Back to Journals » Neuropsychiatric Disease and Treatment » Volume 14
Treatment patterns, health care resource utilization, and costs in Japanese adults with attention-deficit hyperactivity disorder treated with atomoxetine
Authors Imagawa H, Nagar SP , Montgomery W, Nakamura T, Sato M , Davis KL
Received 29 August 2017
Accepted for publication 7 December 2017
Published 22 February 2018 Volume 2018:14 Pages 611—621
DOI https://doi.org/10.2147/NDT.S150261
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Video abstract presented by Saurabh P Nagar.
Views: 38908
Hideyuki Imagawa,1 Saurabh P Nagar,2 William Montgomery,3 Tomomi Nakamura,1 Masayo Sato,1 Keith L Davis2
1Medical Development Unit Japan, Eli Lilly Japan K.K., Kobe, Japan; 2RTI Health Solutions, Research Triangle Park, NC, USA; 3Global Patient Outcomes and Real World Evidence, Eli Lilly Australia, NSW, Australia
Objective: To describe the characteristics and medication treatment patterns of adult patients with attention-deficit/hyperactivity disorder (ADHD) prescribed atomoxetine in Japan.
Materials and methods: A retrospective analysis of insurance claims data was conducted using the Japan Medical Data Center database. Adults (≥18 years) with ADHD who had ≥1 atomoxetine claim from January 1, 2013 to December 31, 2014, and ≥180 to ≤900 days of follow-up were included. First atomoxetine claim defined the index date. Patient characteristics included age, gender, and comorbid conditions. Treatment patterns assessed included rates of atomoxetine discontinuation, switching, persistence, adherence (assessed via the medication possession ratio), and use of concomitant medications.
Results: A total of 457 adults met all the inclusion criteria. Mean (SD) age was 32.7 (10.4) years, and 61.0% of patients were male. Nearly 72.0% of the patients had at least one comorbid mental health condition in the baseline period; depression (43.8%) and insomnia (40.7%) were the most common mental health comorbidities. Most common physical comorbidities were chronic obstructive pulmonary disease (14.4%) and diabetes (12.9%). Non-ADHD-specific psychotropics were prescribed to 59.7% of patients during the baseline period and to 65.9% during the follow-up period; 6.6% were prescribed non-ADHD-specific psychotropics concomitantly with atomoxetine. Overall, 40.0% of adults discontinued atomoxetine during the entire follow-up period and 65.9% were persistent with atomoxetine therapy at 3 months post-index date. Mean (SD) atomoxetine medication possession ratio was 0.57 (0.25), and 25.4% switched to an alternative ADHD therapy; methylphenidate (22.4%) and non-ADHD-specific psychotropics (77.6%) were the most common medication alternatives. Nearly 8% augmented atomoxetine with methylphenidates, non-stimulants, or non-ADHD-specific psychotropics.
Conclusion: In this observational study, a majority of adults with ADHD treated with atomoxetine were still persistent with therapy at 3 months post-index date, with one quarter switching to alternative ADHD therapy. High proportions of mental health comorbidities, along with high use of non-ADHD-specific psychotropic medications in both the baseline and follow-up periods, were observed among patients with ADHD prescribed atomoxetine.
Keywords: ADHD, atomoxetine, treatment patterns, Japan, comorbidity, claims database, adherence, persistence
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a common neuropsychiatric disorder that impairs social, academic, and occupational functioning. Children and adolescents with ADHD struggle in academic functioning, self-esteem, and interpersonal relationships,1–3 and are at higher risk for mental health comorbidities, such as mood disorders and substance abuse disorders.4–6 While much of the study on ADHD focuses on its effects on children and adolescents, ADHD also has a substantial negative impact on the daily life of adults who have been diagnosed with the disorder.7,8
Many children who are diagnosed with ADHD continue to seek treatment into adulthood, with symptoms such as memory loss, poor organizational skills, problems finishing daily activities, and restlessness9,10 persisting beyond childhood in as many as two-thirds of adolescent patients with ADHD.11–13 The prevalence of ADHD among Japanese adults has been reported to be 1.7% in 2013.14 Furthermore, many patients with ADHD are not first diagnosed until adulthood or were misdiagnosed with another psychiatric comorbidity before their ADHD diagnosis.15
Japanese treatment guidelines for ADHD were first published in 2003 by Kanbayashi et al16 who included a key recommendation to reserve the use of ADHD-directed medications for severe cases in which psychotherapy is ineffective. In a subsequent revision to the treatment guidelines noted above, methylphenidate (MPH) was recommended as the standard first-line treatment for ADHD, followed by second-line treatment with mood stabilizers, antipsychotics, and/or antidepressants.17 However, non-medical and illegal uses of MPH lead to an increase in problems such as MPH addiction, adverse events, and medical complications. The increase in cases of MPH abuse triggered the establishment of the Ritalin Distribution Control Panel to introduce in 2007 regulations limiting access to prescribed MPH for ADHD.
After implementation of these regulations, an osmotic controlled-release oral stimulant MPH (OROS MPH) was approved as the only stimulant ADHD drug in Japan. A newer medication, atomoxetine – a non-stimulant, selective inhibitor of the presynaptic norepinephrine transporter – was approved in Japan in 2009 for the treatment of ADHD in children and adolescents, and subsequently for use in adults with ADHD in 2012.18 Previous studies conducted in Asian adults diagnosed with ADHD have reported an acceptable safety, tolerability, and efficacy profile of atomoxetine.19–21 However, despite the favorable safety profile, clinicians are recommended to use electrocardiogram for monitoring adults who are being considered for treatment or who are currently receiving treatment with atomoxetine to ensure the absence of cardiac side effects,22 although these side effects are still expected to be rare.23 Despite approval of an OROS MPH and the demonstrated safety and efficacy of non-stimulant atomoxetine therapy, attitudes of Japanese physicians toward the use of pharmacologic (versus behavioral) treatment of ADHD have remained somewhat negative, possibly due in part to a lack of additional therapeutic options in Japan compared with other countries.24 Moreover, even when pharmacologic treatment has been implemented for ADHD, therapeutic doses, particularly for stimulants, have been observed to be much lower in Japan compared with other countries.24
In addition to the humanistic burden of adult ADHD symptoms, the disorder poses an increased cost burden on health care systems. Previous research from the USA reported $143 to $266 billion in annual incremental costs associated with ADHD compared with non-ADHD controls over a 20-year period.25 Among the factors driving the overall cost burden of ADHD is increased frequency of physician office visits, as evidenced from data collected in an epidemiological survey of Japanese adults, suggesting an increased frequency of health care provider visits compared with patients without ADHD.14
To date, only limited data have been available on how atomoxetine is used in routine practice in Japan for the treatment of ADHD. Notable gaps in the literature include estimates for the prevalence of comorbid mental health and physical conditions, mental health-related medication history, atomoxetine adherence and persistence, and rates of switching to or augmentation with alternative ADHD-related (non-atomoxetine) therapies. Little information is available on overall health care utilization and costs for adult patients with ADHD treated with atomoxetine in Japan. To help address these information gaps, this retrospective cohort study using an insurance claims database was conducted to describe the baseline patient demographic and clinical characteristics, treatment patterns, and diagnostic monitoring among adults with ADHD treated with atomoxetine in regular clinical practice in Japan.
Materials and methods
Data source
Data for this study were derived from the Japan Medical Data Center (JMDC) database.26 The JMDC database comprises retrospective claims data sourced from the Japanese union-managed health insurance system (Health Insurance Association). The authors were not involved in the collection of this data. Retrieval of the data from this database occurred in an unlinked faction. As the data had been anonymized, the Ethical Guidelines for Epidemiological Research (Ministry of Education, Culture, Sports, Science and Technology, and Ministry of Health, Labour and Welfare of Japan), which require ethics approval and informed consent, were not applicable to this study. Based on the Ethical Guidelines on Biomedical Research Involving Human Subjects (Ministry of Education, Culture, Sports, Science and Technology, and Ministry of Health, Labour and Welfare of Japan), pharmacoepidemiological studies conducted on data from medical databases constitute research carried out on pre-existing material and information that did not require any interventions or interactions with patients. For such studies, including this study, obtaining written informed consent from patients is not required.
The database includes information predominantly from persons of working age (ie, <65 years old) employed by middle- to large-sized companies as well as their dependents. The database currently includes >1 million unique persons from 2003 onward and represents ~1% of the total population of Japan. The data are refreshed monthly and carry a lag of 4–5 months. Data elements captured in the JMDC database include patient-level demographic and plan enrollment information (eg, start and stop dates of health plan enrollment), date-stamped (month and year) inpatient and outpatient medical claims (eg, diagnosis codes, procedure codes, provider specialty, cost), and pharmacy claims (eg, prescription fill/refill dates, drug name/code, dosage, cost).
Patient selection and study period
The following inclusion criteria were applied for selection of patients from the JMDC database:
- at least one diagnosis claim for ADHD, defined by International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) diagnosis code F90.xx;
- at least one pharmacy claim for atomoxetine, with the first atomoxetine claim defining the study index date (no atomoxetine claim before the study index date);
- at least 18 years of age at the index date;
- at least 6 months of continuous insurance coverage/enrollment before the index date;
- at least 6 months of continuous insurance coverage/enrollment after the index date to ensure adequate follow-up on study measures.
The study data spanned a longitudinal period of 3 years from July 1, 2012 through June 30, 2015, and the study index date was identified from January 1, 2013 through December 31, 2014 to allow for at least 6 months of follow-up period. Figure 1 presents a graphical representation of the study period and the overall study design.
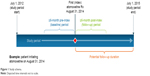  | Figure 1 Study schema. |
Study measures
All demographic characteristics available in the patients’ claims data set were documented, including age and sex. Daily dosage for each patient’s index prescription of atomoxetine and their second atomoxetine prescription (to evaluate the extent of initial dosing adjustment) was also assessed. Medication usage was defined by observation of relevant prescription fills (identified based on generic drug name descriptions containing “atomoxetine [Strattera; Eli Lilly Japan K.K., Kobe, Japan]”) observed in the JMDC pharmacy claims data. The presence of various mental health-related comorbidities, as defined by ICD-10 diagnosis codes, observed during the 6-month pre-index date period, was also assessed. In addition to ADHD-related mental health comorbidities, other chronic medical comorbidities were assessed to obtain a Charlson Comorbidity Index (CCI) score for each patient. The CCI score includes 20 categories of comorbidities, as defined by International Classification of Diseases, 9th Revision, Clinical Modification diagnosis codes (which were converted to ICD-10 codes for the JMDC database), with associated weights corresponding to the severity of the comorbid condition of interest.27 As with the previously noted mental health-related comorbidities, CCI scores were calculated for each patient based on the relevant diagnosis codes occurring during the 6-month period before the index date. Use of other ADHD- and mental health-related medications in the 6 months before the study index date (inclusive of the index date) was also reported. The ADHD- and mental health-related drug classes of interest for this analysis were stimulants (which were limited to MPH formulations in Japan at the time of this study), non-stimulants (other than atomoxetine), and non-ADHD-specific psychotropics (antipsychotics, antidepressants, and anxiolytics).
Various treatment pattern measures, as described in the following, were assessed from the study index date (atomoxetine initiation) until the earliest date of health plan disenrollment (≥6 months from atomoxetine initiation) or the end of the JMDC database extraction period (July 1, 2015).
- Overall atomoxetine discontinuation: Atomoxetine discontinuation was defined as the first day of the first observed atomoxetine refill gap of ≥60 days. The proportion of patients with atomoxetine discontinuation and time to discontinuation (atomoxetine duration) were documented. Discontinuations were classified as “permanent” (ie, no reuptake observed for the duration of available follow-up) or “temporary” (ie, atomoxetine reinitiated at any point after the conclusion of first ≥60-day refill gap).
- Switching: Among patients who discontinued atomoxetine, switching was defined as initiation of an alternative ADHD-related therapy (stimulant, non-stimulant other than atomoxetine, or non-ADHD-specific psychotropic) within 60 days after the atomoxetine discontinuation date. The proportion of patients with a switch, distribution of alternative agent(s) switched to, and time to switch from atomoxetine initiation were assessed.
- Augmentation: Augmentation of atomoxetine therapy was defined as the uptake of an alternative ADHD-related therapy (stimulant, non-stimulant other than atomoxetine, or non-ADHD-specific psychotropic) in concomitant use with the index atomoxetine therapy, with the requirement that patients have an overlapping days’ supply of ≥60 days for both therapies. The proportion of patients with augmentation, distribution of alternative agent(s) added to the index atomoxetine therapy, and time to augmentation from atomoxetine initiation were assessed.
- Atomoxetine persistence: Persistence with atomoxetine was assessed as the proportion of patients who remained on the drug with no refill gaps of ≥60 days from the index date through the end of available follow-up. The proportion of patients who were persistent on atomoxetine therapy (no refill gaps of ≥60 days) through 3 months post-index date also was assessed.
- Atomoxetine adherence: Adherence to atomoxetine therapy was assessed using the medication possession ratio (MPR), defined as the proportion of patients’ time on the drug (ie, days between the index date and the end of days’ supply of the last prescription) with medication supply on hand. Results were reported as the average MPR observed as well as a frequency distribution of MPR decile categories. Patients with an MPR of ≥0.8 (ie, refill adherence of ≥80%) were considered to be “adherent” with therapy.
In addition to the treatment pattern measures described above, various measures of health care utilization were assessed for each patient. This included utilization of cardiovascular monitoring (ie, electrocardiogram [ECG]) and other general health and mental health diagnostics (ie, blood draw/test, electroencephalography, psychological test) from atomoxetine initiation until follow-up end (plan disenrollment or database end), as well as various measures of ADHD-related health care resource utilization and costs for the 6-month post-index period. Specific health care utilization measures assessed were as follows:
- Hospitalizations: Proportion of patients with ≥1 hospitalization, number of hospitalizations per patient, total days hospitalized per patient, and total inpatient costs per patient.
- Outpatient/physician office visits: Proportion of patients with ≥1 outpatient visit, number of unique visits per patient, total days with a visit per patient, and total outpatient costs per patient.
- Pharmacy claims: Total number of prescription fills per patient and total prescription costs per patient.
Cost data were extracted from the JMDC database and were reported as Japanese yen (¥). ADHD-related utilization and costs included encounters and payments associated with claims containing a diagnosis code for ADHD (F90.xx) or pharmacy claims for ADHD-related medications (stimulants, non-stimulants, non-ADHD-specific psychotropics).
All study measures were summarized descriptively through the tabular and graphical display of mean values, SDs, medians, and ranges for continuous variables and frequency distributions for categorical variables.
Results
From January 1, 2013 to December 31, 2014, there were 5,432 patients who had at least one claim for an ADHD diagnosis (Figure 2). Among these patients with ADHD, 1,428 patients had at least one pharmacy claim for atomoxetine from January 1, 2013 to December 31, 2014. Of this population, 1,177 patients had at least 6 months of continuous health plan enrollment before and post-index date. Of these patients, 720 (61.2%) were children and adolescents aged <18 years and 457 (38.8%) were adults aged 18 years and older. Patient demographics are presented in Table 1. Mean (SD) patient age was 32.7 (10.4) years, and a majority (61.0%) were male. Median daily dose of atomoxetine at the index date, at second claim, and at 3 months post-index date were 40, 65, and 80 mg, respectively. Atomoxetine is recommended to be initiated at a total daily dose of 40 mg and increased to a target total daily dose of 80–120 mg in patients who have not achieved an optimal response.
Baseline clinical characteristics are presented in Table 2. Nearly 72.0% of the patients had at least one comorbid mental health condition in the baseline period, with depression (43.8%), insomnia/sleep disturbances (40.7%), schizophrenia (24.5%), bipolar disorder (21.4%), and pervasive developmental disorder (14.4%) being the most common mental health-related comorbidities. Hyperlipidemia (15.3%), chronic pulmonary disease (14.4%), diabetes with or without complications (12.9%), and hypertension (6.6%) were the most common general medical comorbidities. Baseline ADHD and mental health-related medication history is presented in Table 3. MPH was used by 10.1% of patients in the baseline period before atomoxetine initiation. Frequent use of non-ADHD-specific psychotropic medications (59.7%) was observed in patients during the baseline period. Atypical antipsychotics (23.6%), antidepressants (39.6%), and anxiolytics (55.4%) were also common in the baseline period. Among patients with comorbid depression in the baseline period, 67.0% were prescribed antidepressants during the follow-up period; among patients with bipolar disorder in the baseline period, 68.4% were prescribed antidepressants and 86.7% were prescribed anxiolytics during the follow-up period.
  | Table 3 ADHD- and other mental health-related medication utilization |
Treatment patterns during the post-index period are presented in Table 4. Overall, 183 of the 457 subjects studied (40.0%) discontinued atomoxetine during the entire follow-up period; of these, 167 (91.3%) discontinued permanently with no reinitiation during the follow-up period. Among the group of patients who discontinued treatment with atomoxetine, the median time to discontinuation was 49 days. Among all patients, 65.9% were persistent on atomoxetine therapy at 3 months post-index. Among all patients, mean (SD) MPR was 0.57 (0.25), with 20.1% of patients achieving an MPR ≥0.80. Among all patients, 25.4% switched to an alternative ADHD therapy; among patients with treatment switching, MPH (22.4%) and non-ADHD-specific psychotropic medication (77.6%) were the most common medications switched to. Among patients who switched therapy, the median time to switching was 63 days. Among all patients, 7.7% augmented atomoxetine with an alternative ADHD therapy, with MPH (17.1%) and non-ADHD-specific psychotropic medication (85.7%) being the most common agents used in augmentation. Among patients who augmented therapy, the median time to augmentation was 42 days.
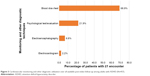
Cardiovascular and other diagnostic monitoring utilization patterns are presented in Figure 3. Overall, 6.6% of patients had at least one encounter for electroencephalogram during the follow-up period. Overall, 21.9% had at least one encounter for psychological testing and 68.9% had at least one encounter for a blood test. Only 2.2% of patients had an encounter for an ECG. ADHD-related health care resource utilization and costs during the 6-month post-index period are presented in Table 5. Nearly 1.5% of patients with ADHD had at least one ADHD-related hospitalization during the 6-month post-index period. Among those few patients who were hospitalized, the mean (SD) number of days hospitalized per patient was 22.0 (6.6), with the mean (SD) hospitalization costs per patient being ¥1,022,447 (¥753,731). The majority of patients (96.3%) in the study sample had at least one outpatient visit, with a mean (SD) number of visits per patient during the 6-month post-index period of 4.9 (1.8); mean (SD) number of days for an outpatient visit per patient was 2.2 (2.4). Mean (SD) outpatient costs per patient in the 6-month post-index period was ¥86,636 (¥139,154). Most (86.7%) patients had at least one ADHD- or mental health-related prescription fill, with a mean (SD) number of fills per patient of 4.9 (2.0). Mean (SD) pharmacy cost per patient in the 6-month post-index period was ¥167,973 (¥133,529). Mean (SD) total cost per patient in the 6-month post-index period, inclusive of ADHD-related hospitalizations, outpatient visits, and prescription fills, was ¥246,160 (¥227,622), or ¥492,320 (¥227,622) annually (Table 5).
Discussion
In this retrospective claims database study, we assessed treatment patterns, health care utilization and associated costs, and cardiovascular and other general health monitoring among adult patients with ADHD in Japan treated with atomoxetine. Results suggest that >70% of patients had at least one comorbid mental health disorder in the 6-month pre-index baseline period, and that depression was the most common mental health comorbidity observed. Most common physical comorbidities were hyperlipidemia, chronic pulmonary disease, diabetes, and hypertension during the 6-month pre-index baseline period. Not much information on the physical comorbidities of patients with ADHD is currently available to physicians in Japan, which could be an important finding for the neurodevelopmental world. Additionally, nearly 60% of adults were on non-ADHD-specific psychotropic medications, defined for this analysis as antipsychotics, antidepressants, and anxiolytics, in the baseline period before initiating atomoxetine. A study using data from an online survey in Japan similarly reported that adult patients with ADHD had a high frequency of mental health-related comorbidities, with depression being the most common.28 Previous studies of adult patients with ADHD conducted in other countries such as the USA and Australia also reported similar results with regard to baseline comorbidities.29–33
Results also suggested that most physicians prescribed antidepressants or anxiolytics during the follow-up period for patients with comorbid depression or bipolar disorder. These results suggest that atomoxetine might be generally used in combination with non-ADHD-specific psychotropics for patients with psychiatric comorbidities such as depression or bipolar disorder. Some physicians have raised concerns on tolerability or efficacy in using antidepressants with atomoxetine as an “antidepressant augmentation strategy” for patients with ADHD comorbid with depression.34 However, our results reported that in the real world, many physicians are actually comfortable prescribing non-ADHD-specific psychotropics with atomoxetine and could provide assurance on tolerability or efficacy. In addition, most patients with comorbid bipolar disorder were also prescribed antidepressants or anxiolytics. Previous studies also reported that treatment with mood stabilizers or antipsychotics is essential for patients with bipolar disorder and ADHD to avoid psychiatric activation if they are treated with atomoxetine simultaneously.35
Adherence and persistence to ADHD medications has been linked to positive treatment outcomes such as improvement in cognitive and academic functionality and reductions in unintentional injury and risk of future substance abuse.36–38 Our results showed that two-thirds of adult patients with ADHD remained persistent with atomoxetine therapy at 3 months, and that one-quarter of patients switched from atomoxetine to an alternative ADHD therapy. The rate of discontinuation of atomoxetine was 40% among adults with ADHD during the entire follow-up period. A placebo-controlled clinical trial study conducted in three Asian countries including Japan reported that a total of 30.9% patients with ADHD who were on atomoxetine treatment for 10 weeks had no response, defined as decrease of 25% in the Conners’ Adult ADHD Rating Scale–Investigator Rated: Screening Version score.39 A German study reported a discontinuation rate of 25.2% and a switch rate to MPH of 26.8% in the first year after treatment initiation; these data are generally consistent with the rates of discontinuation and switching reported here.40 A previous study using pooled data from clinical trials on adult patients with ADHD reported that continuous use of atomoxetine for at least 6 months would result in better outcomes among those who do not respond early to atomoxetine treatment.41 However, the JMDC database does not collect data on the response status or tolerability status of atomoxetine, so this could be a topic of further research. Our study also reported low adherence rates for atomoxetine as measured by MPR. A systematic literature review evaluating medication adherence and persistence among patients with ADHD reported drug adherence to be poor for all age groups and medication classes.42 A recent population-based study conducted among adult patients with ADHD in Taiwan reported mean MPR of 85.3% for those prescribed atomoxetine.43 The higher adherence rate reported in the Taiwanese study should be interpreted in the context of formulary restrictions – the Bureau of National Health Insurance requires patients with ADHD to fail on MPH before being prescribed atomoxetine, and use in adults is reimbursed only as continuation therapy following approval for use when the patient was younger than 18 years at the start of treatment. Taken together, the findings from our study on atomoxetine discontinuation and switch rates indicate that many adult patients being prescribed atomoxetine had a change in therapy, which suggests that this may be an unmet medical need given the importance of adherence and persistence to achieving optimal clinical outcomes.
Our study showed that 2.2% of patients with ADHD received ECG monitoring during all available follow-up. Despite the results from a recent clinical trial in Japanese adults with ADHD reporting significant changes in blood pressure, pulse rate, and body weight after administration of atomoxetine,18 many physicians in Japan may not be well aware of the importance of ECG monitoring before initiating medication and while prescribing this drug. To our knowledge, there is no study from a real-world population in Japan describing the frequency of cardiac testing among patients prescribed atomoxetine medication. Package insert recommends that it is important to conduct cardiovascular monitoring for the patients who might have the risk before initiating atomoxetine therapy.
Some additional observations regarding comorbidities are worth mentioning. Prevalence of diabetes without diabetic complications was almost 12% among the adult ADHD patients in our study. According to the National Nutrition Survey, prevalence of diabetes among general Japanese young adults was found to be 3%–5%, which is much lower than the prevalence reported in our study.44 Early identification of naïve patients in young adults at risk for ADHD among those with type II diabetes might be necessary to ensure that they receive prompt and appropriate treatment.45 While the association between ADHD and obesity is beginning to be documented in the literature, there is a paucity of data examining association of ADHD with obesity-related medical complications such as diabetes and hypertension.46,47
Our results also revealed that ADHD-related total costs were substantial (¥246,160: ~$2,461, where 100 JPY = US$1.00) in the 6-month post-index period, potentially driven by the number of ADHD-related outpatient visits and prescription fills observed in these patients. Consistent with our findings, results from recent surveys of adults with ADHD in Japan also reported high health care utilization (including physician visits, emergency department visits, and hospitalizations), particularly when compared with the general non-ADHD population.14,28 Although hospitalization was a rare event, the mean duration of inpatient stay (22 days) was considerable and may reflect the general trend in Japan for longer inpatient stays compared with most other Organization for Economic Co-operation and Development countries.48 Frequent use of health care services among adults with ADHD was also reported in the USA.49 To our knowledge, there is no literature available from a real-world population in Japan that has assessed health care utilization and costs among adults with ADHD using a health care claims database. Our study helps to fill this information gap and generally confirms the findings of previous survey-based data from Japan that patients with ADHD incur high direct medical costs.
Our study findings should be considered within the context of several limitations inherent in the JMDC data source. First, the ADHD study sample was selected from persons with health care coverage by the Japanese union-managed health insurance system, which may not be representative of all persons with ADHD in Japan who receive health care benefits under other insurance types. Second, the JMDC database contains only the month and year of service at the claim level for inpatient and outpatient encounters. Therefore, it was necessary to assume a within-month service date (last day of the month) for analytic purposes, which may have influenced the accuracy of event time and other duration measures. Third, claims data do not allow identification of intentional, planned breaks or “holidays” from therapy as is commonly implemented in ADHD pharmacotherapy; these types of clinically intentional breaks from therapy may result in prescription refills occurring later than expected, thus potentially biasing our estimates of MPR downward in regard to assessments of atomoxetine adherence. Our study is descriptive and not comparative in nature. The patient’s medical history may influence a physician’s decision to modify treatments. Our study found almost 70% of the study sample had a comorbid psychiatric condition during the baseline period with a significant history of prescriptions for non-ADHD-specific psychotropics. Hence, interpretation of the treatment pattern results should be made with caution. Moreover, the database did not have reasons underlying specific treatment patterns such as discontinuation, switching, and augmentation or the ability to link medication to the indication of use, which could have helped put results into context. In addition, no information on lab test results was available; therefore, assessments of background characteristics such as comorbid hypertension in the CCI score relied solely on ICD-10 coding, which may have resulted in an underestimation of the prevalence of these conditions. The results cannot be generalized to the adult population with ADHD since this study focused only on patients with ADHD who were prescribed atomoxetine. Furthermore, the diagnoses recorded in the claims data were subject to administrative coding errors and could not be validated with a post hoc medical record review.
Conclusion
Many adult patients with ADHD in Japan also have psychiatric comorbidities and were taking non-ADHD-specific psychotropic medications during the baseline period, and patients with comorbid depression or bipolar disorder were likely to continue taking non-ADHD-specific psychotropics in the follow-up period. Among Japanese adults with ADHD treated with atomoxetine, two thirds remained persistent with therapy at 3 months post-index date, with one quarter switching to alternative ADHD therapy. However, the proportion of patients who were fully adherent to treatment was low. Treatment persistence and adherence in patients with ADHD has been well documented to influence treatment outcomes. Strategies aimed at improving both medication persistence and adherence have the potential to improve patient outcomes. Given the low rates of cardiovascular monitoring in the atomoxetine recipients studied here, there may be a need for increased awareness among Japanese health care providers of the value of ECG monitoring, particularly for adverse cardiac effects, in patients with ADHD prescribed atomoxetine therapy. Finally, our study confirmed survey-based findings from other studies of patients with ADHD, suggesting that these patients confer a high health care utilization and cost burden on third-party payers. This is one of the first studies conducted in Japan to evaluate a large cohort of patients from an administrative database and to assess treatment patterns of atomoxetine and health care resource utilization associated with the treatment of adult ADHD. The findings of this study reflect the current dosing and treatment patterns of atomoxetine in a real-world clinical practice in Japan.
Acknowledgment
This study was funded by Eli Lilly Japan K.K.
Disclosures
Hideyuki Imagawa, Tomomi Nakamura, and Masayo Sato were employees and stockholders of Eli Lilly Japan K.K. when this study was conducted. Saurabh P Nagar and Keith L Davis are employees of RTI Health Solutions, Research Triangle Park, NC, USA which has received remuneration from Eli Lilly Japan K.K. William Montgomery was an employee and stockholder of Eli Lilly Australia when this study was conducted. The authors report no other conflicts of interest in this work.
References
Adesman AR. The diagnosis and management of attention-deficit/hyperactivity disorder in pediatric patients. Prim Care Companion J Clin Psychiatry. 2001;3(2):66–77. | ||
Mannuzza S, Klein RG. Long-term prognosis in attention-deficit/hyperactivity disorder. Child Adolesc Psychiatr Clin N Am. 2000;9(3):711–726. | ||
Minde K, Lewin D, Weiss G, Lavigueur H, Douglas V, Sykes E. The hyperactive child in elementary school: a 5 year, controlled, followup. Except Child. 1971;38(3):215–221. | ||
Biederman J, Newcorn J, Sprich S. Comorbidity of attention deficit hyperactivity disorder with conduct, depressive, anxiety, and other disorders. Am J Psychiatry. 1991;148(5):564–577. | ||
Biederman J. Attention-deficit/hyperactivity disorder: a life-span perspective. J Clin Psychiatry. 1998;59 (Suppl 7):S4–S16. | ||
Spencer TJ, Biederman J, Mick E. Attention-deficit/hyperactivity disorder: diagnosis, lifespan, comorbidities, and neurobiology. J Pediatr Psychol. 2007;32(6):631–642. | ||
Antshel KM, Hargrave TM, Simonescu M, Kaul P, Hendricks K, Faraone SV. Advances in understanding and treating ADHD. BMC Med. 2011;9:72. | ||
Rösler M, Casas M, Konofal E, Buitelaar J. Attention deficit hyperactivity disorder in adults. World J Biol Psychiatry. 2010;11(5):684–698. | ||
Faraone SV, Biederman J, Spencer T, et al. Attention-deficit/hyperactivity disorder in adults: an overview. Biol Psychiatry. 2000;48(1):9–20. | ||
Adler L, Cohen J. Diagnosis and evaluation of adults with attention-deficit/hyperactivity disorder. Psychiatr Clin North Am. 2004;27:187–201. | ||
Wilens TE, Morrison NR, Prince J. An update on the pharmacotherapy of attention-deficit/hyperactivity disorder in adults. Expert Rev Neurother. 2011;11(10):1443–1465. | ||
Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;2:159–165. | ||
Klein RG, Mannuzza S, Olazagastil MAR, et al. Clinical and functional outcome of childhood attention-deficit/hyperactivity disorder 33 years later. Arch Gen Psychiatry. 2012;69(12):1295–1303. | ||
Nakamura S, Ohnishi M, Uchiyama S. Epidemiological survey of adult attention deficit hyperactivity disorder (ADHD) in Japan. Jpn J Psychiatr Treat. 2013;28:155–162. | ||
Nylander L, Holmqvist M, Gustafson L, Gillberg C. Attention-deficit/hyperactivity disorder (ADHD) and autism spectrum disorder (ASD) in adult psychiatry. A 20-year register study. Nordic J Psychiatry. 2013;67(5):344–350. | ||
Kanbayashi Y, Saito K, Kita M. Japanese Guideline for Diagnosis and Treatment of AD/HD [in Japanese]. Tokyo: Jiho; 2003. | ||
Saito K, Watanabe K. Japanese Guideline for Diagnosis and Treatment of AD/HD, Revised Edition [in Japanese]. Tokyo: Jiho; 2006. | ||
Hirata Y, Goto T, Takita Y, et al. Long-term safety and tolerability of atomoxetine in Japanese adults with attention deficit hyperactivity disorder. Asia Pac Psychiatry. 2014;6(3):292–301. | ||
Takahashi M, Takita Y, Goto T, et al. An open-label, dose-titration tolerability study of atomoxetine hydrochloride in Japanese adults with attention-deficit/hyperactivity disorder. Psychiatry Clin Neurosci. 2011;65(1):55–63. | ||
Takahashi M, Goto T, Takita Y, Chung S, Wang Y, Shur-Fen Gau S. Open-label, dose-titration tolerability study of atomoxetine hydrochloride in Korean, Chinese, and Taiwanese adults with attention-deficit/hyperactivity disorder. Asia Pac Psychiatry. 2014;6(1):62–70. | ||
Matsui A, Azuma J, Witcher JW, et al. Pharmacokinetics, safety, and tolerability of atomoxetine and effect of CYP2D6*10/*10 genotype in healthy Japanese men. J Clin Pharmacol. 2012;52(3):388–403. | ||
Camporeale A, Upadhyaya H, Ramos-Quiroga JA, et al. Safety and tolerability of atomoxetine hydrochloride in a long-term, placebo-controlled randomized withdrawal study in European and non-European adults with attention-deficit/hyperactivity disorder. Eur J Psychiatry. 2013;27(3):206–224. | ||
Wernicke JF, Faries D, Girod D, et al. Cardiovascular effects of atomoxetine in children, adolescents, and adults. Drug Saf. 2003;26(10):729–740. | ||
Takeda T. Psychopharmacology for attention deficit/hyperactivity disorder in Japan. Curr Attention Dis Rep. 2009;1(1):21–28. | ||
Doshi JA, Hodgkins P, Kahle J, et al. Economic impact of childhood and adult attention-deficit/hyperactivity disorder in the United States. J Am Acad Adolesc Psychiatry. 2012;51(10):990–1002. | ||
Japan Medical Data Center [homepage on the Internet]. Tokyo: Japan Medical Data Center Co., Ltd. Available from: https://www.jmdc.co.jp/en/about/database.html. Accessed July 6, 2017. | ||
Charlson ME, Charlson RE, Peterson JC, Marinopoulos SS, Briggs WM, Hollenberg JP. The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol. 2008;61(12):1234–1240. | ||
Kirino E, Imagawa H, Goto T, Montgomery W. Sociodemographics, comorbidities, healthcare utilization and work productivity in Japanese patients with adult ADHD. PLoS One. 2015;10(7):e0132233. | ||
Secnik K, Swensen A, Lage MJ. Comorbidities and costs of adult patients diagnosed with attention-deficit hyperactivity disorder. Pharmacoeconomics. 2005;23(1):93–102. | ||
Perwien AR, Hall J, Swensen A, Swindle R. Stimulant treatment patterns and compliance in children and adults with newly treated attention-deficit/hyperactivity disorder. J Manag Care Pharm. 2004;10(2):122–129. | ||
Biederman J, Faraone S, Milberger S, et al. A prospective 4-year follow-up study of attention-deficit hyperactivity and related disorders. Arch Gen Psychiatry. 1996;53(5):437–446. | ||
Kooij JS, Huss M, Asherson P, et al. Distinguishing comorbidity and successful management of adult ADHD. J Atten Disord. 2012;16(5 Suppl):3S–19S. | ||
Das D, Cherbuin N, Butterworth P, Anstey KJ, Easteal S. A population-based study of attention deficit/hyperactivity disorder symptoms and associated impairment in middle-aged adults. PLoS One. 2012;7(2):e31500. | ||
McIntosh D, Kutcher S, Binder C, Levitt A, Fallu A, Rosenbluth M. Adult ADHD and comorbid depression: a consensus-derived diagnostic algorithm for ADHD. Neuropsychiatr Dis Treat. 2009;5:137–150. | ||
Chang K, Nayar D, Howe M, Rana M. Atomoxetine as an adjunct therapy in the treatment of co-morbid attention-deficit/hyperactivity disorder in children and adolescents with bipolar I or II disorder. J Child Adolesc Psychopharmacol. 2009;19(5):547–551. | ||
Barkley RA. Challenges in diagnosing adults with ADHD. J Clin Psychiatry. 2008;69(12):e36. | ||
Buckley PF, Foster AE, Patel NC, Wermert A. Adherence to Mental Health Treatment. New York, NY: Oxford University Press; 2009. | ||
Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. | ||
Goto T, Hirata Y, Takita Y, et al. Efficacy and safety of atomoxetine hydrochloride in Asian adults with ADHD: a multinational 10-week randomized double-blind placebo-controlled Asian study. J Atten Disord. 2017;21(2):100–109. | ||
Garbe E, Mikolajczyk RT, Banaschewski T, et al. Drug treatment patterns of attention-deficit/hyperactivity disorder in children and adolescents in Germany: results from a large population-based cohort study. J Child Adolesc Psychopharmacol. 2012;22(6):452–458. | ||
Sobanski E, Leppämäki S, Bushe C, Berggren L, Casillas M, Deberdt W. Patterns of long-term and short-term responses in adult patients with attention-deficit/hyperactivity disorder in a completer cohort of 12 weeks or more with atomoxetine. Eur Psychiatry. 2015;30(8):1011–1020. | ||
Gajria K, Lu M, Sikirica V, Greven P, Zhong Y, Qin P, Xie J. Adherence, persistence, and medication discontinuation in patients with attention-deficit/hyperactivity disorder–a systematic literature review. Neuropsychiatr Dis Treat. 2014;10:1543. | ||
Cheng YS, Shyu YC, Lee SY, Yuan SS, Yang CJ, Yang KC, Lee TL, et al. Trend, characteristics, and pharmacotherapy of adults diagnosed with attention-deficit/hyperactivity disorder: a nationwide survey in Taiwan. Neuropsychiatr Dis Treat. 2017;13:643. | ||
Neville SE, Boye KS, Montgomery WS, Iwamoto K, Okamura M, Hayes RP. Diabetes in Japan: a review of disease burden and approaches to treatment. Diabetes Metab Res Rev. 2009;25(8):705–716. | ||
Chen HJ, Lee YJ, Yeh GC, Lin HC. Association of attention-deficit/hyperactivity disorder with diabetes: a population-based study. Pediatr Res. 2013;73(4 Pt 1):492–496. | ||
Krause I, Cleper R, Kovalski Y, Sinai L, Davidovits M. Changes in behavior as an early symptom of renovascular hypertension in children. Pediatr Nephrol. 2009;24(11):2271–2274. | ||
Levitt Katz LE, Swami S, Abraham M, et al. Neuropsychiatric disorders at the presentation of type 2 diabetes mellitus in children. Pediatr Diabetes. 2005;6(2):84–89. | ||
OECD. Length of Hospital Stay (Indicator); 2017. Available from: https://data.oecd.org/healthcare/length-of-hospital-stay.htm. Accessed November 21, 2017. | ||
Wasserstein J. Diagnostic issues for adolescents and adults with ADHD. J Clin Psychol. 2005;61(5):535–547. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.