulose acetate dialysis bags Sigma-Aldrich with a cut-off of 14 kDa. The release medium was sampled in 10 points of time interval (10, 30, and 60 minutes and 3, 6, 12, 18, 24, 36, and 48 hours).36 A mixture of ethanol:water (1:1, v/v) (150 mL) was used as release medium to keep the sink condition.14 The drug release data were modeled to determine the best release profile, according to the monoexponential and biexponential models to define the best adjustment by mathematical modeling.35 MicroMath Scientist® was used to analyze the profiles, and the model was determined according to the best correlation coefficient, the best model selection criteria, and the best graphic adjustment. The morphology of the nanocapsules (NCBC and NCAC) were evaluated by transmission electronic microscopy37 using a JEM 1200 Exll, operated at 80 kV and stained with uranyl acetate solution (2% w/v).
Minimum inhibitory concentration (MIC) in bacteria
Bacterial MICs were determined in liquid growth media Mueller Hinton against Pseudomonas aeruginosa (ATCC 27853), Escherichia coli (ATCC 25922), and Staphylococcus aureus (ATCC 25923), using serial microdilution, in 96-well plates.38 In order to avoid natural turbidity caused by the nanocapsules, which could interfere in visual inspection, a spectrophotometer was employed to measure the turbidimetry. To determine BG, the measures were taken before (T0) and after 24 hours of incubation (T24). Therefore, absorbance results of T0 were subtracted from T24, according to Equation 1. MICs were obtained considering the lowest concentration which had no statistically significant difference between T24 and T0 (p>0.05). Inoculums and serial microdilution were prepared according to CLSI 200338 in microdilution plates (96 U-shaped wells), and the wavelength used to measure the turbidity was 625 nm, with T0 as initial value.
BG = T24 − T0 | (1) |
MIC in yeast
The MIC determination of Candida albicans (ATCC 24433) for different formulations was made in liquid growth media RPMI-MOPS, using serial microdilution, according to CLSI 2008.39 MTT assay was used to determine viability detection,40 which had an absorbance in the two wavelengths evaluated (570 and 690 nm). Breakpoints were determined as the minor concentration, which reached 80% of cellular damage. Inoculums and microdilution were prepared following CLSI 200839 and the time of incubation was 48 hours. To verify any possible interference due to redox reaction, and consequently, formazan precipitation, all samples were incubated in growth media for 24 hours without the presence of inoculums.40
Zeta potential of P. aeruginosa before and after contact with nanocapsules
Zeta potential values were evaluated for pure inoculum of P. aeruginosa and for inoculum in contact with nanocapsules in the same proportion (1:1) to simulate the same conditions as in the first microdilution well. The inoculum with nanocapsules was homogenized and left in contact for 15 minutes to ensure a proper interaction. Dilutions in 10 mmol/L NaCl aqueous solutions were made for the nanocapsule analysis, as described earlier. Results were expressed as a mean of 3 independent measurements,41 and bacterial concentration was fixed at 5×105 CFU/mL to reproduce the first well of the serial microdilution testing condition.
Number of nanocapsules per colony forming unit (CFU)
To determine the number of nanocapsules that could interact with each CFU, a theoretical ratio calculation (R), Equation 2, was preformed using the particle number density (nanocapsules per milliliter, dNP), as described earlier. The number of CFU was established during inoculum preparation (~5×105 CFU/mL).
R = dNP/CFU | (2) |
Nanocapsules incorporation into wound dressing
To verify the feasibility of incorporating the NCAC into the hemicellulose wound dressing (Veloderm®), a spraying method was applied. Briefly, the wound dressing was cut into small rectangles (2×6 cm2), weighted before and after spraying the nanocapsule formulation, and dried (24 hours protected from the wind). Spraying process was carried out two times with a commercial spray (Brand New®) at 15 cm distance. The product was named WD-NCAC, and triclosan from incorporated NCAC was extracted from dried wound dressing with pure acetonitrile (3 mL) and quantified by HPLC (λ=280 nm) with a validated method.
Challenge test
To verify the maintenance of the antimicrobial effect, the challenge test was performed. The strains tested were Escherichia coli ATCC 8739 (in MacConkey agar), Staphylococcus aureus ATCC 25923 (Baird-Parker agar), Pseudomonas aeruginosa ATCC 27853 (cetrimide agar), and Candida albicans ATCC 10231 (potato glycosylated agar). The procedure was adapted from a previously reported methodology,42 by dilution in buffered sodium chloride-peptone solution, adjusted to pH 6.0–8.0, in the same proportion (1:9). Samples were taken at time intervals (24 hours and 7, 14, 21, and 28 days) after incubation and cultivated for 24 hours; visual quantifications were made. This experiment was performed for NCAC suspension and NCAC incorporated into a wound dressing.
Statistical analysis
Significant differences between measurements were detected by two-way ANOVA, followed by Bonferroni’s multiple comparison test. Differences between comparisons were considered to be significant at p<0.05. All analyzes were performed using GraphPad Prism 5.0® software (GraphPad Software, Inc., San Diego, CA, USA).
Results and discussion
Development of nanocapsules
The nanocapsule formulations containing triclosan (NCBC and NCAC) showed narrow size distribution profiles by laser diffraction with similar D[4,3] and polydispersity (Table 2). NCAC showed a calculated median diameter by number of particles [d(0.5)n] of 130±2 nm. NCBC and NCAC analyzed by dynamic light scattering (DLS) (Table 2) had similar Dh with narrow size distributions, since PDIs were below 0.15. Zeta potential was negative before chitosan coating (NCBC) and it was reverted to a positive value after the interfacial reaction (NCAC) (Table 2), corroborating a previous study.22 Potentiometry analyses showed that pH was neutral for NCBC formulation (7.04±0.17); however, after adding the chitosan solution, the pH values decreased to 4.09±0.1 (NCAC). The acidity increased due to the presence of acetic acid used to disperse chitosan in water, as previously reported.22 Values of pH between 4 and 5 can be suitable for a topical application, since skin surface has slight acidity.43 Nanocapsules prepared without triclosan and with MCT in the place of α-bisabolol (NCBL) showed similar physicochemical attributes than those presented by NCAC.
After preparation, NCAC had an experimental triclosan content of 0.86±0.02 mg/mL, which was close (95.5%) to the theoretical concentration (0.9 mg/mL). After 30 days, this formulation showed a similar (p>0.05) triclosan content (0.82±0.01 mg/mL). Particle number density for NCAC was (7.88±0.96) ×1013 nanoparticles per milliliter. This value is ~10 times higher than the one observed for other nanocapsule formulation.35 The difference is based on the use of ethanol to prepare the former nanocapsules compared to the ethanol-free process generally employed for the latter, which particles have mean size >100 nm.
No triclosan was detected by HPLC in the ultrafiltrate for NCAC irrespective of whether samples were undiluted or diluted, indicating an EE% of 100%. NCAC are polymeric nanocapsules prepared with other nucleous than the lipid-core nanocapsules. However, this result corroborated our previous study,12 in which we proposed the use of log D as the main parameter to estimate the mechanism of drug encapsulation in polysorbate 80-coated lipid-core nanocapsules with a core composed of MCT and sorbitan monostearate. For polysorbate 80-lipid-core nanocapsules, drugs with log D >4 are concentrated in the nanocapsule core. Triclosan (log D 5.17) is likely to concentrate within the core of NCAC, which is composed of α-bisabolol.
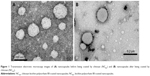
Transmission electron microscopy was used to show the morphological characteristics of the nanoparticles (Figure 1). NCBC are spheroids with a higher pigment density at the corona (Figure 1A). This characteristic was previously observed for lecithin-polysorbate 80-lipid-core nanocapsules,44 with a corona formed by spherical and cylindrical micellar structures. NCAC are also spheroids having a corona pigmentation of lower intensity with a characteristic fringe (Figure 1B) due to the presence of chitosan in the formulation.
Regarding the release experiment, the triclosan dialyzed from the polysorbate 80 dispersion formulation (TP80) was ~80%, in 24 hours. In contrast, for NCAC, the triclosan released in 24 hours was 64%, showing a controlled profile, which reached a plateau in 36 hours (Figure 2). The results fitted to a monoexponential first-order model for both formulations TP80 (k= (3.2±1.5) ×10−3/min and t1/2=4.9±2.6 hours) and NCAC (k= (9.2±3.3) ×10−4/min and t1/2=14.1±4.2 hours). These results showed that nanoencapsulated formulations were able to control the drug releasing rate (k), 3 times slower, and half-life (t1/2), 3 times higher, when compared to TP80.
MIC
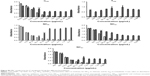
MIC results are described based on the concentrations of triclosan (MICT), α-bisabolol (MICα), and chitosan (MICCHI) (Table 3 and Figures S1–S4). The strains were chosen for being the most common pathogens found in infected wounds. S. aureus colonization may occur within the first 48 hours and P. aeruginosa and E. coli within the first 72 hours, while Candida sp. is the most common cause of fungal infections.44 For S. aureus and considering triclosan, NCBC showed a slight reduction in sensitivity compared to the free drug dispersed in polysorbate 80 (TP80). Conversely, after coating the nanocapsules with chitosan, NCAC showed a sensitivity increase close to 4-folds compared to TP80. Considering α-bisabolol, both nanocapsule formulations (NCBC and NCAC) were more effective than the free drug dispersed in polysorbate 80 (αP80) (917-folds and 7,333-folds, respectively). It is worth noting the difference between the formulations containing or not containing chitosan as the coating material. The presence of chitosan was a determinant to increase the sensitivity of S. aureus against triclosan and α-bisabolol. Our previous study45 showed a better antimicrobial activity of chitosan-lecithin-polysorbate 80-coated lipid-core nanocapsules against S. aureus, which was attributed to the antimicrobial activity of this polysaccharide against Gram-positive and Gram-negative bacteria.
For E. coli, the nanoencapsulation of triclosan in NCBC or NCAC has slightly reduced the strain sensitivity compared to TP80 (Table 3), while the nanoencapsulation of α-bisabolol in NCBC and in NCAC increased the strain sensitivity compared to aP80 (113-folds and 449-folds, respectively). The results suggest that a formulation containing only α-bisabolol, as an anti-inflammatory drug, is very promising for further studies.
For P. aeruginosa, TP80 did not show any activity as previously reported for triclosan (free drug).46,47 In fact, P. aeruginosa is reported13 to be highly resistant to triclosan, reaching values >1 mg/mL, due to its efflux pump in the outer membrane.15,48,49 In contrast, the nanoencapsulation of triclosan showed MICT of 220 mg/mL (NCBC) and 56 mg/mL (NCAC) (Table 3). NCBC demonstrated a reduction in strain sensitivity compared to aP80, while NCAC showed an increase of 1.8-folds compared to the free drug dispersed in polysorbate 80. Once again, the effect of chitosan as a coating is an important parameter to obtain results. Thereby, the main explanation relies on the cationic character of the particles, once lipopolysaccharide-mediated resistance of Gram-negative bacteria to neutral and anionic detergents were overcome by cationic nanoemulsions.6 A study conducted with Al2O3-cationic nanoparticles suggested that those structures may have an easier interaction with negative cell membrane, enhancing their penetration into the cell,3 a hypothesis that can be taken into consideration.
For C. albicans, NCBC did not show any inhibitory effect in relation to both TP80 and aP80 (Table 3). The slight negative zeta potential could be a barrier to prevent NCBC interaction with the bacterial membrane, which also has negative zeta potential.41 On the other hand, NCAC promoted an increase of 1.6-folds and 1.8-folds in comparison to TP80 and aP80, respectively. In a study performed with positive PCL nanocapsules containing chlorhexidine (free base), it was suggested that the cationic nanoparticles were able to interact with bacteria, due to their opposite charges, diffusing the drug from the core of the nanocapsules to the bacterial cell membrane.4
For all strains, αP80 showed high MICa values (Table 3). Actually, this drug is more remarkably known as a potentiator of antibiotic activity by disarranging the cell membrane structure18 than by having an antimicrobial activity.50 Nevertheless, MICa values for NCAC were reduced compared to αP80, remarkably in the case of S. aureus and E. coli.
The MICs of chitosan (MICCHI) against S. aureus, E. coli, P. aeruginosa, and C. albicans were determined using chitosan-coated blank-nanocapsule formulation (NCBL) (Table 3). The most relevant result was the antimicrobial effect against P. aeruginosa, considering the comparison between NCBL and NCAC (MICCHI 44 μg/mL for both). The inhibitory effect of NCAC observed for NCAC was exclusive from the presence of chitosan at the nanocapsule surface suggesting a possible reduction in triclosan and α-bisabolol doses in the formulation. Previously, studies in the literature reported the antimicrobial effect of chitosan.45,51–53 Regarding S. aureus, E. coli, and C. albicans, the inhibitory effect observed for NCBL was lower than that observed for NCAC.
The increased susceptibility reversal of triclosan resistance by the nanoencapsulation should be highlighted as an important achievement, since P. aeruginosa is among the most common burn infectious agents, possessing many intrinsic and acquired resistance mechanisms, which makes burn wounds infected by these bacteria difficult to treat.54 Therefore, it was selected as the activation strain model to test the hypothesis of electrostatic interaction.
Zeta potential of P. aeruginosa before and after contact with the nanocapsules
This experiment was performed to analyze the zeta potential alterations of pure inoculum and inoculum after contact with NCAC. In general, Gram-negative bacteria, such as P. aeruginosa, possess negative zeta potential due to the presence of lipopolysaccharides, phospholipids, and membrane proteins.55 The zeta potential determined for the P. aeruginosa inoculum was −4.18±1.6 mV (pure inoculum). Negative potential enhanced the interaction with positive ions,41 such as cationic-coated nanocapsules. The zeta potential of P. aeruginosa inoculum shifted to +3.19±0.27 mV after contact with NCAC (Figure 3). The positive surface potential enhabled NCAC to bind to the P. aeruginosa surface.
Number of nanocapsules per CFU
The results of the ratio between NCAC and CFUs are shown in Table 4. These results may be applied to any bacterial strain, whereas the number of CFU is the same for all of them. In experimental conditions, the nanocapsules have displayed, even in small concentration (initial dose 0.39%), high magnificence (106 nanocapsules/CFU). The high number of cationic nanocapsules interacting with the bacterial surface might destabilize or affect the membrane leading to the leakage of intracellular components and, consequently, to cell death.1 The adsorption of bacteria by the nanocapsules may prevent the electrostatic interaction between the bacteria and the surface, disabling bacterial fixation.16 Another possibility would be a great number of nanocapsules diffusing their antimicrobial drug from the core directly to the cytoplasm of microorganisms.4
  | Table 4 Ratio between the number of nanocapsules (NCAC) and the number of colony forming units (CFUs) in each dilution |
Incorporation of NCAC into wound dressing and challenge test
NCAC was incorporated into the wound dressing (WD-NCAC) showing a drug recovery of 93.28%±7.27%, corresponding to 23.41±2.54 μg/unit. The challenge test was performed in order to verify the antimicrobial effect of WD-NCAC after production and after 28 days of storage. Results shown in Table 5 confirm that after dilution (1:9), in experimental condition, the triclosan concentration (90 μg/mL) was able to inhibit microorganism growth within 28 days. This result was expected once all MIC values obtained from serial microdilution were <90 μg/mL. However, when we take into account the triclosan concentration in WD-NCAC after dilution (2.34 μg/mL), we observed that MIC obtained for P. aeruginosa (56.25 μg/mL) was ~24 times higher and C. albicans had its growth totally inhibited after 21 days.
Due to the importance of protecting the wounds toward contamination, Veloderm® with the NCAC incorporated could act in two ways. On the first contact, the nanocapsules would be able to kill microrganisms and inhibit growth of bacteria for at least 28 days. While a single application of wound dressing seals the wound bed, avoiding exposure and contact with new infectious agents, making a suitable environment for proper healing. It is important to note that both systems are complementary.
Conclusion
The present study described the development of a cationic nanostructured system, presenting highly homogeneous size of nanoparticles, without any micrometric contaminants, with acceptable pH for cutaneous use, and with the ability to control the release of triclosan. In terms of MIC results, the nanocapsules after chitosan coating (NCAC) presented the best results when compared to all controls. The wound dressing containing those nanocapsules maintained antimicrobial activity. The results also included species with high resistance to free triclosan, such as P. aeruginosa, which became susceptible to a dose nearly 8-folds smaller. In order to understand the mechanism of action of NCAC, physicochemical tests were performed revealing a large number of nanocapsules per CFU with an inversion of zeta potential after adding the formulation into the bacterial inoculum. Considering the state of the art, the results give us a light over a possible mechanism of action of NCAC and the promising use of those nanocapsules as a platform to develop novel drug delivery systems intended to increase microorganism susceptibility.
Acknowledgments
The authors appreciate the financial support provided by the following Brazilian agencies: FAPERGS, CNPq (PRONEX), and CAPES.
Disclosure
The authors report no conflict of interest in this work.
References
Qi L, Xu Z, Jiang X, Hu C, Zou X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr Res. 2004;339(16):2693–2700. | ||
Li Q, Mahendra S, Lyon DY, et al. Antimicrobial nanomaterials for water disinfection and microbial control: potential applications and implications. Water Res. 2008;42(18):4591–4602. | ||
Simon-Deckers A, Loo S, Mayne-L’hermite M, et al. Size-, composition- and shape-dependent toxicological impact of metal oxide nanoparticles and carbon nanotubes toward bacteria. Environ Sci Technol. 2009;43(21):8423–8429. | ||
Lboutounne H, Chaul JF, Ploton C, Falson F, Pirot F. Sustained ex vivo skin antiseptic activity of chlorhexidine in poly(e-caprolactone) nanocapsule encapsulated form and as a digluconate. J Control Release. 2002;82(2–3):319–334. | ||
Couvreur P, Fattal E, Alphandary H, Puisieux F, Andremont A. Intracellular targeting of antibiotics by means of biodegradable nanoparticles. J Control Release. 1992;19(1–3):259–268. | ||
LiPuma JJ, Rathinavelu S, Foster BK, et al. In vitro activities of a novel nanoemulsion against Burkholderia and other multidrug-resistant cystic fibrosis-associated bacterial species. Antimicrob Agents Chemother. 2009;53(1):249–255. | ||
Nicolosi D, Scalia M, Nicolosi VM, Pignatello R. Encapsulation in fusogenic liposomes broadens the spectrum of action of vancomycin against Gram-negative bacteria. Int J Antimicrob Agents. 2010;35(6):553–558. | ||
Huang C, Chen C, Pornpattananangkul D, et al. Eradication of drug resistant Staphylococcus aureus by liposomal oleic acids. Biomaterials. 2011;32(1):214–221. | ||
Chakraborty SP, Sahu SK, Mahapatra SK, et al. Nanoconjugated vancomycin: new opportunities for the development of anti-VRSA agents. Nanotechnology. 2010;21(10):1–9. | ||
Turos E, Reddy GSK, Greenhalgh K, et al. Penicillin-bound polyacrylate nanoparticles: restoring the activity of beta-lactam antibiotics against MRSA. Bioorg Med Chem Lett. 2007;17(12):3468–3472. | ||
Huh AJ, Kwon YJ. “Nanoantibiotics”: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J Control Release. 2011;156(2):128–145. | ||
Oliveira CP, Venturini CG, Donida B, Poletto FS, Guterres SS, Pohlmann AR. An algorithm to determine the mechanism of drug distribution in lipid-core nanocapsule formulations. Soft Matter. 2013;9(4):1141–1150. | ||
Bhargava HN, Leonard PA. Triclosan: applications and safety. Am J Infect Control. 1996;24(3):209–218. | ||
SCCS (Scientific Committee on Consumer Safety). Opinion on Triclosan (Antimicrobial Resistance). SCCS; 2010. | ||
Mima T, Joshi S, Gomez-Escalada M, Schweizer HP. Identification and characterization of TriABC-OpmH, a triclosan efflux pump of Pseudomonas aeruginosa requiring two membrane fusion proteins. J Bacteriol. 2007;189(21):7600–7609. | ||
Darra E, Abdel-Azeim S, Manarac A, et al. Insight into the apoptosis-inducing action of α-bisabolol towards malignant tumor cells: involvement of lipid rafts and Bid. Arch Biochem Biophys. 2008;476(2):113–123. | ||
Kamatou GPP, Viljoen AM. A review of the application and pharmacological properties of α-bisabolol and α-bisabolol-rich oils. J Am Oil Chem Soc. 2010;87(1):1–7. | ||
Brehm-Stecher BF, Johnson EA. Sensitization of Staphylococcus aureus and Escherichia coli to antibiotics by the sesquiterpenoids nerolidol, farnesol, bisabolol, and apritone. Antimicrob Agents Chemother. 2003;47(10):3357–3360. | ||
Cavalieri F, Tortora M, Stringaro A, Colone M, Baldassarri L. Nanomedicines for antimicrobial interventions. J Hosp Infect. 2014;88(4):183–190. | ||
Seil JT, Webster TJ. Antimicrobial applications of nanotechnology: methods and literature. Int J Nanomedicine. 2012;7:2767–2781. | ||
Mazzarino L, Travelet C, Ortega-Murillo S, et al. Elaboration of chitosan-coated nanoparticles loaded with curcumin for mucoadhesive applications. J Colloid Interface Sci. 2012;370(1):58–66. | ||
Bender EA, Adorne MD, Colomé LM, Abdalla DSP, Guterres SS, Pohlmann AR. Hemocompatibility of poly(ε-caprolactone) lipid-core nanocapsules stabilized with polysorbate 80-lecithin and uncoated or coated with chitosan. Int J Pharm. 2012;426(1–2):271–279. | ||
Kong M, Chen XG, Xing K, Park HJ. Antimicrobial properties of chitosan and mode of action: a state of the art review. Int J Food Microbiol. 2010;144(1):51–63. | ||
Zhu X, Radovic-Moreno AF, Wua J, Langer R, Shia J. Nanomedicine in the management of microbial infection – overview and perspectives. Nano Today. 2014;9(4):478–498. | ||
Archana D, Singh BK, Dutta J, Dutta PK. Chitosan-PVP-nano silver oxide wound dressing: in vitro and in vivo evaluation. Int J Biol Macromol. 2015;73:49–57. | ||
Hebeish A, El-Rafie MH, EL-Sheikh MA, Seleem AA, El-Naggar ME. Antimicrobial wound dressing and anti-inflammatory efficacy of silver nanoparticles. Int J Biol Macromol. 2014;65:509–515. | ||
Melandri D, De Angelis A, Orioli R, et al. Use of a new hemicellulose dressing (Veloderm®) for the treatment of split-thickness skin graft donor sites A within-patient controlled study. Burns. 2006;32(8):964–972. | ||
Kothamasu P, Kanumur H, Ravur N, Maddu C, Parasuramrajam R, Thangavel S. Nanocapsules: the weapons for novel drug delivery systems. Bioimpacts. 2012;2(2):71–81. | ||
Ricci EB, Cassino R, Di Campli C. Microcrystalline cellulose membrane for re-epithelisation of chronic leg wounds: a prospective open study. Int Wound J. 2010;7(6):438–447. | ||
Fessi H, Puisieux F, Devissaguet J, Ammoury N, Benita S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int J Pharm. 1989;55(1):1–4. | ||
Jornada DS, Friedrich RB, Ferrarini S, et al. Lipid-core nanocapsules: reducing the aqueous phase volume to increase encapsulation efficiency and to reduce the energy and time consuming of the production process. J Colloid Sci Biotechnol. 2015;4(1):79–85. | ||
Mayer FQ, Adorne MD, Bender EA, et al. Laronidase-functionalized multiple-wall lipid-core nanocapsules: promising formulation for a more effective treatment of mucopolysaccharidosis type I. Pharm Res. 2015;32(3):941–954. | ||
Paese K, Jäger A, Poletto FS, et al. Semisolid formulation containing a nanoencapsulated sunscreen: effectiveness, in vitro photostability and immune response. J Biomed Nanotechnol. 2009;5(3):240–246. | ||
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), Validation of analytical procedures: methodology, 1996. | ||
Poletto FS, Jäger E, Cruz L, Pohlmann AR, Guterres SS. The effect of polymeric wall on the permeability of drug-loaded nanocapsules. Mater Sci Eng C. 2008;28(4):472–478. | ||
Fontana MC, Coradini K, Guterres SS, Pohlmann AR, Beck RC. Nanoencapsulation as a way to control the release and to increase the photostability of clobetasol propionate: influence of the nanostructured system. J Biomed Nanotechnol. 2009;5(3):254–263. | ||
da Silva AL, Contri RV, Jornada DS, Pohlmann AR, Guterres SS. Vitamin K1-loaded lipid-core nanocapsules: physicochemical characterization and in vitro skin permeation. Skin Res Technol. 2013;19(1):e223–e230. | ||
Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacterial that Grow Aerobically. 6th ed. CLSI Document M07-A6. PA, USA: CLSI; 2003. | ||
Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard. 3rd ed. CLSI Document M27-A3. PA, USA: CLSI; 2008. | ||
Pippi B, Lana AJD, Moraes RC, et al. In vitro evaluation of the acquisition of resistance, antifungal activity and synergism of Brazilian red propolis with antifungal drugs on Candida spp. J Appl Microbiol. 2015;118(4):839–850. | ||
Domingues MM, Silva PM, Franquelim HG, Carvalho FA, Castanho MARB, Santos NC. Antimicrobial protein rBPI21-induced surface changes on Gram-negative and Gram-positive bacteria. Nanomedicine. 2014;10(3):543–551. | ||
United States Pharmacopeia (USP 35); Antimicrobial Effectiveness Testing. 2012;51:52–54. | ||
Schmid-Wendtner M, Korting HC. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol Physiol. 2006;19(6):296–302. | ||
D’Avignon LC, Chung KK, Saffle JR, Renz EM, Cancio LC; Prevention of Combat-Related Infections Guidelines Panel. Prevention of infections associated with combat-related burn injuries. J Trauma. 2011;71(2 Suppl 2):S282–S289. | ||
Cé R, Marchi JG, Bergamo VZ, et al. Chitosan-coated dapsone-loaded lipid-core nanocapsules: growth inhibition of clinical isolates, multidrug-resistant Staphylococcus aureus and Aspergillus ssp. Colloids Surf A Physicochem Eng Asp. 2016;511:153–161. | ||
Koburger T, Hübner N-O, Braun M, Siebert J, Kramer A. Standardized comparison of antiseptic efficacy of triclosan, PVP–iodine, octenidine dihydrochloride, polyhexanide and chlorhexidine digluconate. J Antimicrob Chemother. 2010;65(8):1712–1719. | ||
Hernández-Richter T, Schardey HM, Lohlein F, et al. Binding kinetics of triclosan (Irgasan®) to alloplastic vascular grafts: an in vitro study. Ann Vasc Surg. 2000;14(4):370–375. | ||
Chuanchuen R, Karkhoff-Schweizer RAR, Schweizer HP. High-level triclosan resistance in Pseudomonas aeruginosa is solely a result of efflux. Am J Infect Control. 2003;31(2):124–127. | ||
Kumar A, Schweizer HP. Bacterial resistance to antibiotics: active efflux and reduced uptake. Adv Drug Deliv Rev. 2005;57(10):1486–1513. | ||
Duke JA, Bogenschutz-Godwin MJ, DuCellier J, Duke P-AK. Handbook of Medicinal Herbs. 2nd ed. Boca Raton: CRC Press; 2002. | ||
Wang X, Du Y, Fan L, Liu H, Hu Y. Chitosan-metal complexes as antimicrobial agent: synthesis, characterization and structure-activity study. Polym Bull. 2005;55(1–2):105–113. | ||
Wang X, Du Y, Liu H. Preparation, characterization and antimicrobial activity of chitosan–Zn complex. Carbohydr Polym. 2004;56(1):21–26. | ||
No HK, Young Park N, Ho Lee S, Meyers SP. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int J Food Microbiol. 2002;74(1–2):65–72. | ||
Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev. 2006;19(2):403–434. | ||
Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harb Perspect Biol. 2010;2(5):a000414. |
Supplementary materials
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.