Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 15
Treatment Regimens and Care Models for Older Patients Living with HIV: Are We Doing Enough?
Authors Frey E , Johnston CD , Siegler EL
Received 10 March 2023
Accepted for publication 24 April 2023
Published 29 April 2023 Volume 2023:15 Pages 191—208
DOI https://doi.org/10.2147/HIV.S311613
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Olubunmi Akindele Ogunrin
Emily Frey,1 Carrie D Johnston,2 Eugenia L Siegler3
1Department of Medicine, Weill Cornell/New York Presbyterian Hospital, New York, NY, USA; 2Division of Infectious Diseases, Weill Cornell Medicine, New York, NY, USA; 3Division of Geriatrics and Palliative Medicine, Weill Cornell Medicine, New York, NY, USA
Correspondence: Emily Frey, Department of Medicine, Weill Cornell Medicine, 505 East 70th Street, New York, NY, 10021, USA, Tel +1 212 746 4749, Fax +1 212 746 4609, Email [email protected]
Abstract: With improved access to antiretroviral therapy throughout the world, people are aging with HIV, and a large portion of the global population of people with HIV (PWH) is now age 50 or older. Older PWH experience more comorbidities, aging-related syndromes, mental health challenges, and difficulties accessing fundamental needs than the population of older adults without HIV. As a result, ensuring that older PWH are receiving comprehensive healthcare can often be overwhelming for both PWH and the providers. Although there is a growing literature addressing the needs of this population, gaps remain in care delivery and research. In this paper, we suggest seven key components to any healthcare program designed to address the needs of older people with HIV: management of HIV, comorbidity screening and treatment, primary care coordination and planning, attention to aging related-syndromes, optimization of functional status, support of behavioral health, and improved access to basic needs and services. We review many of the difficulties and controversies related to the implementation of these components, which include the absence of screening guidelines for this population and the challenges of care integration, and we suggest key next steps.
Keywords: older people with HIV, care integration, antiretroviral therapy, multimorbidity, aging-related syndromes
Introduction
For people living with HIV (PWH), consistent access and adherence to effective antiretroviral therapy (ART) has transformed HIV from a fatal illness to a chronic disease. PWH on treatment now experience near normal lifespans.1,2 The UNAIDS global 90-90-90 targets, launched in 2014, aimed to ensure that by 2020 90% of people infected with HIV would be aware of their status, 90% of those diagnosed would be initiated on ART, and 90% of people on ART would achieve viral suppression.3 The program has achieved significant success in improving access to testing and to ART around the world, and by 2020, eight countries had met or exceeded these targets. In 2021, these targets were increased to 95-95-95 (already met by 2 countries) in anticipation of continued improvements in care and access.3,4
With effective ART, the demographics of the population of PWH have shifted, and a growing number of people are aging with HIV. Globally, an estimated 7.5 million people aged 50 and older live with HIV, making up approximately one-fifth of the total population of PWH.3 In the US, over half of PWH are at least age 50.5 Similarly, in the Netherlands, it is estimated that approximately 70% of PWH will be 50 years of age or older by 2030.6
The aging of the population with HIV is a worldwide phenomenon.7 Even though people 50 and over make up the greatest percentage of those living with HIV in high-income countries (HIC), the majority of older PWH (defined in this review as 50 years of age or older) reside in low- and middle-income countries (LMIC).8 Although in 2018 approximately 15% of PWH in sub-Saharan Africa were age 50 or older, this region accounts for the largest total number of older PWH in the world; models estimate that the percentage of PWH who are 50+ will grow to 27% by 2040.9–11
Although HIV-related mortality has decreased in individuals treated with ART, older PWH experience more comorbidities and geriatric syndromes than their age-matched HIV-negative peers despite pharmacologic suppression of HIV viremia.12,13 In addition, this population may experience more issues related to social isolation, stigma, mental health challenges, food insecurity and housing instability than the corresponding HIV-negative cohort.14–17
The significant impact of non-HIV related illness in this burgeoning population of older PWH has led some to call for the addition of a 4th 90 to the initial UNAIDS targets, a “90” that focuses on healthy aging for people living with HIV.14 For older PWH, healthcare must extend far beyond the management of HIV and HIV-related illness and must seek to integrate various components of care, moving away from the current piecemeal strategies to address needs as they arise.9,18 An ever-growing body of research has been initiated to better define the specific healthcare concerns, to identify best practices to prevent, diagnose, and treat various conditions, and to develop comprehensive care models to better address the needs of older PWH. However, much of this work has occurred at academic institutions in large cities in HIC and relies on ease of access to resources that are associated with such institutions.18 Although many of these projects have produced innovative solutions to better address the needs of older PWH, such programs may have limited accessibility and generalizability outside of these urban hubs, necessitating additional efforts to identify creative solutions for older PWH who receive care in small, nonacademic programs, in rural areas of HIC or in LMIC countries, where the majority of older PWH reside.
With this in mind, treatment regimens and care models developed for older PWH must be relevant and adaptable in various cultures and contexts. In the absence of a universal model of care for older PWH, we can begin by describing key components appropriate for any care model developed to support the healthy aging of older PWH (Figure 1) and discuss current practices, considerations and challenges that surround the implementation of these components, concluding with recommendations for next steps.
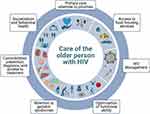 |
Figure 1 Components of care of the older person with HIV. Notes: Adapted from “7 panels in a circular layout”, by BioRender.com (2020). Retrieved from https://app.biorender.com/biorender-templates. Created with BioRender.com. |
Components of Care
HIV Management
A recent meta-analysis of older adults with HIV worldwide demonstrated a wide range of HIV prevalence rates, and in many regions, sub-optimal adherence to care in the setting of barriers to access, medical comorbidities and polypharmacy. This highlights the central importance of global equity to optimize HIV treatment in this population19 and the importance of careful consideration of optimal antiretroviral medication for older PWH.
Initial ART Regimens in Older PWH
ART is recommended for all people with HIV, regardless of T-cell count or prior treatment history. In fact, older adults may experience the greatest relative survival benefit due to a reduction in lower all-cause and non-AIDS mortality.20,21 As of early 2023, there are 34 approved medications to treat HIV, falling into 6 broad mechanistic classes: 1) nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors (PIs), integrase strand transfer inhibitors (INSTIs), entry inhibitors, and capsid inhibitors. Current first-line ART regimens from the International Antiviral Society-USA panel and the World Health Organization (WHO) include the use of an integrase inhibitor with 1–2 NRTIs, listed in Table 1.22,23 The choice of initial therapy for an older person with HIV should be tailored to their health status and, if any, medical comorbidities.22,23
 |
Table 1 Recommended Initial Regimens for HIV Treatment |
A new factor to be considered when initiating HIV treatment is prior use of pre-exposure prophylaxis (PrEP) medications prior to HIV acquisition, as this may increase the chance of viral resistance. Current options for PrEP include tenofovir-based oral tablet regimens or injectable cabotegravir; those who took tenofovir alafenamide (TAF) or tenofovir disoproxil fumarate (TDF) based regimens for PrEP may have viral resistance to TAF or TDF but can be initiated on other NRTI pending genotype testing. Those who were using cabotegravir should not initiate treatment on any integrase inhibitor.24
Taking Multimorbidity and Polypharmacy into Consideration
Older PWH are at higher risk for multiple medical comorbidities compared to younger adults; all comorbidities must be evaluated when selecting and continuing an ART regimen (Table 2). Many older adults with HIV have been living with HIV for many years and have experienced treatment with earlier generations of antiretrovirals, often with greater toxicity and lower barriers to resistance; consideration and review of prior HIV resistance mutations is key. Because of the higher prevalence of multimorbidity, older PWH are at risk of polypharmacy and drug interactions.25,26 Additionally, pill size may be a factor, as some patients have difficulty swallowing the relatively large co-formulated single-tablet regimens. Although multiple drug interaction-support web resources are available (including but not limited to LexiComp, Clinical Pharmacology, Micromedex, Liverpool Drug Interactions, and Toronto General Hospital Drug Interaction Tables), a study comparing the use of these resources for ART interactions yielded inconsistent results.27 Hence, active involvement by a pharmacist to thoroughly review complicated cases of ART-related drug interactions is preferred and has been shown to improve ART adherence and outcomes.28
 |
Table 2 Considerations When Prescribing Antiretrovirals for Older People with HIV |
Pursuing New, Simpler Treatment Regimens
Monitoring HIV treatment effectiveness in older adults should include special attention to the greater risk for adverse effects on renal, liver, cardiovascular, CNS, bone health, and metabolism. Some older PWH may wish to maintain a potentially outdated ART regimen, citing the rationale, “if it’s not broken, why fix it?” Revisiting medical decisions about an ART regimen with a shared decision-making approach can include a review of potential side-effect burden of older ART regimens and highlight the potential advantages to newer ART regimens in the setting of comorbidities.
Example: promoting bone health. Data suggest that ART regimens that contain TDF, boosted protease inhibitors, or both, are associated with greater loss of bone mineral density as compared to ART regimens with INSTIs and other NRTIs.29,30 Considering these factors, expert guidance recommends not only bone density monitoring in men aged 50 and older and postmenopausal cisgender women and for those at risk for fragility fractures but also switching from TDF and/or boosted PIs to other ART regimens.31
Example: avoiding drug interactions: In general, pharmacokinetic enhancing (“booster”) medications like cobicistat and ritonavir have the greatest potential for drug–drug interactions and may not be an optimal choice in the setting of polypharmacy. All drug–drug interactions should be assessed in maintenance and clinical decisions to switch ART regimens.
Example: Avoiding weight gain. Post-marketing surveillance can uncover unexpected side effects. A relatively newly-recognized side effect of certain components of ART is the finding of weight gain, especially central/visceral adiposity, with the use of INSTIs and/or TAF as compared to TDF.32 With increased rates of obesity worldwide,33 this is an important consideration for all people with HIV, and especially important for older adults who are at increased risk for frailty, immobility, and weight gain.34 In addition to ART, counseling regarding diet, exercise, and smoking cessation is paramount.
Inadequate Representation of Older People in ART Research
Early ART regimens, developed in the mid 1980s, were characterized by many side effects, complicated dosing schedules, and a high pill burden. With scientific advances, HIV treatment has evolved into a myriad of options ranging from one tablet per day co-formulated regimens to every two-month long-acting injectable regimens for many patients.35 Although HIV treatment may evolve dramatically with the uptake of such bi-monthly injections, clinical implementation is challenging, as health systems must adapt to support injection visits, and adherence to these follow-up visits is paramount. The large, international, Phase III clinical trial35 that demonstrated non-inferiority of long-acting cabotegravir-rilpivirine (compared to dolutegravir–abacavir–lamivudine) consisted of 809 participants, with an age range from 18 to 68 years, and a median age of 34 years old. Pharmacokinetic data in older PWH are limited, especially as new delivery methods such as nanoformulated long-acting injectable regimens and other delivery systems are developed. Future advances may include novel delivery methods such as microneedle patches.36 In addition, older PWH may have unique considerations with such injectable therapies; for example, lack of sufficient gluteal tissue in which to inject long-acting cabotegravir-rilpivirine may be an issue for some with sarcopenia and muscle tissue loss.
Previously, people with drug-resistant HIV often had few tolerable treatment options available, and for this population new therapies are desperately needed. Many older adults with HIV have been living with HIV for over three decades, and projection data indicate that a growing proportion of PWH age 60 and over will have been taking ART for 30 years or more.37 The newest HIV treatment medication to be approved for use is lenacapavir, a novel HIV capsid inhibitor with potent activity and a relatively long half-life administered as a sub-cutaneous injection and studied for use in individuals who are treatment-experienced with evidence of multi-drug resistant HIV. The median age of participants in the clinical trial informing the approval of lenacapavir was 52 years old (range 23–78), reflecting that highly treatment-experienced individuals tended to be over age 50.38
Older PWH have historically been underrepresented in clinical trials of therapeutic agents for HIV. Age-limit cutoffs and exclusion due to comorbid conditions that disproportionately affect older adults result in exclusion of this important population from clinical trials, limiting data regarding the use of crucial HIV treatment medications in older populations.
Management of Comorbidities
Like their HIV-negative peers, older PWH are at risk of the onset of non-communicable diseases (NCDs) as they age; however, HIV independently confers an increased risk of developing these conditions. Specifically, being HIV-positive is independently associated with an increased risk of diabetes, dyslipidemia, cardiovascular disease, osteoporosis, liver disease, chronic kidney disease and non-AIDS defining malignancies.9,18,39,40 In addition, PWH experience a greater degree of multimorbidity overall when compared to the HIV-negative population.9,18,39,40
Although these age-related non-communicable diseases have typically been described in resource-rich settings in HIC, with successful HIV treatment, PWH in LMIC are aging and have also been noted to experience these conditions. Most PWH live in LMIC, which now account for the majority of the morbidity and mortality stemming from these NCDs.41 As the population of older PWH grows in these countries, the impact of managing both HIV and of NCDs will challenge their healthcare systems.
Where ART is accessible with high uptake and adherence in both HIC and LMIC, HIV is suppressed; these non-HIV related conditions, rather than the HIV itself, are a major driver of morbidity and mortality in older PWH.9 Addressing NCDs in an HIV care setting offers opportunities to link people to care they otherwise could not pursue.42,43 It is therefore crucial that every site caring for older PWH has programs in place to prevent, diagnose, treat, and monitor these conditions.9
Few PWH-Specific Comorbidity Screening Guidelines
Although screening guidelines for non-HIV related comorbidities in PWH are available, they are often largely consistent with those guidelines established for the general HIV-negative population, raising some concerns that these algorithms may underestimate disease in PWH.44
For example, cardiovascular disease screening guidelines for PWH recommend lipid lowering intervention thresholds consistent with those for the general population. Even so, studies assessing screening tools that include additional criteria to reflect the independent risk of cardiovascular disease associated with HIV infection have demonstrated that standard guidelines underestimate the risk of disease, and therefore likely lead to disease undertreatment in PWH.44,45 Unfortunately, no consensus regarding the best revised method for risk assessment has been reached.46
Diabetes screening recommendations are generally consistent with those for the general population but do suggest that providers “pay special attention” to weight gain and change in weight distribution in older PWH, with consideration given to the contribution of ART regimen.44,47 Guidelines regarding screening for chronic kidney disease44 and heart failure45 have minimal differences in recommendations from those for the population without HIV, aside from having a “low threshold” for referral or testing.
Screening guidelines for cancers are varied. Generally, guidelines for cervical, anal, and liver cancers are modified to better reflect the increased risk of these malignancies associated with HIV. In contrast, screening for most other cancers follow the guidelines established for the general population.44
The difficulties in assessing, managing, and coordinating comorbidities led to the development in 2009 of an HIV specialist clinic for older PWH in England to focus on comorbidity care.47 While existing screening guidelines were utilized for some conditions (cancers, CKD, and metabolic disease), the clinic elected to modify guidelines for diseases whose risk they felt existing screening algorithms underestimated.47 Given the substantial evidence of increased risk for cardiovascular disease in older PWH, the clinic elected to add screening with CT Coronary Artery Calcium Score (CACS) to better identify atherosclerosis and risk stratify patients. Similarly, despite local guidelines recommending osteoporosis screening via the Fracture Risk Assessment (FRAX) tool (https://frax.shef.ac.uk/FRAX/index.aspx) alone, the clinic screened PWH using FRAX and dual-energy x-ray absorptiometry (DEXA) every three years.47 The majority of patients found to have osteoporosis on DEXA would not have qualified for treatment based on FRAX alone had the additional screening not been completed.47 More recent guidelines recommend DEXA screening,31 but adherence is by no means universal.48
The valid concern that standard guidelines are underestimating disease prevalence has led some providers to attempt to incorporate additional testing.44,45,47 While appropriate, this complicates an already complex area and leads to inconsistencies across institutions, localities, and countries and inhibits the development of algorithms that can be applied universally. Further efforts should be made to develop comprehensive screening guidelines that consider resource limitations, user skills, and the likelihood of uptake to adequately risk-stratify patients.
Difficulty Ensuring Appropriate Treatment of Multimorbidity
For the majority of older PWH in HIC, care of comorbidities is managed by their primary care provider (either an HIV specialist or general practitioner). If a condition requires more advanced or specialized care, the primary provider may refer patients to subspecialists, generally housed at different clinic sites. Subspecialists then assess patients with regard to the specific concern and may make amendments to a care plan, schedule additional testing, and modify medication regimens. This may be appropriate for individuals with a few, relatively straightforward issues. In the setting of increasing numbers of comorbidities, however, patients and providers are challenged to navigate issues related to a greater burden of polypharmacy and drug–drug interactions while on ART, increased reliance on subspeciality consultation, and complex coordination of care. For those individuals with the aging-related syndrome of multimorbidity (two or more chronic comorbidities), care delivery can be piecemeal and disconnected, increasing the possibility of complications and creating a heavy burden for patients.44,49
Older PWH are not only at risk of adverse outcomes from individual conditions but from multimorbidity itself. The syndrome of multimorbidity is associated with increased frailty and functional decline, as well as higher rates of hospitalization.50,51 Even if HIV-specific guidelines are developed for managing the most common comorbidities seen in older people with HIV, when implemented together the guidelines may conflict, overwhelming and potentially endangering the patient. As care becomes more complex, older PWH are at risk for adverse reactions from increasing medications, decreased adherence to extensive treatment plans, and poorer quality of life overall.52
The management of and complications from multimorbidity are discussed at length in the aging literature for the general population. One approach to multimorbidity that providers may use is the framework outlined by the American Geriatric Society. In this framework, providers assess five domains when evaluating patients with multiple comorbidities: patient preferences, evidence-based guidelines, patient prognosis, feasibility, and optimization of the treatment and care plan.52 This process can be time-intensive but may improve outcomes and be more congruent with patient goals.
Attention to Geriatric Syndromes and Optimization of Functional Status
In addition to NCDs and multimorbidity, older PWH have been shown to be at increased risk of other aging-related syndromes (ie, geriatric syndromes), including frailty, functional impairment, polypharmacy, falls, and cognitive impairment.49,53 As with NCDs, providers caring for older PWH face challenges related to screening, prevention, and management of aging-related syndromes in their patients.
Screening for Aging-Related Syndromes and Functional Limitations
Frailty is widely discussed in the aging literature. Generally, it describes individuals experiencing physical, cognitive, or social conditions that lead to reduced ability to respond to stress. This puts individuals at increased risk of adverse health outcomes including comorbidities, falls, cognitive decline, quality of life, hospitalization, and mortality.54,55 PWH are at higher risk of frailty than their HIV-negative peers.56 In addition, there is an association between pre-frailty and frailty and functional limitation in PWH.54
Multiple studies in both HIC and LMIC have shown that PWH are at increased risk of functional limitations, consequently relying on others for assistance with ADLs and IADLs.10 As with frailty, functional limitation puts patients at increased risk for falls, hospitalization, disability and mortality.10
While older PWH are at increased risk of frailty and functional limitations at younger ages and, thus, are at increased risk of morbidity and mortality, studies have demonstrated both modifiable risk factors and a degree of reversibility for these conditions.54,57 With this in mind, providers caring for older PWH should screen for these conditions and, where applicable, should endeavor to prevent and manage them.
In an effort to identify older PWH at risk of developing frailty and functional limitation, various bodies have recommended the incorporation of frailty and functional limitation screening guidelines into the care of older PWH.54 For example, the European AIDS Clinical Society (EACS) has recommended annual frailty screening for all older PWH beginning at age 50.58 However, while numerous screening tools for frailty and functional limitations exist, no consensus has been reached regarding the most appropriate tool or tools in this patient population, when to start, whom to target, or how to address frailty or pre-frailty. Older PWH may face significant barriers even to basic exercise activities.59 The value of screening for frailty is diminished if there are no opportunities beyond a brief course of physical therapy to address it.
Neurocognitive Impairment in Older PWH
Neurocognitive impairment has long been associated with HIV. Prior to widely available, effective ART, HIV-associated neurocognitive disorder (HAND) was often seen in young PWH without significant comorbidities and was often severe, secondary to CNS viral infection and associated inflammation.60 Since the widespread initiation of ART, the presentation of neurocognitive impairment has shifted, as have the underlying etiologies. Neurocognitive impairment is now often subtler at the time of presentation and is more common in older PWH with comorbidities. These individuals often have multiple conditions including HIV, cardiovascular disease, depression, substance use, and advanced age that may contribute to the presentation of cognitive impairment.60 As they age, older PWH may be caring for aging relatives, and caregiving, especially by those under stress, may also increase the already high risk of cognitive impairment in older PWH.61
Although it is estimated that up to 50% of older PWH experience some degree of impairment attributed to the spectrum of HAND, the aging of PWH increases the likelihood of neurodegenerative disorders like Alzheimer’s disease; distinguishing between HAND and Alzheimer-type cognitive impairment is an area of active investigation and has important implications for patient care.60,62,63
Cognitive impairment has devastating consequences; it has been associated with increased rates of pre-frailty, difficulty with ADLS and IADLS, incontinence and depression.62 Many older PWH live alone and may be socially isolated (discussed below), and screening for impairment is fundamental, as is referral for evaluation and support when impairment is identified. As there are few effective treatments for neurodegenerative disorders, linkage to systems of support is essential, and often the greatest challenge.
Involving a Geriatrician in the Care of Older PWH
Care for older PWH experiencing multiple comorbidities and aging-related syndromes is increasingly complex and thorough evaluation and care coordination can require significant time investment from providers.49 In this setting, providers trained in the principles of geriatrics may offer assistance to the primary providers.
Geriatric expertise can be incorporated in different ways both within and outside the primary care setting.64 In particular, geriatricians or others with clinical expertise in aging can lead a comprehensive geriatric assessment (CGA) for those patients identified as at risk of aging-related syndromes. CGA can offer a better sense of a patient’s functional status and goals, and in turn, better inform next step planning, prevention strategies, and management goals.49,65,66
Unfortunately, there are a number of challenges to incorporating geriatric care into the care of older PWH:
- Lack of access to providers trained in geriatric principles, especially outside of HIC academic institutions. The geriatric workforce is not adequate to meet the needs of the general population globally67–69 and lacks training in HIV care.70 As with many of the proposed interventions, opportunities for connecting patients with geriatricians often occur in HIC in large cities with academic institutions, limiting the generalizability of such interventions and calling attention to the importance of equity in designing programs.
- Lack of consensus about who should be referred to geriatricians and when. We do not know which screening tools to use, the age at which screening should begin, or the frequency with which screens should be conducted.65
- Patient reluctance to engage with geriatrics. Older PWH may feel negatively about being labeled as geriatric at younger ages than those typically associated with geriatric care.49
- Lack of primary care provider interest in referral when a geriatrician is available. When patients are evaluated, there may be an inconsistent response by the primary providers to recommendations from consulted geriatricians.71
Access to Food, Housing, and Services
Healthy aging requires attention to fundamental needs. Older PWH face numerous negative social determinants of health or the structural and contextual conditions in which patients live. These challenges limit their ability to maintain health and navigate a complex healthcare system. Structural barriers to health for older PWH include but are not limited to, housing insecurity, food insecurity, employment insecurity, absence of affordable transportation, and limited access to safe spaces for exercise and relaxation.72–74
Generally, PWH experience higher rates of food and housing insecurity than those without HIV. Food and housing insecurity are associated with poor health outcomes including decreased ART adherence, increased serious illness, poor quality of life and increased mortality.72–75 Food insecurity is found worldwide, but there are marked variations in prevalence. In the US, as many as 50% of PWH have been food insecure.76 The REPRIEVE study of cardiovascular health, for example, found that more than 60% had optimal diet in South Asia, whereas only 6% did in sub-Saharan Africa.77 The literature on interventions to address food insecurity is mixed.78 Regarding housing, as of 2016, it was estimated that greater then 10% of PWH of all ages had unmet housing needs.72 Improving housing status has been shown to improve health outcomes.79
These basic needs may be even more difficult to meet as aging individuals become less able to engage in the workforce, face increased social isolation, and experience issues associated with functional or cognitive limitations. Yet providing support to help facilitate stable access to these basic needs is fundamental to basic health and should not be neglected due to a focus on more medical concerns.
Socialization and Behavioral Health
PWH have high rates of mental health issues including depression, anxiety, substance use, and loneliness. As PWH age, these existing conditions can be further exacerbated by aging-specific issues including isolation, reduced social support, and reduced employment. These are compounded by stress experienced in the setting of stigmatization of older PWH in certain communities due to HIV status, sexual orientation, gender identity, race/ethnicity, and aging.14 The social and behavioral health needs of older PWH have been well described.80,81 This section will touch upon areas of specific importance in the general clinical care of older PWH.
Special Needs of the Older Newly Diagnosed PWH
In Europe, approximately 17% of new HIV diagnoses occur in people 50 years of age and older.82 Newly diagnosed older adults are more likely to be diagnosed at later stages of the disease and have higher mortality rates than people diagnosed at younger ages.82,83 In addition to medical and social concerns voiced by long-term survivors, these older PWH may require intensive counseling as they cope with a new HIV diagnosis, disrupted family relationships, and recovery from infectious complications; there is little research to guide their care.
Depression and Substance Use
Older PWH experience depression at higher rates than their HIV-negative peers. Depression in older PWH is associated with decreased ART adherence, increased HIV-related illness, increased non-HIV related comorbidities, and a significantly increased risk of mortality.84,85 In addition, older PWH have higher rates of substance use disorders with particularly high rates of tobacco, and alcohol use.17,86 These three conditions are frequently seen in combination and not only carry individual risk of adverse outcomes but have been shown to be associated with higher mortality risk when in combination.86
Providers caring for older PWH should incorporate regular screening for depression and substance use into their practice. Once identified, patients should be encouraged to engage with psychotherapy and pharmacotherapy as appropriate. Remission of one condition is associated with the increased likelihood of remission of others. In efforts to streamline treatments and reduce regimen complexity, providers should consider those pharmacologic treatments that may address more than one of the conditions.86
Impact of Stigma and Isolation
PWH experience stigma on multiple levels due to their HIV status and other identities with which they may associate, including race/ethnicity, sexual orientation, gender identity and socioeconomic status.15 Older PWH may also experience additional stigma associated with the process of aging, making them hesitant to share their HIV status with others for fear of rejection, violence and loss of livelihood.15 This may translate into a reluctance to seek necessary treatment, develop, or maintain important relationships.15
The impact of stigma on this population is far-reaching and affects both physical and mental health. Stigma is associated with depression and anxiety and can lead to behaviors of isolation. Older PWH are generally more likely to have reduced social networks, fewer friends and live alone and in the setting of experiencing multiple levels of stigmatization, this isolation may be exacerbated or further entrenched.7,15 Without good social support and in the setting of anxiety and/or depression, older PWH may be less adherent to treatment regimens and may experience worsening of their physical conditions.15 Trauma and PTSD from life events, the diagnosis of HIV itself, and the ongoing impact of stigma are related mental health conditions that must be recognized.87
Ideally, practices should invest in addressing stigma, trauma, and social isolation. Providers can directly ask older PWH about their attitudes toward aging and healthy relationships; they should enable open discussions about sexual health, including menopause.88–90 Clinical sites should help patients access resources to improve socialization and connection and assist with caregiving. Although older PWH face multiple stresses, they seek meaning as well. They wish to give back to others.91,92 Despite their own health needs, they may be providing caregiving to others and deriving purpose from it. Although the literature on caregiving by people with HIV is limited,93 a recent study or Ugandan women showed an association between caregiving and better quality of life.94
Additional education and efforts to shape policy should be pursued to assist in reducing the stigma associated with HIV, aging, and other identities with which individuals in this population may be associated.
Unfortunately, clinical sites without external funding usually cannot create internal programs providing physical and social benefits that address identified aging-related syndromes and functional limitations. The absence of funding for non-pharmacological approaches to improve socialization and mitigate aging-related syndromes in the setting of social vulnerability limits the impact of screening. In the US, for example, the law forbids programs from using Ryan White funds (a federal program for low-income people with HIV) for socialization, recreation, or interventions such as Tai Chi.95
Primary Care
Before effective ART, care for PWH was largely hospital-based and was focused on identifying tolerable treatment regimens and managing opportunistic infections and other HIV-associated conditions. Today, care for PWH is largely outpatient. The advent of safe, effective, and accessible ART has enabled sustained viral suppression, and older PWH have growing primary care needs beyond care for HIV, as previously described. These shifting needs are reflected in various guidelines developed for the primary care of PWH,96–98 which should serve as the basis for the primary care of PWH of any age. This review is intended not to replace but to supplement them.
The US Institute of Medicine defines primary care as, “the provision of integrated, accessible health care services by clinicians who are accountable for addressing a large majority of personal health care needs, developing a sustained partnership with patients, and practicing in the context of family and community”.99 The demands on primary care providers are generally challenging and time consuming.100 The additional needs of people aging with HIV add to the complexity.
Depending on local practices, individuals providing primary care for older PWH have traditionally been general practitioners or infectious disease specialists.18 These providers are now tasked with managing comorbidities (including communications with specialists), aging-related syndromes, counseling, and coordinating care for complicated social needs within traditional models of primary care,49 as discussed above. As the population with HIV ages, primary care also includes anticipation of future needs, in particular, advance care planning and long-term care.
Discussion of Advance Care Planning, Connection to Palliative Care, and End-of-Life Decision Making
Advance care planning (ACP) “enables individuals to define goals and preferences for future medical treatment and care, to discuss these goals and preferences with family and health-care providers, and to record and review these preferences if appropriate”.101 Ideally, ACP begins in adulthood and is revisited throughout the life course.102 In the absence of consensus about when or how these conversations should be initiated, for older PWH, ACP is critical regardless of state of health. In multiple studies regarding ACP in PWH, rates for associated documentation range from about 10–50%, similar to that of the HIV-negative population.103 A variety of barriers to ACP for PWH in the US such as low income, low education, identifying as Black or Hispanic, and history of IV drug use are associated with lower rates of ACP. Sangarlangkarn et al found that a large portion of the ACP was recorded in acute care settings,103 suggesting missed opportunities by primary care providers despite evidence that PWH are open to engaging in this topic.104 Providers caring for older PWH should ensure that ACP conversations are included as part of regular care for this population.
Though not isolated to end-of-life care, palliative care offers substantial potential benefits to older PWH. As older PWH age and experience the impact of multiple comorbidities, they may experience an array of non-specific symptoms including fatigue, insomnia, and pain. Although primary providers should attempt to treat the full range of symptoms experienced by older PWH, they may also benefit from connection to palliative care services to better address these non-specific symptoms.105 Older PWH who engage with palliative care services may see improved quality of life, even in the absence of terminal disease, pain and multimorbidity fall within the palliative care treatment continuum.105,106
Long-Term Care Needs
As PWH age, they will need long-term care. Stigma has led to an increased dependence on formal long-term care.107 In the US, the proportion of long-stay nursing home residents is increasing.108 PWH may require nursing home care at a younger age than those without HIV.109 Currently, management of HIV care in long-term facilities is suboptimal. One recent study documented that fewer than 80% of PWH in nursing homes were receiving ART; workforce education is essential to ensure care of older PWH will be medically optimized and culturally sensitive.107,110 We can anticipate that home care utilization will accelerate even more quickly than institutional care and that PWH may have different needs from their counterparts without HIV.111
Existing Care Models and Care Integration
Optimal care of individuals with HIV, high comorbidity burden, aging-related syndromes, and multiple negative social determinants of health requires sophisticated integration that promotes access to care and services, and respects individuals’ priorities.112,113 In HIC, traditional physician-centered clinic-based models of care for PWH may be challenged to provide the sort of integrated care that older PWH require. The COVID-19 pandemic has also necessitated creative responses to disruptions in care.114 In response to these changing needs, a number of innovative clinical models attempting some level of care integration have been developed or proposed:
Multimorbidity-Focused Clinic Within HIV Primary Care Setting
Providers identified a high degree of comorbidities and advanced disease in older PWH at the Chelsea and Westminster Hospital NHS Foundation Trust Clinic. This prompted the development of several co-specialty clinics that focus on cardiology, nephrology, metabolic disease, and menopause and facilitate the evaluation of older PWH with a particular comorbid condition in a single clinic visit, leveraging expertise from both an HIV provider and a specialist. These visits allow foster collaboration and serve as a sort of “one stop shop” for patients.47
Consultative Clinic External to Primary Care
The Silver Clinic in Brighton, UK, runs out of an existing HIV clinic and includes an HIV consultant, geriatric consultant, nursing staff, and pharmacist with expertise in infectious diseases/HIV. Older PWH with multimorbidity, polypharmacy, frailty, falls, or difficulties with ADLs are referred by HIV providers to the clinic where they undergo CGA by a team of providers.115 The multidisciplinary, individualized care plan is communicated to the referring primary provider.115
Metabolic Clinic
The Modena (Italy) HIV Metabolic Clinic was initially developed in 2003 to treat metabolic disease, providing access to comprehensive care targeting lipodystrophy syndrome.116 Patients consult with infectious disease physicians, cardiologists, endocrinologists, nephrologists, geriatricians, nutritionists, physical therapists, surgeons, and psychologists in order to address metabolic disease.116
Model That Combines Socialization and Medical Care
Golden Compass, in San Francisco, USA, was developed within Ward 86, a safety net HIV clinic, to provide comprehensive care older people living with HIV referred by their primary care provider. The center includes a multidisciplinary team comprising an HIV geriatrician, cardiologist, pharmacist, and social workers. The program also provides patients with classes on subjects relevant to aging with HIV, evaluated patients for frailty and functional limitations, offered opportunities to create social connections, and specialty referrals as needed.117 In follow-up surveys, patients and providers saw improvements across multiple domains, including quality of life, as a result of participation in Golden Compass.118
Although LMIC may lack the resources to create these kinds of multidisciplinary programs, care integration has become a cornerstone of treatment. A recent review identified 219 models (for people with and without HIV) integrating management of NCDs and/or neuropsychiatric problems into primary care.119 Some LMIC countries have initiated formal plans for care integration of HIV and NCDs, but as of yet there are few data about the cost-effectiveness of these programs.120
Differentiated Service Delivery (DSD)
DSD “is an adaptive approach that aims to efficiently use limited resources by tailoring health services to local context, and patients’ clinical status and preferences”.121 It was originally developed to scale up HIV testing and ART distribution and monitoring when ART demand increased after the change in guidelines promoting treatment of all PWH. While comprehensive clinical programs are feasible within hospital systems in well-resourced locales, the vast majority of older PWH live in areas that with fewer resources.8–11 The rapid growth of multimorbidity in LMIC irrespective of HIV status is outpacing countries’ abilities to provide care.9 However, LMIC may be able to leverage DSD platforms to treat comorbidities, not only to support the population of PWH but expanded to include treatment for the general population.8,9,122
Alternatively, Hussain et al propose that it may be reasonable to work to establish metabolic clinics, like that described above, in Africa to address the significant impact of metabolic disease.123 Although it may not be feasible to treat all older PWH with metabolic disease in these clinics, as in the other HIV care models, it may be possible to focus referrals for those individuals who are found to have the greatest need during community-based screening and treatment.122,123
Discussion: Are We Doing Enough?
There is nothing aging-specific about the fundamental importance of care integration and patient-centeredness in the care of older PWH.124 The challenge of creating optimal programs for older PWH is the extraordinary complexity of care, which requires a much more multifaceted process of ensuring access and quality, while at the same time anticipating future medical and long-term care needs as people age.
A second challenge is recognizing that location, resources, and culture may all influence what older PWH prioritize and what they feel they need from health care. A recent study surveying 680 participants from 4 different regions around the globe demonstrated very different priorities, quality of life assessments, and psychosocial needs between these locations.125
Are we doing enough? Of course not. And we must be cognizant of the growing frustration of older people with HIV who feel their voices are not being heard. The Glasgow Manifesto is one recent example of an angry plea for attention, respect, and investment.126
But there has been a sea change in how care for older PWH is being prioritized. A recent review in the Lancet described the challenges faced internationally from a health systems perspective when preparing to care for older PWH irrespective of funding source—PEPFAR, single payer, or US medical system—and is an important call to action.18
Many other programs have been initiated; government incentives and demonstration projects offer opportunities to create novel models or care and improve recognition and screening.127 In another sign of commitment to improving care of older PWH, the US Health Services Resource Administration (HRSA, which funds the Ryan White Programs) has recently funded 10 demonstration projects through its Special Project of National Significance for Emerging Strategies (SPNS) Program designed to Improve Health Outcomes for People Aging with HIV.128
Unfortunately, large development grants are available to only a small number of programs in a limited number of locations; moreover, they are usually short term. The fundamental long-term target is a universal standard of care applied irrespective of country, region, or program size, with the following goals:
- Incorporation of the concept of optimizing functional ability into the primary care model. Any program can begin with a simple screen. The World Health Organization's ICOPE guidance is an excellent example of this approach.129
- Active involvement of older PWH in decisions about priorities and structure of clinical programs. Ultimately, all care is local, and older PWH must be given a voice in the program that serves them.
- Development of screening tools that accurately reflect the risk of comorbidity in older PWH and of referral protocols that are feasible for programs of different sizes and locations. Creation of guidelines that assist in the management of multimorbidity in older PWH will be key to safe and effective care.
- Integration of care and other services. Studies have highlighted the importance of provider respect and “one-stop” care.130
- Creation of a clearinghouse for models of care at every level. Programs for older PWH are being implemented throughout the world, but the medical literature rarely offers opportunities to describe innovative programs.50 Clinical sites need a forum to share their experiences, develop best practices, and learn from one another, and researchers must know about these programs in order to study their effectiveness.
- Recognition that access to good nutrition, exercise, socialization, and safe housing promotes healthy aging and is essential to every program; at the local level, true integration to promote optimal health should be the goal, taking the care into the community and adapting to the clients’ needs. Health care systems and their payment mechanisms are not yet flexible enough to underwrite these programs. Funders, whether from government or foundations, should support models that include these services and the means of accessing them. The Covid pandemic and its disruption of healthcare services have served as an impetus for clinical programs in HIC to look to DSD as a means of delivering tailored care, as well.114 A recent HIV Medicine White Paper also calls for innovative care models in the US based on DSD and “Street Medicine”.131
- Unsiloing of aging-related social services, HIV-related social services, and medical care. Adaptation of geriatric social programs for older people with HIV can serve as an intermediate step. In the US, these programs can be comprehensive, like existing PACE (Program of all-inclusive Care of the Elderly) programs132 or smaller, local innovative demonstration projects such as the adaptation of the Villages model by the University of California at San Diego.133
- Inclusion of older PWH in antiretroviral drug trials. Older PWH have historically been underrepresented in clinical trials of therapeutic agents for HIV. Age limit cutoffs and exclusion due to comorbid conditions that disproportionately affect older adults result in inadequate representation of this important population from clinical trials. Drug development at all stages, and especially clinical trial design, must consider adequate inclusion of older adults a priori to increase equity and generalizability to the global population of people with HIV. Research should consider addressing age-specific efficacy, adherence, pharmacokinetics/pharmacodynamics, and side effects that may be unique to older adults.
- Preparation for long-term care needs to maintain older PWH in the community and to create safe and caring environments in long-term care facilities.134
Conclusion
The global population of older PWH is rapidly growing. Supporting healthy aging for this population in the setting of stretched healthcare budgets presents a unique set of challenges for healthcare providers, public health experts, governments, and PWH themselves. Older PWH deserve healthcare that is comprehensive—incorporating aspects of prevention, diagnosis and management for HIV, non-communicable diseases, and aging-related syndromes while facilitating access to services that support a variety of social needs.
Providing integrated and optimal care across these components can be difficult in traditional models of healthcare. In response, many groups are starting to identify new and innovative solutions to improve delivery of care in both HIC and LMIC. While a universal model of care is unlikely to address the vast diversity across individual populations and healthcare systems, we can learn from demonstration programs, ongoing research, and models used in other fields like geriatrics to develop best practices that can be adapted to the varied contexts in which older PWH live and obtain healthcare.
Author Contributions
All 3 authors have made a significant contribution to the conception and design of the review. Have written and substantially revised the article. Have agreed on the journal to which the article will be submitted. Reviewed and agreed on all versions of the article before submission. Agree to take responsibility and be accountable for the contents of the article.
Disclosure
Dr Siegler received support from the Wayen Foundation for the preparation of this manuscript and reported grants from Gilead Sciences, Inc., outside the submitted work. Dr Johnston is supported by NIH K23AG072960 and reports personal fees from TheraTechnologies, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Edwards JK, Cole SR, Breger TL, et al. Mortality among people entering HIV care compared to the general US population: an observational study. Ann Intern Med. 2021;174(9):1197–1206. doi:10.7326/M21-0065
2. Edwards JK, Cole SR, Breger TL, et al. Five-year mortality for adults entering human immunodeficiency virus care under universal early treatment compared with the general US population. Clin Infect Dis. 2022;75(5):867–874. doi:10.1093/cid/ciab1030
3. Frescura L, Godfrey-Faussett P, Feizzadeh AA, El-Sadr W, Syarif O, Ghys PD; on and behalf of the 2025 testing treatment target Working Group. Achieving the 95 95 95 targets for all: a pathway to ending AIDS. PLoS One. 2022;17(8):e0272405. doi:10.1371/journal.pone.0272405
4. Farley SM, Wang C, Bray RM, et al. Progress towards the UNAIDS 90-90-90 targets among persons aged 50 and older living with HIV in 13 African countries. J Int AIDS Soc. 2022;25(S4). doi:10.1002/jia2.26005
5. Center for Disease Control. HIV surveillance reports; 2022. Available from: https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html.
6. Smit M, Brinkman K, Geerlings S, et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis. 2015;15(7):810–818. doi:10.1016/S1473-3099(15)00056-0
7. Autenrieth CS, Beck EJ, Stelzle D, Mallouris C, Mahy M, Ghys P. Global and regional trends of people living with HIV aged 50 and over: estimates and projections for 2000–2020. PLoS One. 2018;13(11):e0207005. doi:10.1371/journal.pone.0207005
8. Godfrey C, Vallabhaneni S, Shah MP, Grimsrud A. Providing differentiated service delivery to the ageing population of people living with HIV. J Int AIDS Soc. 2022;25(Suppl4):e26002. doi:10.1002/jia2.26002
9. Harris TG, Rabkin M, El-Sadr WM. Achieving the fourth 90: healthy aging for people living with HIV. AIDS Lond Engl. 2018;32(12):1563–1569. doi:10.1097/QAD.0000000000001870
10. Bernard C, Font H, Diallo Z, et al. Prevalence and factors associated with physical function limitation in older West African people living with HIV. PLoS One. 2020;15(10):e0240906. doi:10.1371/journal.pone.0240906
11. Hontelez JA, de Vlas SJ, Baltussen R, et al. The impact of antiretroviral treatment on the age composition of the HIV epidemic in sub-Saharan Africa. AIDS Lond Engl. 2012;26(01):S19–S30. doi:10.1097/QAD.0b013e3283558526
12. Verheij E, Boyd A, Wit FW, et al. Long-term evolution of comorbidities and their disease burden in individuals with and without HIV as they age: analysis of the prospective AGEhIV cohort study. Lancet HIV. 2023;10:S2352-3018(22)00400–3. doi:10.1016/S2352-3018(22)00400-3
13. Greene M, Covinsky KE, Valcour V, et al. Geriatric syndromes in older HIV-infected adults. J Acquir Immune Defic Syndr. 2015;69(2):161–167. doi:10.1097/QAI.0000000000000556
14. Weinstein ER, Lee JS, Mendez NA, Harkness A, Safren SA, El-Sadr W. HIV/AIDS and aging: the new frontier for HIV/AIDS research and care. AIDS Lond Engl. 2021;35(12):2043–2045. doi:10.1097/QAD.0000000000003000
15. Brennan-Ing M. Diversity, stigma, and social integration among older adults with HIV. Eur Geriatr Med. 2019;10(2):239–246. doi:10.1007/s41999-018-0142-3
16. Emlet CA. “You’re awfully old to have this disease”: experiences of stigma and ageism in adults 50 years and older living with HIV/AIDS. Gerontologist. 2006;46(6):781–790. doi:10.1093/geront/46.6.781
17. Havlik RJ, Brennan M, Karpiak SE. Comorbidities and depression in older adults with HIV. Sex Health. 2011;8(4):551–559. doi:10.1071/SH11017
18. Kiplagat J, Tran DN, Barber T, et al. How health systems can adapt to a population ageing with HIV and comorbid disease. Lancet HIV. 2022;9(4):e281–e292. doi:10.1016/S2352-3018(22)00009-1
19. Bhatta M, Nandi S, Dutta N, Dutta S, Saha MK. HIV care among elderly population: systematic review and meta-analysis. AIDS Res Hum Retroviruses. 2020;36(6):475–489. doi:10.1089/AID.2019.0098
20. Edwards JK, Cole SR, Westreich D, et al. Age at entry into care, timing of antiretroviral therapy initiation, and 10-year mortality among HIV-seropositive adults in the United States. Clin Infect Dis. 2015;61(7):1189–1195. doi:10.1093/cid/civ463
21. Lodi S, Costagliola D, Sabin C, et al. Effect of immediate initiation of antiretroviral treatment in HIV-positive individuals aged 50 years or older. J Acquir Immune Defic Syndr. 2017;76(3):311–318. doi:10.1097/QAI.0000000000001498
22. Gandhi RT, Bedimo R, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2022 Recommendations of the International Antiviral Society–USA Panel. JAMA. 2023;329(1):63–84. doi:10.1001/jama.2022.22246
23. World Health Organization. Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach. World Health Organization; 2021.
24. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Department of Health and Human Services. Available from: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv.
25. Freedman SF, Johnston C, Faragon JJ, Siegler EL, Del Carmen T. Older HIV-infected adults. Complex patients (III): polypharmacy. Eur Geriatr Med. 2019;10(2):199–211. doi:10.1007/s41999-018-0139-y
26. Back D, Marzolini C. The challenge of HIV treatment in an era of polypharmacy. J Int AIDS Soc. 2020;23(2):e25449. doi:10.1002/jia2.25449
27. Drwiega EN, Badowski ME, Michienzi S. Antiretroviral drug-drug interactions: a comparison of online drug interaction databases. J Clin Pharm Ther. 2022;47(10):1720–1724. doi:10.1111/jcpt.13750
28. Hazen RJ, Halbur D, Mills B, Kirkham HS, Hou J; Patient-Centered HIV Care Model Team. Evaluation of medication therapy Issues, resolutions, and adherence among persons with HIV in the pharmacist-led patient-centered HIV care model. J Acquir Immune Defic Syndr. 2021;88(1):96–102. doi:10.1097/QAI.0000000000002732
29. Stellbrink HJ, Orkin C, Arribas JR, et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis. 2010;51(8):963–972. doi:10.1086/656417
30. Duvivier C, Kolta S, Assoumou L, et al. Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. AIDS Lond Engl. 2009;23(7):817–824. doi:10.1097/QAD.0b013e328328f789
31. Brown TT, Hoy J, Borderi M, et al. Recommendations for evaluation and management of bone disease in HIV. Clin Infect Dis. 2015;60(8):1242–1251. doi:10.1093/cid/civ010
32. Mallon PW, Brunet L, Hsu RK, et al. Weight gain before and after switch from TDF to TAF in a U.S. cohort study. J Int AIDS Soc. 2021;24(4):e25702. doi:10.1002/jia2.25702
33. World Health Organiziaton. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. Tech Rep Ser. 2000;894:i–253.
34. Afshin A, Forouzanfar MH; GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27. doi:10.1056/NEJMoa1614362
35. Orkin C, Arasteh K, Górgolas Hernández-Mora M, et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med. 2020;382(12):1124–1135. doi:10.1056/NEJMoa1909512
36. Rajabi M, Roxhed N, Shafagh RZ, et al. Flexible and stretchable microneedle patches with integrated rigid stainless steel microneedles for transdermal biointerfacing. PLoS One. 2016;11(12):e0166330. doi:10.1371/journal.pone.0166330
37. Marty L, Diawara Y, Rachas A, Grabar S, Costagliola D, Supervie V. Projection of age of individuals living with HIV and time since ART initiation in 2030: estimates for France. J Int AIDS Soc. 2022;25(Suppl4):e25986. doi:10.1002/jia2.25986
38. Segal-Maurer S, DeJesus E, Stellbrink HJ, et al. Capsid inhibition with lenacapavir in multidrug-resistant HIV-1 infection. N Engl J Med. 2022;386(19):1793–1803. doi:10.1056/NEJMoa2115542
39. Kong AM, Pozen A, Anastos K, Kelvin EA, Nash D. Non-HIV comorbid conditions and polypharmacy among people living with HIV age 65 or older compared with HIV-negative individuals age 65 or older in the United States: a retrospective claims-based analysis. AIDS Patient Care STDs. 2019;33(3):93–103. doi:10.1089/apc.2018.0190
40. Schouten J, Wit FW, Stolte IG, et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis. 2014;59(12):1787–1797. doi:10.1093/cid/ciu701
41. Achwoka D, Mutave R, Oyugi JO, Achia T. Tackling an emerging epidemic: the burden of non-communicable diseases among people living with HIV/AIDS in sub-Saharan Africa. Pan Afr Med J. 2020;36:271. doi:10.11604/pamj.2020.36.271.22810
42. Kintu A, Sando D, Okello S, et al. Integrating care for non-communicable diseases into routine HIV services: key considerations for policy design in sub-Saharan Africa. J Int AIDS Soc. 2020;23(Suppl1):e25508. doi:10.1002/jia2.25508
43. Manne-Goehler J, Montana L, Gómez-Olivé FX, et al. The ART advantage: health care utilization for diabetes and hypertension in rural South Africa. J Acquir Immune Defic Syndr. 2017;75(5):561–567. doi:10.1097/QAI.0000000000001445
44. American Academy of HIV Medicine. Recommended treatment strategies for clinicians managing older patients with HIV. Available from: https://aahivm.org/hiv-and-aging/.
45. Feinstein MJ, Hsue PY, Benjamin LA, et al. Characteristics, prevention, and management of cardiovascular disease in people living with HIV: a scientific statement from the American Heart Association. Circulation. 2019;140(2):e98–e124. doi:10.1161/CIR.0000000000000695
46. Douglas PS, Umbleja T, Bloomfield GS, et al. Cardiovascular risk and health among people with Human Immunodeficiency Virus (HIV) eligible for primary prevention: insights from the REPRIEVE Trial. Clin Infect Dis. 2021;73(11):2009–2022. doi:10.1093/cid/ciab552
47. Pereira B, Mazzitelli M, Milinkovic A, et al. Evaluation of a clinic dedicated to people aging with HIV at Chelsea and Westminster Hospital: results of a 10-year experience. AIDS Res Hum Retroviruses. 2022;38(3):188–197. doi:10.1089/AID.2021.0083
48. Turin CG, Khanjee N, Breaux K, Armamento-Villareal R, Rodriguez-Barradas MC, Clark EH. Evaluation of adherence to guideline-based bone mineral density screening in veterans with HIV. AIDS Res Hum Retroviruses. 2022;38(3):216–221. doi:10.1089/AID.2021.0095
49. Singh HK, Del Carmen T, Freeman R, Glesby MJ, Siegler EL. From one syndrome to many: incorporating geriatric consultation into HIV care. Clin Infect Dis. 2017;65(3):501–506. doi:10.1093/cid/cix311
50. Siegler EL, Burchett CO, Glesby MJ. Older people with HIV are an essential part of the continuum of HIV care. J Int AIDS Soc. 2018;21(10):e25188. doi:10.1002/jia2.25188
51. Lorenz DR, Mukerji SS, Misra V, et al. Multimorbidity networks associated with frailty among middle aged and older people with HIV. AIDS Lond Engl. 2021;10. doi:10.1097/QAD.0000000000003040
52. Balavoine JF. Guiding principles for the care of older adults with multimorbidity: an approach for clinicians: American Geriatrics Society Expert Panel on the care of older adults with multimorbidity. J Am Geriatr Soc. 2012;60(10):E1–E25. doi:10.1111/j.1532-5415.2012.04188.x
53. Guaraldi G, Rockwood K. Geriatric-HIV medicine Is born. Clin Infect Dis. 2017;65(3):507–509. doi:10.1093/cid/cix316
54. Kehler DS, Milic J, Guaraldi G, Fulop T, Falutz J. Frailty in older people living with HIV: current status and clinical management. BMC Geriatr. 2022;22:919. doi:10.1186/s12877-022-03477-7
55. Brañas F, Torralba M, Antela A, et al. Effects of frailty, geriatric syndromes, and comorbidity on mortality and quality of life in older adults with HIV. BMC Geriatr. 2023;23(1):4. doi:10.1186/s12877-022-03719-8
56. Kooij KW, Wit FW, Schouten J, et al. HIV infection is independently associated with frailty in middle-aged HIV type 1-infected individuals compared with similar but uninfected controls. AIDS Lond Engl. 2016;30(2):241–250. doi:10.1097/QAD.0000000000000910
57. Piggott DA, Bandeen-Roche K, Mehta SH, et al. Frailty transitions, inflammation, and mortality among persons aging with HIV infection and injection drug use. AIDS Lond Engl. 2020;34(8):1217–1225. doi:10.1097/QAD.0000000000002527
58. Frailty. EACS guidelines version 11; 2022. Available from: https://eacs.sanfordguide.com/prevention-non-infectious-co-morbidities/frailty.
59. Chetty L, Cobbing S, Chetty V. The perceptions of older people living with HIV/AIDS towards physical activity and exercise. AIDS Res Ther. 2022;19(1):67. doi:10.1186/s12981-022-00500-0
60. Underwood J, Winston A. Guidelines for evaluation and management of cognitive disorders in HIV-positive individuals. Curr HIV/AIDS Rep. 2016;13(5):235–240. doi:10.1007/s11904-016-0324-x
61. Vance DE, Lee Y, Batey DS, et al. Risk factors of cognitive decline in older caregivers with HIV: an emerging hypothesis. J Assoc Nurses AIDS Care. 2022;33:676–681. doi:10.1097/JNC.0000000000000349
62. Hosaka KRJ, Greene M, Premeaux TA, et al. Geriatric syndromes in older adults living with HIV and cognitive impairment. J Am Geriatr Soc. 2019;67(9):1913–1916. doi:10.1111/jgs.16034
63. Rubin LH, Sundermann EE, Moore DJ. The current understanding of overlap between characteristics of HIV-associated neurocognitive disorders and Alzheimer’s disease. J Neurovirol. 2019;25(5):661–672. doi:10.1007/s13365-018-0702-9
64. Davis AJ, Greene M, Siegler E, et al. Strengths and challenges of various models of geriatric consultation for older adults living with HIV. Clin Infect Dis. 2021;ciab682. doi:10.1093/cid/ciab682
65. Brañas F, Ryan P, Troya J, Sánchez-Conde M. Geriatric-HIV medicine: the geriatrician’s role. Eur Geriatr Med. 2019;10(2):259–265. doi:10.1007/s41999-018-0144-1
66. Siegler EL, Moxley JH, Glesby MJ. Aging-related concerns of people living with HIV referred for geriatric consultation. HIVAIDS Auckl NZ. 2021;13:467–474. doi:10.2147/HIV.S306532
67. U.S. Department of Health and Human Services, Health Resources and Services Administration, National Center for Health Workforce Analysis. National and Regional Projections of Supply and Demand for Geriatricians: 2013–2025. Rockville, Maryland: U.S. Department of Health and Human Services, Health Resources and Services Administration, National Center for Health Workforce Analysis; 2017.
68. Dotchin CL, Akinyemi RO, Gray WK, Walker RW. Geriatric medicine: services and training in Africa. Age Ageing. 2013;42(1):124–128. doi:10.1093/ageing/afs119
69. Pati S, Sharma A, Pati S, Zodpey S. Teaching of geriatric health in India: mapping the terrain. Gerontol Geriatr Educ. 2017;38(1):92–103. doi:10.1080/02701960.2016.1232590
70. Jones HT, Barber TJ. How do geriatricians feel about managing older people living with HIV? A scoping review. Eur Geriatr Med. 2022;13(4):987–997. doi:10.1007/s41999-022-00642-4
71. Bitas C, Jones S, Singh HK, Ramirez M, Siegler E, Glesby M. Adherence to recommendations from comprehensive geriatric assessment of older individuals with HIV. J Int Assoc Provid AIDS Care. 2019;18:2325958218821656. doi:10.1177/2325958218821656
72. Aidala AA, Wilson MG, Shubert V, et al. Housing status, medical care, and health outcomes among people living with HIV/AIDS: a systematic review. Am J Public Health. 2016;106(1):e1–e23. doi:10.2105/AJPH.2015.302905
73. Hessol NA, Zepf R, Zobell E, Weiser SD, John MD. Food insecurity and aging outcomes in older adults living with HIV. AIDS Behav. 2017;21(12):3506–3514. doi:10.1007/s10461-017-1838-y
74. Mitchell BD, Utterback L, Hibbeler P, et al. Patient-identified markers of quality care: improving HIV service delivery for older African Americans. J Racial Ethn Health Disparities. 2023;10(1):475–486. doi:10.1007/s40615-022-01237-2
75. Sok P, Gardner S, Bekele T, et al. Unmet basic needs negatively affect health-related quality of life in people aging with HIV: results from the Positive Spaces, Healthy Places study. BMC Public Health. 2018;18(1):644. doi:10.1186/s12889-018-5391-z
76. McKay FH, Lippi K, Dunn M. Investigating responses to food insecurity among HIV positive people in resource rich settings: a systematic review. J Community Health. 2017;42(5):1062–1068. doi:10.1007/s10900-017-0351-6
77. Fitch KV, McCallum SA, Erlandson KM, et al. Diet in a global cohort of adults with HIV at low-to-moderate traditional cardiovascular disease risk. AIDS Lond Engl. 2022;36(14):1997–2003. doi:10.1097/QAD.0000000000003344
78. De Marchis EH, Torres JM, Benesch T, et al. Interventions addressing food insecurity in health care settings: a systematic review. Ann Fam Med. 2019;17(5):436–447. doi:10.1370/afm.2412
79. Rajabiun S, Tryon J, Feaster M, et al. The influence of housing status on the HIV continuum of care: results from a multisite study of patient navigation models to build a medical home for people living with HIV experiencing homelessness. Am J Public Health. 2018;108(S7):S539–S545. doi:10.2105/AJPH.2018.304736
80. Ruiz EL, Greene KY, Galea JT, Brown B. From surviving to thriving: the current status of the behavioral, social, and psychological issues of aging with HIV. Curr Opin HIV AIDS. 2022;17(2):55–64. doi:10.1097/COH.0000000000000725
81. Emlet CA, Brennan-Ing M. Is there no place for us? The psychosocial challenges and rewards of aging with HIV. J Elder Policy. 2020;1(1). doi:10.18278/jep.1.1.4
82. Tavoschi L, Gomes Dias J, Pharris A; EU/EEA HIV Surveillance Network. New HIV diagnoses among adults aged 50 years or older in 31 European countries, 2004–15: an analysis of surveillance data. Lancet HIV. 2017;4(11):e514–e521. doi:10.1016/S2352-3018(17)30155-8
83. Yee WH, Li WP, Bador MK, et al. Poor HIV-related outcomes in older adults newly diagnosed with HIV: a four-year retrospective analysis from a single site in Asia. J Acquir Immune Defic Syndr. 2023. doi:10.1097/QAI.0000000000003169
84. Yu X, Giordano TP, Baillargeon J, et al. Assessing incident depression among older people with and without HIV in U.S. Soc Psychiatry Psychiatr Epidemiol. 2023;58(2):299–308. doi:10.1007/s00127-022-02375-y
85. So‐Armah K, Gupta S, Kundu S, et al. Depression and all‐cause mortality risk in HIV‐infected and HIV‐uninfected US veterans: a cohort study. HIV Med. 2019;20(5):317–329. doi:10.1111/hiv.12726
86. Chichetto NE, Kundu S, Freiberg MS, et al. Association of syndemic unhealthy alcohol use, cigarette use, and depression with all-cause mortality among adults living with and without HIV infection: Veterans Aging Cohort Study. Open Forum Infect Dis. 2019;6(6):ofz188. doi:10.1093/ofid/ofz188
87. Verhey R, Chibanda D, Vera A, Manda E, Brakarsh J, Seedat S. Perceptions of HIV-related trauma in people living with HIV in Zimbabwe’s Friendship Bench Program: a qualitative analysis of counselors’ and clients’ experiences. Transcult Psychiatry. 2020;57(1):161–172. doi:10.1177/1363461519850337
88. Savoy M, O’Gurek D, Brown-James A. Sexual health history: techniques and tips. Am Fam Physician. 2020;101(5):286–293.
89. Granville L, Pregler J. Women’s sexual health and aging. J Am Geriatr Soc. 2018;66(3):595–601. doi:10.1111/jgs.15198
90. Narasimhan M, Payne C, Caldas S, Beard JR, Kennedy CE. Ageing and healthy sexuality among women living with HIV. Reprod Health Matters. 2016;24(48):43–51. doi:10.1016/j.rhm.2016.11.001
91. Emlet CA, Harris L. Giving back is receiving: the role of generativity in successful aging among HIV-positive older adults. J Aging Health. 2020;32(1):61–70. doi:10.1177/0898264318804320
92. Burchett CO, Shen MJ, Freeman R, et al. Using focus group feedback to identify patient-centered initiatives for older persons with HIV. Clin Gerontol. 2022;45(3):661–672. doi:10.1080/07317115.2020.1769245
93. Lee Y, Batey DS, Clay OJ, Emlet CA, Fazeli PL, Vance DE. Older caregivers with HIV: an unrecognized gap in the literature. J Assoc Nurses AIDS Care. 2021;32(1):29–36. doi:10.1097/JNC.0000000000000180
94. Mugisha J, Scholten F, Owilla S, et al. Caregiving responsibilities and burden among older people by HIV status and other determinants in Uganda. AIDS Care. 2013;25(11):1341–1348. doi:10.1080/09540121.2013.765936
95. Ryan White Guidance for Part B Contractors. HRSA Ryan White HIV/AIDS program; 2012. Available from: https://www.health.ny.gov/diseases/aids/providers/regulations/ryan_white/part_b/docs/guidance_for_contractors.pdf.
96. Thompson MA, Horberg MA, Agwu AL, et al. Primary care guidance for persons with Human Immunodeficiency Virus: 2020 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2021;73(11):e3572–e3605. doi:10.1093/cid/ciaa1391
97. Dyer M, Kerr C, McGowan JP, et al. Comprehensive Primary Care for Adults with HIV. Baltimore (MD): Johns Hopkins University; 2021.
98. EACS guidelines version 11. 2022. Available from: https://eacs.sanfordguide.com.
99. Donaldson MS, Yordy KD, Lohr KN, Vanselow NA. Institute of Medicine (US) Committee on the future of primary care. Primary care: America’s Health in a new era. Washington DC: National Academies Press (US); 1996. Available from: http://www.ncbi.nlm.nih.gov/books/NBK232643/.
100. Porter J, Boyd C, Skandari MR, Laiteerapong N. Revisiting the time needed to provide adult primary care. J Gen Intern Med. 2023;38(1):147–155. doi:10.1007/s11606-022-07707-x
101. Rietjens JAC, Sudore RL, Connolly M, et al. Definition and recommendations for advance care planning: an international consensus supported by the European Association for Palliative Care. Lancet Oncol. 2017;18(9):e543–e551. doi:10.1016/S1470-2045(17)30582-X
102. Brull J. Advance Care Planning: how to have the conversation you want with your patients. Fam Pract Manag. 2019;26(6):18–22.
103. Sangarlangkarn A, Merlin JS, Tucker RO, Kelley AS. Advance care planning and HIV infection in the era of antiretroviral therapy: a review. Top Antivir Med. 2016;23(5):174–180.
104. Nguyen AL, Park BY, Thayer E, Bailey J, Christensen C, Taylor J. Comparing advance care planning between older adults with and without HIV. AIDS Care. 2022;1–3. doi:10.1080/09540121.2022.2126961
105. Rangaraj A, Connor S, Harding R, Pinto C, Chitembo L, Ford N. Advanced HIV disease and health-related suffering-exploring the unmet need of palliative care. Lancet HIV. 2023;10(2):e126–e133. doi:10.1016/S2352-3018(22)00295-8
106. Goodkin K, Kompella S, Kendell SF. End-of-Life care and bereavement issues in Human Immunodeficiency Virus-AIDS. Nurs Clin North Am. 2018;53(1):123–135. doi:10.1016/j.cnur.2017.10.010
107. Siou K, Mahan M, Cartagena R, Chan Carusone S. A growing need—HIV education in long-term care. Geriatr Nurs. 2017;38(3):199–206. doi:10.1016/j.gerinurse.2016.10.014
108. Miller SC, Cai S, Daiello LA, Shireman TI, Wilson IB. Nursing home residents by human immunodeficiency virus status: characteristics, dementia diagnoses, and antipsychotic use. J Am Geriatr Soc. 2019;67(7):1353–1360. doi:10.1111/jgs.15949
109. Cai S, Miller SC, Wilson IB. Trajectory of physical functioning among persons living with HIV in nursing homes. J Am Med Dir Assoc. 2019;20(4):497–502. doi:10.1016/j.jamda.2019.01.126
110. Olivieri-Mui B, McGuire J, Cahill S, Griffith J, Briesacher B. People living with HIV in U.S. nursing homes in the fourth decade of the epidemic. J Assoc Nurses AIDS Care. 2019;30(1):20–34. doi:10.1097/JNC.0000000000000033
111. Foebel AD, Hirdes JP, Lemick R, Tai JWY. Comparing the characteristics of people living with and without HIV in long-term care and home care in Ontario, Canada. AIDS Care. 2015;27(10):1343–1353. doi:10.1080/09540121.2015.1058893
112. Dowdy DW, Powers KA, Hallett TB. Towards evidence-based integration of services for HIV, non-communicable diseases and substance use: insights from modelling. J Int AIDS Soc. 2020;23(Suppl1):e25525. doi:10.1002/jia2.25525
113. Siegler EL, Brennan-Ing M. Adapting systems of care for people aging with HIV. J Assoc Nurses AIDS Care. 2017;28(5):698–707. doi:10.1016/j.jana.2017.05.006
114. Schmalzle SA, Viviano NA, Mohanty K, et al. People aging with HIV - protecting a population vulnerable to effects of COVID-19 and its control measures. AIDS Care. 2022;34(11):1355–1363. doi:10.1080/09540121.2021.2020208
115. Levett T, Alford K, Roberts J, Adler Z, Wright J, Vera JH. Evaluation of a combined HIV and geriatrics clinic for older people living with HIV: the Silver Clinic in Brighton, UK. Geriatrics. 2020;5(4):81. doi:10.3390/geriatrics5040081
116. Guaraldi G, Orlando G, Squillace N, et al. Multidisciplinary approach to the treatment of metabolic and morphologic alterations of HIV-related lipodystrophy. HIV Clin Trials. 2006;7(3):97–106. doi:10.1310/EYWJ-8B5K-X7VQ-9CPE
117. Greene ML, Tan JY, Weiser SD, et al. Patient and provider perceptions of a comprehensive care program for HIV-positive adults over 50 years of age: the formation of the Golden Compass HIV and aging care program in San Francisco. PLoS One. 2018;13(12):e0208486. doi:10.1371/journal.pone.0208486
118. Tan JY, Greene M, Blat C, et al. Examining the impact of the golden compass clinical care program for older people with HIV: a qualitative study. AIDS Behav. 2022;26(5):1562–1571. doi:10.1007/s10461-021-03509-0
119. Adler AJ, Drown L, Boudreaux C, et al. Understanding integrated service delivery: a scoping review of models for noncommunicable disease and mental health interventions in low-and-middle income countries. BMC Health Serv Res. 2023;23(1):99. doi:10.1186/s12913-023-09072-9
120. Adeyemi O, Lyons M, Njim T, et al. Integration of non-communicable disease and HIV/AIDS management: a review of healthcare policies and plans in East Africa. BMJ Glob Health. 2021;6(5):e004669. doi:10.1136/bmjgh-2020-004669
121. Collins LF, Colasanti JA, Nguyen ML, et al. The COVID-19 pandemic as a catalyst for differentiated care models to end the HIV epidemic in the United States: applying lessons from high-burden settings. AIDS. 2021;35(2):337–341. doi:10.1097/QAD.0000000000002746
122. Duncombe C, Rosenblum S, Hellmann N, et al. Reframing HIV care: putting people at the centre of antiretroviral delivery. Trop Med Int Health. 2015;20(4):430–447. doi:10.1111/tmi.12460
123. Husain NE, Noor SK, Elmadhoun WM, et al. Diabetes, metabolic syndrome and dyslipidemia in people living with HIV in Africa: re-emerging challenges not to be forgotten. HIVAIDS Auckl NZ. 2017;9:193–202. doi:10.2147/HIV.S137974
124. UNAIDS. 2025 AIDS Targets; 2020. Available from: https://aidstargets2025.unaids.org/#section-targets.
125. Grosso TM, Hernández-Sánchez D, Dragovic G, et al. Identifying the needs of older people living with HIV (≥ 50 years old) from multiple centres over the world: a descriptive analysis. AIDS Res Ther. 2023;20(1):10. doi:10.1186/s12981-022-00488-7
126. EATG. The Glasgow Manifesto by the International Coalition of older people with HIV (iCOPe HIV). Available from: https://www.eatg.org/press-releases-and-statements/the-glasgow-manifesto-by-The-international-coalition-of-older-people-with-hiv-icope-hiv/.
127. Siegler E, Belander D, Hartigan J, Steinbock C, Wikiera J, Gonzalez C. New York State AIDS Institute initiatives to improve care of older people with HIV and long-term survivors: an example of a government-academic-community partnership. 13th Annual International Workshop on HIV and Aging. Rev Antiviral Ther Infect Dis. 2022;11:12.
128. Part F: Special Projects of National Significance (SPNS) program [website]. HRSA Ryan White HIV/AIDS program. Available from: https://ryanwhite.hrsa.gov/about/parts-and-initiatives/part-f-spns.
129. World Health Organization. Integrated Care for Older People (ICOPE): Guidance for Person-Centred Assessment and Pathways in Primary Care. Geneva, Switzerland: World Health Organization; 2019.
130. Norberg A, Nelson J, Holly C, Jewell ST, Lieggi M, Salmond S. Experiences of HIV-infected adults and healthcare providers with healthcare delivery practices that influence engagement in US primary healthcare settings: a qualitative systematic review. JBI Database Syst Rev Implement Rep. 2019;17(6):1154–1228. doi:10.11124/JBISRIR-2017-003756
131. Providers, advocates call for new strategies to improve access to HIV care; 2023. Available from: https://www.hivma.org/news_and_publications/hivma_news_releases/2023/providers-advocates-call-for-new-strategies-to-improve-access-to-hiv-care/.
132. Arku D, Felix M, Warholak T, Axon DR. Program of All-Inclusive Care for the Elderly (PACE) versus other programs: a scoping review of health outcomes. Geriatrics. 2022;7(2):31. doi:10.3390/geriatrics7020031
133. Karris MY. 2nd AC+: leveraging technology to create communities of care around older people living with HIV. NLM identifier: NCT04323176. Available from: https://clinicaltrials.gov/ct2/show/NCT04323176.
134. Justice AC, Akgün KM. What does aging with HIV mean for nursing homes? J Am Geriatr Soc. 2019;67(7):1327. doi:10.1111/jgs.15950
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
